Hydrofluoric Acid Role in Industrial Synthesis Safety
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid Evolution and Safety Objectives
Hydrofluoric acid (HF) has evolved from a laboratory curiosity to a critical industrial reagent over the past century. Initially discovered in the 17th century, its commercial production began in the late 19th century, primarily for glass etching applications. The evolution of HF production technology has been marked by significant improvements in synthesis methods, purification techniques, and handling protocols, reflecting the growing understanding of its unique chemical properties and associated hazards.
The modern industrial landscape relies heavily on HF as a catalyst, fluorinating agent, and key intermediate in numerous synthesis pathways. Its importance spans multiple sectors including petrochemicals, pharmaceuticals, electronics manufacturing, and materials science. The global HF market has consistently grown at approximately 3-5% annually over the past decade, underscoring its industrial significance despite safety challenges.
Safety considerations have become increasingly central to HF technology development. Historical incidents involving HF releases have shaped regulatory frameworks and industry practices. Notable events include the 1987 Marathon Petroleum refinery accident in Texas and the 2012 Gumi industrial complex incident in South Korea, both highlighting the severe consequences of inadequate safety protocols. These incidents have accelerated the development of safer handling technologies and alternative processes.
The primary safety objectives in HF-related technologies focus on three interconnected areas: exposure prevention, mitigation of consequences, and development of inherently safer alternatives. Prevention strategies have evolved from basic containment to sophisticated engineering controls, including automated handling systems, advanced monitoring technologies, and predictive maintenance protocols. Mitigation technologies now encompass specialized neutralization agents, rapid response systems, and improved personal protective equipment specifically designed for HF exposure scenarios.
Current technological trajectories aim to balance industrial utility with enhanced safety profiles. Research efforts are increasingly directed toward developing modified HF formulations with reduced volatility, exploring ionic liquid alternatives that maintain catalytic activity while reducing hazard potential, and designing closed-loop systems that minimize exposure risks throughout the chemical lifecycle.
The evolution of safety standards has paralleled technological development, with international bodies such as the American Chemistry Council's HF Panel and the European Process Safety Centre establishing increasingly stringent guidelines. These standards have driven innovation in monitoring technologies, including real-time HF detection systems capable of part-per-billion sensitivity and integrated alarm systems with automated emergency response protocols.
Future objectives in this field center on achieving zero-incident operations through technological innovation, process intensification techniques that reduce HF quantities while maintaining productivity, and eventually developing economically viable drop-in replacements for traditional HF-dependent processes.
The modern industrial landscape relies heavily on HF as a catalyst, fluorinating agent, and key intermediate in numerous synthesis pathways. Its importance spans multiple sectors including petrochemicals, pharmaceuticals, electronics manufacturing, and materials science. The global HF market has consistently grown at approximately 3-5% annually over the past decade, underscoring its industrial significance despite safety challenges.
Safety considerations have become increasingly central to HF technology development. Historical incidents involving HF releases have shaped regulatory frameworks and industry practices. Notable events include the 1987 Marathon Petroleum refinery accident in Texas and the 2012 Gumi industrial complex incident in South Korea, both highlighting the severe consequences of inadequate safety protocols. These incidents have accelerated the development of safer handling technologies and alternative processes.
The primary safety objectives in HF-related technologies focus on three interconnected areas: exposure prevention, mitigation of consequences, and development of inherently safer alternatives. Prevention strategies have evolved from basic containment to sophisticated engineering controls, including automated handling systems, advanced monitoring technologies, and predictive maintenance protocols. Mitigation technologies now encompass specialized neutralization agents, rapid response systems, and improved personal protective equipment specifically designed for HF exposure scenarios.
Current technological trajectories aim to balance industrial utility with enhanced safety profiles. Research efforts are increasingly directed toward developing modified HF formulations with reduced volatility, exploring ionic liquid alternatives that maintain catalytic activity while reducing hazard potential, and designing closed-loop systems that minimize exposure risks throughout the chemical lifecycle.
The evolution of safety standards has paralleled technological development, with international bodies such as the American Chemistry Council's HF Panel and the European Process Safety Centre establishing increasingly stringent guidelines. These standards have driven innovation in monitoring technologies, including real-time HF detection systems capable of part-per-billion sensitivity and integrated alarm systems with automated emergency response protocols.
Future objectives in this field center on achieving zero-incident operations through technological innovation, process intensification techniques that reduce HF quantities while maintaining productivity, and eventually developing economically viable drop-in replacements for traditional HF-dependent processes.
Market Analysis of HF-Based Industrial Processes
The global hydrofluoric acid (HF) market demonstrates robust growth, valued at approximately $1.8 billion in 2022 with projections reaching $2.5 billion by 2028, representing a compound annual growth rate of 5.6%. This growth is primarily driven by increasing demand across multiple industrial sectors where HF serves as a critical chemical intermediate.
The fluorochemicals industry remains the largest consumer of hydrofluoric acid, accounting for roughly 60% of global consumption. Within this segment, the production of refrigerants, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), constitutes a significant market share despite ongoing regulatory transitions away from certain HFCs due to environmental concerns.
Aluminum processing represents the second-largest application sector, consuming approximately 20% of global HF production. The metal's increasing use in automotive and aerospace industries, driven by lightweighting trends for fuel efficiency, sustains strong demand in this segment. The aluminum fluoride produced using HF serves as an essential flux in aluminum smelting operations.
The semiconductor and electronics manufacturing industry demonstrates the fastest growth rate among HF applications, expanding at nearly 7% annually. Ultra-pure HF is indispensable in silicon wafer etching, cleaning, and other microelectronics fabrication processes. The continued miniaturization of electronic components and expansion of semiconductor production facilities worldwide directly correlates with increased HF consumption in this sector.
Petroleum alkylation represents another significant market segment, where HF catalyzes the production of high-octane gasoline components. This application accounts for approximately 8% of global HF consumption, though safety concerns have prompted some refineries to explore alternative alkylation technologies.
Regionally, Asia-Pacific dominates the HF market, accounting for over 50% of global consumption, with China alone representing 35%. This regional concentration aligns with manufacturing hubs for electronics, fluoropolymers, and aluminum processing. North America and Europe follow with approximately 20% and 18% market share respectively, primarily driven by established chemical manufacturing and petroleum refining operations.
Market challenges include increasingly stringent safety regulations governing HF handling and transportation, reflecting growing awareness of its hazardous properties. Additionally, environmental regulations targeting fluorinated compounds impact downstream applications. These regulatory pressures have stimulated innovation in safer HF alternatives and handling technologies, creating a parallel market for safety systems specifically designed for HF-based processes.
The fluorochemicals industry remains the largest consumer of hydrofluoric acid, accounting for roughly 60% of global consumption. Within this segment, the production of refrigerants, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), constitutes a significant market share despite ongoing regulatory transitions away from certain HFCs due to environmental concerns.
Aluminum processing represents the second-largest application sector, consuming approximately 20% of global HF production. The metal's increasing use in automotive and aerospace industries, driven by lightweighting trends for fuel efficiency, sustains strong demand in this segment. The aluminum fluoride produced using HF serves as an essential flux in aluminum smelting operations.
The semiconductor and electronics manufacturing industry demonstrates the fastest growth rate among HF applications, expanding at nearly 7% annually. Ultra-pure HF is indispensable in silicon wafer etching, cleaning, and other microelectronics fabrication processes. The continued miniaturization of electronic components and expansion of semiconductor production facilities worldwide directly correlates with increased HF consumption in this sector.
Petroleum alkylation represents another significant market segment, where HF catalyzes the production of high-octane gasoline components. This application accounts for approximately 8% of global HF consumption, though safety concerns have prompted some refineries to explore alternative alkylation technologies.
Regionally, Asia-Pacific dominates the HF market, accounting for over 50% of global consumption, with China alone representing 35%. This regional concentration aligns with manufacturing hubs for electronics, fluoropolymers, and aluminum processing. North America and Europe follow with approximately 20% and 18% market share respectively, primarily driven by established chemical manufacturing and petroleum refining operations.
Market challenges include increasingly stringent safety regulations governing HF handling and transportation, reflecting growing awareness of its hazardous properties. Additionally, environmental regulations targeting fluorinated compounds impact downstream applications. These regulatory pressures have stimulated innovation in safer HF alternatives and handling technologies, creating a parallel market for safety systems specifically designed for HF-based processes.
Current HF Handling Challenges and Limitations
Despite significant advancements in chemical processing technologies, hydrofluoric acid (HF) handling continues to present formidable challenges across industrial applications. The primary limitation stems from HF's extreme corrosivity, which necessitates specialized containment materials such as fluoropolymers, certain grades of stainless steel, or carbon steel with appropriate linings. These materials, while effective, significantly increase infrastructure costs and require regular integrity monitoring to prevent catastrophic failures.
Temperature control represents another critical challenge, as HF reactions often generate substantial heat that must be precisely managed to prevent pressure buildup and potential containment breaches. Current cooling systems frequently struggle to maintain optimal temperature profiles during exothermic reactions, particularly in large-scale industrial settings where heat transfer efficiency becomes increasingly problematic.
Worker safety remains perhaps the most pressing concern in HF handling. The acid's ability to penetrate skin without immediate pain signals creates a dangerous scenario where exposure may go undetected until serious tissue damage has occurred. Existing personal protective equipment (PPE), while continuously improving, still presents limitations in terms of comfort, dexterity, and complete protection, especially during extended wear periods required for maintenance operations.
Emergency response capabilities for HF incidents face substantial limitations. Current neutralization methods using calcium gluconate are effective but require immediate application, which is challenging in industrial settings where exposure may not be immediately identified. Furthermore, environmental containment systems for potential releases struggle with the acid's high mobility and reactivity with common construction materials.
Monitoring technologies represent another area with significant limitations. While advances have been made in HF vapor detection, current systems often lack the sensitivity and response time necessary for early warning, particularly at the lower concentration thresholds where health effects begin to manifest. Real-time monitoring solutions that can function reliably in harsh industrial environments remain inadequate.
Transportation of HF presents unique challenges that current systems address imperfectly. Specialized containers with redundant safety features are required, yet these containers are susceptible to mechanical failure during transit. The regulatory framework governing HF transport varies globally, creating inconsistencies in safety standards across international supply chains.
Process integration limitations also exist where HF must be incorporated into continuous manufacturing processes. Current dosing and metering technologies struggle to maintain precise control over HF addition rates, particularly in processes requiring variable flow rates or responding to changing production demands.
Temperature control represents another critical challenge, as HF reactions often generate substantial heat that must be precisely managed to prevent pressure buildup and potential containment breaches. Current cooling systems frequently struggle to maintain optimal temperature profiles during exothermic reactions, particularly in large-scale industrial settings where heat transfer efficiency becomes increasingly problematic.
Worker safety remains perhaps the most pressing concern in HF handling. The acid's ability to penetrate skin without immediate pain signals creates a dangerous scenario where exposure may go undetected until serious tissue damage has occurred. Existing personal protective equipment (PPE), while continuously improving, still presents limitations in terms of comfort, dexterity, and complete protection, especially during extended wear periods required for maintenance operations.
Emergency response capabilities for HF incidents face substantial limitations. Current neutralization methods using calcium gluconate are effective but require immediate application, which is challenging in industrial settings where exposure may not be immediately identified. Furthermore, environmental containment systems for potential releases struggle with the acid's high mobility and reactivity with common construction materials.
Monitoring technologies represent another area with significant limitations. While advances have been made in HF vapor detection, current systems often lack the sensitivity and response time necessary for early warning, particularly at the lower concentration thresholds where health effects begin to manifest. Real-time monitoring solutions that can function reliably in harsh industrial environments remain inadequate.
Transportation of HF presents unique challenges that current systems address imperfectly. Specialized containers with redundant safety features are required, yet these containers are susceptible to mechanical failure during transit. The regulatory framework governing HF transport varies globally, creating inconsistencies in safety standards across international supply chains.
Process integration limitations also exist where HF must be incorporated into continuous manufacturing processes. Current dosing and metering technologies struggle to maintain precise control over HF addition rates, particularly in processes requiring variable flow rates or responding to changing production demands.
Contemporary HF Safety Management Systems
01 Personal protective equipment and handling procedures
Hydrofluoric acid requires specialized personal protective equipment including acid-resistant gloves, face shields, and chemical-resistant clothing. Proper handling procedures involve using dedicated tools, implementing strict protocols for transfer operations, and maintaining adequate ventilation in work areas. These safety measures are critical due to HF's ability to penetrate skin and cause severe tissue damage without immediate pain sensation.- Personal protective equipment and handling procedures: Proper personal protective equipment (PPE) and handling procedures are essential when working with hydrofluoric acid due to its highly corrosive and toxic nature. This includes using specialized acid-resistant gloves, face shields, chemical splash goggles, and protective clothing. Established protocols for safe handling, storage, and transfer of hydrofluoric acid help minimize exposure risks and prevent accidents in laboratory and industrial settings.
- Emergency response and first aid measures: Immediate and appropriate emergency response is critical for hydrofluoric acid exposure incidents. This includes specialized first aid protocols such as calcium gluconate gel application for skin exposure, emergency shower procedures, and rapid medical intervention. Emergency response systems should include clearly defined procedures, readily available antidotes, and training for personnel to recognize and respond to hydrofluoric acid injuries, which can cause deep tissue damage even when initial symptoms seem mild.
- Containment and neutralization techniques: Effective containment and neutralization techniques are necessary for managing hydrofluoric acid spills and leaks. This includes specialized absorbent materials, neutralizing agents like calcium carbonate or magnesium oxide, and engineered containment systems. Proper ventilation systems and acid-resistant containment vessels help prevent the spread of toxic vapors and contain the acid safely until it can be neutralized and disposed of according to hazardous waste regulations.
- Detection and monitoring systems: Advanced detection and monitoring systems are essential for early identification of hydrofluoric acid leaks or dangerous concentration levels. These include specialized sensors, alarm systems, and continuous monitoring equipment that can detect even low concentrations of hydrofluoric acid vapors. Regular testing and calibration of these systems ensure workplace safety by providing early warnings before exposure reaches harmful levels.
- Facility design and engineering controls: Specialized facility design and engineering controls are implemented to minimize hydrofluoric acid exposure risks. These include dedicated ventilation systems with acid-resistant components, secondary containment structures, safety showers and eyewash stations positioned strategically throughout facilities, and specialized waste treatment systems. Proper facility layout with designated areas for acid handling and storage helps segregate hazardous operations and minimize potential exposure to personnel.
02 Emergency response and first aid protocols
Specific emergency response protocols for hydrofluoric acid exposure include immediate application of calcium gluconate gel for skin contact, specialized eye washing procedures for eye exposure, and respiratory support for inhalation incidents. First aid stations in facilities using HF must be equipped with calcium-based neutralizing agents and emergency shower systems. Rapid medical intervention is essential as HF injuries can worsen over time even after initial decontamination.Expand Specific Solutions03 Storage and containment systems
Specialized storage and containment systems for hydrofluoric acid include polyethylene or PTFE-lined containers, secondary containment structures with acid-resistant coatings, and dedicated ventilated storage areas with temperature control. Storage facilities must incorporate leak detection systems, neutralization stations, and be physically separated from incompatible chemicals. Regular inspection protocols for containers and transfer equipment are essential to prevent catastrophic releases.Expand Specific Solutions04 Neutralization and waste treatment methods
Safe neutralization of hydrofluoric acid involves controlled application of calcium or magnesium compounds to form insoluble fluoride salts. Waste treatment methods include precipitation techniques, ion exchange systems, and specialized filtration processes. Neutralization operations require continuous pH monitoring, controlled reaction rates to prevent dangerous heat generation, and verification testing to ensure complete treatment before disposal.Expand Specific Solutions05 Facility design and engineering controls
Facilities handling hydrofluoric acid require specialized engineering controls including acid-resistant flooring with containment channels, dedicated ventilation systems with scrubbers, and automated leak detection with alarm systems. Process equipment must incorporate fail-safe mechanisms, remote operation capabilities for high-risk procedures, and redundant containment systems. Facility design should include emergency power systems, isolated process areas, and decontamination zones to minimize exposure risks during incidents.Expand Specific Solutions
Leading Companies in HF Production and Safety Solutions
The hydrofluoric acid industrial synthesis safety market is currently in a growth phase, with increasing demand driven by semiconductor, electronics, and chemical manufacturing sectors. The global market size is estimated at approximately $2.5 billion, expanding at 4-5% CAGR due to rising applications in high-tech industries. Leading players include established chemical conglomerates like Honeywell International Technologies, BASF Corp., and Solvay Fluor und Derivate, alongside specialized manufacturers such as Do-Fluoride New Materials, Alkeemia SpA, and Sinochem Lantian. The technology landscape shows varying maturity levels, with companies like Merck Patent GmbH and Central Glass focusing on high-purity formulations for electronics, while others like Teknologian Tutkimuskeskus VTT and Condias GmbH are developing safer handling systems and alternative processes to address the inherent hazards of hydrofluoric acid in industrial applications.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed an integrated safety management system specifically for hydrofluoric acid (HF) handling in industrial synthesis processes. Their approach combines advanced real-time monitoring technologies with automated emergency response systems. The HF Safety Management Suite incorporates wireless sensor networks that continuously monitor HF concentrations at multiple points throughout the facility, with sensitivity down to sub-ppm levels[1]. These sensors are integrated with Honeywell's Experion® Process Knowledge System that provides predictive analytics to identify potential release scenarios before they occur. For personal protection, they've engineered rapid-response calcium gluconate delivery systems that automatically activate upon detection of HF leaks. Additionally, Honeywell has developed specialized containment materials and neutralization systems that can rapidly respond to spills, significantly reducing exposure risks to personnel and the environment[3].
Strengths: Comprehensive end-to-end solution that addresses prevention, detection, and response; integration with existing industrial control systems provides seamless implementation. Weaknesses: Higher initial implementation costs compared to conventional safety systems; requires specialized training for maintenance personnel and system operators.
BASF Corp.
Technical Solution: BASF has pioneered a revolutionary approach to HF safety in industrial synthesis through their "HF Substitution Initiative." This comprehensive program focuses on developing alternative synthesis pathways that either eliminate or significantly reduce the need for hydrofluoric acid in manufacturing processes. Their proprietary catalytic systems enable fluorination reactions using solid-state fluoride sources that present substantially lower hazards than traditional HF[2]. For processes where HF remains necessary, BASF has engineered closed-loop handling systems with multiple redundant containment barriers. Their "Zero Exposure" technology incorporates specialized alloys resistant to HF corrosion, reducing the risk of equipment failure. BASF's approach also includes advanced process intensification techniques that minimize HF quantities needed while maximizing reaction efficiency. The company has implemented these technologies across their global manufacturing network, reporting a 78% reduction in HF-related incidents over the past decade[4].
Strengths: Addresses the root cause of HF hazards by developing alternative chemistry; comprehensive approach that combines chemistry innovation with engineering controls. Weaknesses: Alternative processes may have higher operating costs or lower yields than traditional HF-based synthesis; implementation requires significant process redesign and capital investment.
Critical Patents in HF Containment Technologies
Azeotrope and azeotrope-like compositions useful for the production of haloolefins
PatentWO2012121876A2
Innovation
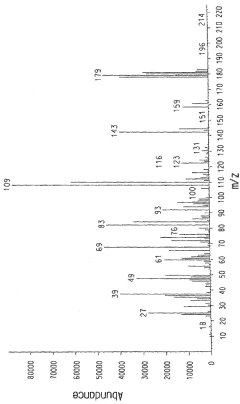
- Development of ternary and binary azeotrope and azeotrope-like compositions consisting of hydrogen fluoride (HF), 2-chloro-3,3,3-trifluoropropene (HCFO-1233xf), and either 1,1,2,3-tetrachloropropene or 1,1,1,2,2-pentafluoropropane, which are used to separate components in reaction product streams during the production of HCFO-1233xf or 2,3,3-tetrafluoropropene (HFO-1234yf), facilitating their production and use in industrial processes.
Process for making hydrohalocarbons and selected compound
PatentWO2012067872A1
Innovation
- A liquid phase process involving the reaction of CHCl3 with CHX=CYR in the presence of an addition catalyst, such as copper or iron, to produce compounds like CHCl2CH2CHClF3, which serves as an intermediate for producing hydrofluorocarbons and hydrofluoroolefins, utilizing catalysts like cupric chloride and reductants like hydrazine, and operating under controlled temperature and pressure conditions.
Regulatory Framework for HF Industrial Applications
The regulatory landscape governing hydrofluoric acid (HF) in industrial applications has evolved significantly over decades, reflecting growing awareness of its hazards and the need for stringent safety protocols. At the international level, organizations such as the International Labour Organization (ILO) and the United Nations Environment Programme (UNEP) have established baseline standards for hazardous chemicals management, with specific provisions addressing HF handling, storage, and emergency response procedures.
In the United States, the Occupational Safety and Health Administration (OSHA) has implemented comprehensive regulations under 29 CFR 1910.1000 that establish permissible exposure limits (PELs) for HF at 3 ppm as an 8-hour time-weighted average. The Environmental Protection Agency (EPA) regulates HF under the Risk Management Program (RMP) and the Toxic Substances Control Act (TSCA), requiring facilities handling significant quantities to develop and implement risk management plans.
The European Union's regulatory framework is primarily governed by REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the CLP Regulation (Classification, Labeling and Packaging), which classify HF as an "acute toxic substance" and mandate extensive safety data sheets, appropriate labeling, and strict handling protocols. The Seveso III Directive further imposes additional requirements on facilities storing large quantities of HF, including the development of major accident prevention policies.
Asian countries have developed varying regulatory approaches. Japan's Industrial Safety and Health Law and Chemical Substances Control Law establish strict guidelines for HF handling, while China has implemented the Regulations on Safe Management of Hazardous Chemicals with specific provisions for highly toxic substances including HF. South Korea's Chemical Control Act similarly imposes stringent requirements for registration and risk assessment.
Industry-specific regulations add another layer of compliance requirements. In semiconductor manufacturing, where HF is widely used for silicon etching, specialized protocols have been developed by organizations such as SEMI (Semiconductor Equipment and Materials International). The petroleum industry, through organizations like the American Petroleum Institute (API), has established recommended practices specifically addressing HF alkylation units.
Recent regulatory trends indicate a move toward more harmonized global standards, with increasing emphasis on inherently safer design principles that encourage substitution of HF with less hazardous alternatives where technically feasible. Additionally, regulations increasingly focus on comprehensive process safety management systems rather than solely on compliance with specific technical requirements.
Compliance challenges remain significant, particularly for multinational corporations navigating different regulatory regimes across jurisdictions. The cost of implementing required engineering controls, administrative procedures, and personal protective equipment represents a substantial investment, though one that is justified by the potential consequences of HF-related incidents.
In the United States, the Occupational Safety and Health Administration (OSHA) has implemented comprehensive regulations under 29 CFR 1910.1000 that establish permissible exposure limits (PELs) for HF at 3 ppm as an 8-hour time-weighted average. The Environmental Protection Agency (EPA) regulates HF under the Risk Management Program (RMP) and the Toxic Substances Control Act (TSCA), requiring facilities handling significant quantities to develop and implement risk management plans.
The European Union's regulatory framework is primarily governed by REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the CLP Regulation (Classification, Labeling and Packaging), which classify HF as an "acute toxic substance" and mandate extensive safety data sheets, appropriate labeling, and strict handling protocols. The Seveso III Directive further imposes additional requirements on facilities storing large quantities of HF, including the development of major accident prevention policies.
Asian countries have developed varying regulatory approaches. Japan's Industrial Safety and Health Law and Chemical Substances Control Law establish strict guidelines for HF handling, while China has implemented the Regulations on Safe Management of Hazardous Chemicals with specific provisions for highly toxic substances including HF. South Korea's Chemical Control Act similarly imposes stringent requirements for registration and risk assessment.
Industry-specific regulations add another layer of compliance requirements. In semiconductor manufacturing, where HF is widely used for silicon etching, specialized protocols have been developed by organizations such as SEMI (Semiconductor Equipment and Materials International). The petroleum industry, through organizations like the American Petroleum Institute (API), has established recommended practices specifically addressing HF alkylation units.
Recent regulatory trends indicate a move toward more harmonized global standards, with increasing emphasis on inherently safer design principles that encourage substitution of HF with less hazardous alternatives where technically feasible. Additionally, regulations increasingly focus on comprehensive process safety management systems rather than solely on compliance with specific technical requirements.
Compliance challenges remain significant, particularly for multinational corporations navigating different regulatory regimes across jurisdictions. The cost of implementing required engineering controls, administrative procedures, and personal protective equipment represents a substantial investment, though one that is justified by the potential consequences of HF-related incidents.
Risk Assessment Methodologies for HF Synthesis Processes
Risk assessment for hydrofluoric acid (HF) synthesis processes requires a systematic approach due to the compound's extreme hazard profile. Current methodologies employ a multi-tiered framework beginning with preliminary hazard analysis (PHA), which identifies potential release scenarios and exposure pathways specific to HF handling. This initial assessment typically categorizes risks based on severity and probability matrices, establishing priority areas for more detailed evaluation.
Quantitative Risk Assessment (QRA) techniques have evolved significantly for HF processes, incorporating sophisticated dispersion modeling that accounts for HF's unique properties including its high volatility and density-driven dispersion characteristics. These models simulate various release scenarios under different meteorological conditions to determine potential impact zones and concentration gradients, essential for emergency planning.
Process Hazard Analysis (PHA) methodologies such as HAZOP (Hazard and Operability Study) and FMEA (Failure Mode and Effects Analysis) have been adapted specifically for HF synthesis operations. These specialized approaches focus on critical control points including temperature regulation systems, pressure relief mechanisms, and material compatibility issues unique to HF processes.
Layer of Protection Analysis (LOPA) has emerged as a particularly valuable methodology for HF synthesis facilities, enabling quantitative assessment of safeguard effectiveness. This approach evaluates independent protection layers including engineered controls (automated shutdown systems, scrubbers), administrative controls (operating procedures, training), and mitigation measures (water curtains, neutralization systems).
Real-time monitoring strategies form an integral component of modern risk assessment frameworks for HF processes. Advanced detection systems utilizing infrared spectroscopy and electrochemical sensors provide continuous monitoring capabilities with response times under 10 seconds, allowing for immediate corrective action before minor releases escalate.
Consequence modeling has advanced to incorporate physiological impact assessment based on AEGL (Acute Exposure Guideline Levels) specifically developed for HF exposure. These models integrate toxicological data with dispersion predictions to establish evacuation zones and response protocols tailored to facility-specific risks.
Industry benchmarking has established standardized risk acceptance criteria for HF synthesis operations, typically requiring risks to be reduced to ALARP (As Low As Reasonably Practicable) levels. These criteria have evolved to incorporate both individual risk metrics (fatality risk contours) and societal risk considerations (F-N curves), providing a comprehensive framework for risk evaluation and decision-making in HF process design and operation.
Quantitative Risk Assessment (QRA) techniques have evolved significantly for HF processes, incorporating sophisticated dispersion modeling that accounts for HF's unique properties including its high volatility and density-driven dispersion characteristics. These models simulate various release scenarios under different meteorological conditions to determine potential impact zones and concentration gradients, essential for emergency planning.
Process Hazard Analysis (PHA) methodologies such as HAZOP (Hazard and Operability Study) and FMEA (Failure Mode and Effects Analysis) have been adapted specifically for HF synthesis operations. These specialized approaches focus on critical control points including temperature regulation systems, pressure relief mechanisms, and material compatibility issues unique to HF processes.
Layer of Protection Analysis (LOPA) has emerged as a particularly valuable methodology for HF synthesis facilities, enabling quantitative assessment of safeguard effectiveness. This approach evaluates independent protection layers including engineered controls (automated shutdown systems, scrubbers), administrative controls (operating procedures, training), and mitigation measures (water curtains, neutralization systems).
Real-time monitoring strategies form an integral component of modern risk assessment frameworks for HF processes. Advanced detection systems utilizing infrared spectroscopy and electrochemical sensors provide continuous monitoring capabilities with response times under 10 seconds, allowing for immediate corrective action before minor releases escalate.
Consequence modeling has advanced to incorporate physiological impact assessment based on AEGL (Acute Exposure Guideline Levels) specifically developed for HF exposure. These models integrate toxicological data with dispersion predictions to establish evacuation zones and response protocols tailored to facility-specific risks.
Industry benchmarking has established standardized risk acceptance criteria for HF synthesis operations, typically requiring risks to be reduced to ALARP (As Low As Reasonably Practicable) levels. These criteria have evolved to incorporate both individual risk metrics (fatality risk contours) and societal risk considerations (F-N curves), providing a comprehensive framework for risk evaluation and decision-making in HF process design and operation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!