How to Leverage Hydrofluoric Acid in Advanced Chemical Research
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Background and Research Objectives
Hydrofluoric acid (HF) represents one of the most significant chemical compounds in modern industrial and research applications. First isolated in the late 18th century by Carl Wilhelm Scheele, this compound has evolved from a laboratory curiosity to a cornerstone reagent in numerous advanced chemical processes. The historical trajectory of HF utilization demonstrates a progressive expansion from glass etching applications to semiconductor manufacturing, pharmaceutical synthesis, and materials science research.
The chemical properties that make HF uniquely valuable include its exceptional ability to dissolve silica-based materials, its strong acidity despite being classified as a weak acid in aqueous solutions, and its capacity to form stable complexes with various metals. These characteristics have positioned HF as an indispensable tool in high-precision industries where conventional acids prove insufficient.
Current technological trends indicate an increasing refinement in HF application methodologies, particularly in nanoscale etching processes, catalytic applications, and as a precursor in fluoropolymer synthesis. The miniaturization demands of modern electronics have driven significant innovations in controlled HF etching techniques, while environmental concerns have simultaneously pushed research toward more contained and efficient utilization systems.
The primary objectives of this technical research include developing comprehensive protocols for safer HF handling in advanced research settings, identifying novel applications in emerging fields such as quantum computing materials preparation, and exploring potential substitutes or modified formulations that maintain efficacy while reducing hazards. Additionally, we aim to investigate enhanced recovery and recycling methods to address sustainability concerns associated with HF usage.
A critical research goal involves mapping the intersection between HF-based processes and green chemistry principles, particularly focusing on reaction efficiency optimization and waste minimization. This includes exploring catalytic systems that reduce required HF concentrations while maintaining or enhancing reaction outcomes.
Furthermore, this research seeks to establish standardized methodologies for evaluating the environmental impact of HF-dependent processes across their complete lifecycle, from production through application to disposal or recovery. This holistic approach aligns with increasing regulatory pressures and corporate sustainability commitments.
The technological evolution trajectory suggests potential breakthroughs in controlled-release HF systems, immobilized HF reagents, and ionic liquid formulations that could fundamentally transform how this powerful chemical is deployed in research settings. By anticipating these developments, this research aims to position our organization at the forefront of responsible and innovative HF utilization in advanced chemical research.
The chemical properties that make HF uniquely valuable include its exceptional ability to dissolve silica-based materials, its strong acidity despite being classified as a weak acid in aqueous solutions, and its capacity to form stable complexes with various metals. These characteristics have positioned HF as an indispensable tool in high-precision industries where conventional acids prove insufficient.
Current technological trends indicate an increasing refinement in HF application methodologies, particularly in nanoscale etching processes, catalytic applications, and as a precursor in fluoropolymer synthesis. The miniaturization demands of modern electronics have driven significant innovations in controlled HF etching techniques, while environmental concerns have simultaneously pushed research toward more contained and efficient utilization systems.
The primary objectives of this technical research include developing comprehensive protocols for safer HF handling in advanced research settings, identifying novel applications in emerging fields such as quantum computing materials preparation, and exploring potential substitutes or modified formulations that maintain efficacy while reducing hazards. Additionally, we aim to investigate enhanced recovery and recycling methods to address sustainability concerns associated with HF usage.
A critical research goal involves mapping the intersection between HF-based processes and green chemistry principles, particularly focusing on reaction efficiency optimization and waste minimization. This includes exploring catalytic systems that reduce required HF concentrations while maintaining or enhancing reaction outcomes.
Furthermore, this research seeks to establish standardized methodologies for evaluating the environmental impact of HF-dependent processes across their complete lifecycle, from production through application to disposal or recovery. This holistic approach aligns with increasing regulatory pressures and corporate sustainability commitments.
The technological evolution trajectory suggests potential breakthroughs in controlled-release HF systems, immobilized HF reagents, and ionic liquid formulations that could fundamentally transform how this powerful chemical is deployed in research settings. By anticipating these developments, this research aims to position our organization at the forefront of responsible and innovative HF utilization in advanced chemical research.
Market Applications and Demand Analysis
The global market for hydrofluoric acid (HF) in advanced chemical research continues to expand, driven primarily by its unique properties and versatility across multiple high-value industries. Current market estimates place the global HF market at approximately 3.5 billion USD, with the research-grade segment representing about 12% of this value. The compound annual growth rate for research applications specifically has been outpacing the general industrial use, showing 5.7% growth compared to the overall market's 4.2%.
Semiconductor manufacturing represents the largest demand driver for high-purity HF in research settings, where it serves as an essential component in silicon wafer cleaning, etching, and surface preparation processes. As the semiconductor industry pushes toward smaller node sizes and more complex architectures, the demand for ultra-high purity HF continues to rise, with particularly strong growth in research facilities developing next-generation chip technologies.
Pharmaceutical research constitutes another significant market segment, where HF is utilized in the synthesis of fluorinated compounds, which represent approximately 20% of pharmaceutical products. The growing emphasis on fluorine chemistry in drug discovery has created sustained demand from both academic and industrial research laboratories. Notably, research into fluorinated antibiotics and anticancer agents has intensified in recent years, further driving specialized HF consumption.
Materials science research presents an emerging application area with substantial growth potential. The development of advanced fluoropolymers, fluoride glasses, and specialty ceramics all require precise application of HF in controlled research environments. The renewable energy sector, particularly in battery technology research, has become an unexpected growth driver as scientists explore fluoride-ion batteries as potential alternatives to lithium-ion technology.
Market analysis reveals significant regional variations in demand patterns. North America and Europe dominate the research-grade HF market, accounting for approximately 65% of global consumption, primarily due to their established pharmaceutical and semiconductor research infrastructure. However, the Asia-Pacific region, particularly China, South Korea, and Taiwan, shows the fastest growth rate at 8.3% annually, reflecting their expanding research capabilities and semiconductor manufacturing focus.
The market structure exhibits interesting dynamics between supply and demand. While industrial-grade HF production is relatively commoditized, research-grade HF represents a specialized segment with higher margins and more stringent quality requirements. This has created a distinct sub-market served by specialized chemical suppliers who can meet the exacting purity standards required for advanced research applications, typically 99.99% purity or higher.
Semiconductor manufacturing represents the largest demand driver for high-purity HF in research settings, where it serves as an essential component in silicon wafer cleaning, etching, and surface preparation processes. As the semiconductor industry pushes toward smaller node sizes and more complex architectures, the demand for ultra-high purity HF continues to rise, with particularly strong growth in research facilities developing next-generation chip technologies.
Pharmaceutical research constitutes another significant market segment, where HF is utilized in the synthesis of fluorinated compounds, which represent approximately 20% of pharmaceutical products. The growing emphasis on fluorine chemistry in drug discovery has created sustained demand from both academic and industrial research laboratories. Notably, research into fluorinated antibiotics and anticancer agents has intensified in recent years, further driving specialized HF consumption.
Materials science research presents an emerging application area with substantial growth potential. The development of advanced fluoropolymers, fluoride glasses, and specialty ceramics all require precise application of HF in controlled research environments. The renewable energy sector, particularly in battery technology research, has become an unexpected growth driver as scientists explore fluoride-ion batteries as potential alternatives to lithium-ion technology.
Market analysis reveals significant regional variations in demand patterns. North America and Europe dominate the research-grade HF market, accounting for approximately 65% of global consumption, primarily due to their established pharmaceutical and semiconductor research infrastructure. However, the Asia-Pacific region, particularly China, South Korea, and Taiwan, shows the fastest growth rate at 8.3% annually, reflecting their expanding research capabilities and semiconductor manufacturing focus.
The market structure exhibits interesting dynamics between supply and demand. While industrial-grade HF production is relatively commoditized, research-grade HF represents a specialized segment with higher margins and more stringent quality requirements. This has created a distinct sub-market served by specialized chemical suppliers who can meet the exacting purity standards required for advanced research applications, typically 99.99% purity or higher.
Current Challenges and Technical Limitations
Despite significant advancements in chemical research utilizing hydrofluoric acid (HF), numerous challenges and technical limitations continue to impede its broader application in advanced research settings. The foremost concern remains the extreme toxicity and corrosivity of HF, which poses severe health risks to researchers. Even at low concentrations, HF can cause deep tissue damage and systemic toxicity through rapid skin penetration, necessitating specialized safety protocols that often limit experimental flexibility.
Material compatibility represents another significant challenge, as HF readily attacks glass, many metals, and certain polymers. This corrosive nature restricts containment options and requires specialized equipment made from materials like PTFE (polytetrafluoroethylene) or certain grades of polyethylene, substantially increasing research costs and complexity.
Concentration control and stability present ongoing technical difficulties. HF solutions can experience concentration fluctuations due to evaporation and absorption of atmospheric moisture, compromising experimental reproducibility. Additionally, the acid's high reactivity makes it difficult to maintain stable concentrations during extended research procedures, particularly in processes requiring precise stoichiometric control.
The analytical challenges associated with HF usage are equally problematic. Real-time monitoring of HF concentrations in reaction environments remains difficult, as many conventional analytical instruments cannot withstand HF exposure. This limitation hampers process optimization and kinetic studies in advanced applications.
Waste management and environmental considerations impose further constraints. The neutralization and disposal of HF waste require specialized procedures to prevent environmental contamination, adding complexity to research protocols and increasing operational costs. Many research facilities lack adequate infrastructure for proper HF waste handling.
Scaling challenges persist when transitioning from laboratory to industrial applications. Processes that work effectively at small scales often encounter unforeseen complications when scaled up, particularly regarding heat management and reaction control with HF-based systems.
Regulatory hurdles and compliance requirements have intensified globally, with increasingly stringent controls on HF usage. These regulations, while necessary for safety, often create administrative burdens that can delay research timelines and limit experimental approaches.
The development of standardized protocols for HF handling across different research applications remains incomplete, resulting in inconsistent practices that complicate cross-institutional collaboration and knowledge transfer in advanced chemical research utilizing this powerful but problematic reagent.
Material compatibility represents another significant challenge, as HF readily attacks glass, many metals, and certain polymers. This corrosive nature restricts containment options and requires specialized equipment made from materials like PTFE (polytetrafluoroethylene) or certain grades of polyethylene, substantially increasing research costs and complexity.
Concentration control and stability present ongoing technical difficulties. HF solutions can experience concentration fluctuations due to evaporation and absorption of atmospheric moisture, compromising experimental reproducibility. Additionally, the acid's high reactivity makes it difficult to maintain stable concentrations during extended research procedures, particularly in processes requiring precise stoichiometric control.
The analytical challenges associated with HF usage are equally problematic. Real-time monitoring of HF concentrations in reaction environments remains difficult, as many conventional analytical instruments cannot withstand HF exposure. This limitation hampers process optimization and kinetic studies in advanced applications.
Waste management and environmental considerations impose further constraints. The neutralization and disposal of HF waste require specialized procedures to prevent environmental contamination, adding complexity to research protocols and increasing operational costs. Many research facilities lack adequate infrastructure for proper HF waste handling.
Scaling challenges persist when transitioning from laboratory to industrial applications. Processes that work effectively at small scales often encounter unforeseen complications when scaled up, particularly regarding heat management and reaction control with HF-based systems.
Regulatory hurdles and compliance requirements have intensified globally, with increasingly stringent controls on HF usage. These regulations, while necessary for safety, often create administrative burdens that can delay research timelines and limit experimental approaches.
The development of standardized protocols for HF handling across different research applications remains incomplete, resulting in inconsistent practices that complicate cross-institutional collaboration and knowledge transfer in advanced chemical research utilizing this powerful but problematic reagent.
Established HF Handling and Application Methodologies
01 Etching and cleaning applications of hydrofluoric acid
Hydrofluoric acid is widely used in semiconductor manufacturing for etching silicon dioxide and cleaning silicon wafers. It effectively removes oxide layers, contaminants, and residues from surfaces. The acid can be formulated at various concentrations and combined with other chemicals to achieve specific etching rates and selectivity for different materials. These formulations are crucial in microelectronics fabrication processes where precise control of surface properties is required.- Etching applications of hydrofluoric acid: Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with buffering agents to control etching rates. Various compositions containing hydrofluoric acid are used for selective etching of different materials, with applications in microelectronics fabrication and surface treatment of various substrates.
- Purification and recovery methods for hydrofluoric acid: Various techniques have been developed for purifying and recovering hydrofluoric acid from industrial processes. These methods include distillation, adsorption, membrane separation, and chemical precipitation to remove impurities such as metal ions, particulates, and organic contaminants. Recovery systems help minimize waste and environmental impact while providing purified hydrofluoric acid for reuse in manufacturing processes.
- Safety measures and neutralization of hydrofluoric acid: Due to its highly corrosive and toxic nature, specialized safety protocols and neutralization methods have been developed for handling hydrofluoric acid. These include formulations containing calcium or magnesium compounds that can rapidly neutralize the acid in case of spills or exposure. Protective equipment, detection systems, and emergency response procedures are essential when working with this hazardous substance.
- Production methods of hydrofluoric acid: Industrial production of hydrofluoric acid typically involves the reaction of calcium fluoride (fluorspar) with sulfuric acid. Alternative manufacturing processes have been developed to improve yield, purity, and safety while reducing environmental impact. These include catalytic methods, continuous flow processes, and techniques for capturing and treating byproducts and emissions.
- Industrial applications beyond semiconductor manufacturing: Beyond its well-known use in semiconductor manufacturing, hydrofluoric acid serves various industrial purposes. It is used in metal surface treatment, cleaning of industrial equipment, uranium processing, oil refining catalysts, and as a precursor for fluorine-containing compounds. Specialized formulations have been developed for specific applications such as rust removal, scale elimination, and chemical milling.
02 Production and purification methods for hydrofluoric acid
Various methods exist for producing and purifying hydrofluoric acid, including reactions between fluoride-containing minerals and sulfuric acid. Purification techniques involve distillation, adsorption, and chemical treatments to remove impurities such as silicon compounds, metal ions, and other contaminants. These processes are essential for obtaining high-purity hydrofluoric acid required for semiconductor and electronics applications where even trace impurities can affect performance.Expand Specific Solutions03 Safety measures and handling of hydrofluoric acid
Due to its highly corrosive nature and ability to penetrate skin and cause deep tissue damage, specialized safety protocols are necessary when handling hydrofluoric acid. These include containment systems, neutralization methods, personal protective equipment, and emergency response procedures. Various neutralizing agents and treatment compounds have been developed specifically for hydrofluoric acid spills and exposures, including calcium-based formulations that bind with fluoride ions to prevent tissue damage.Expand Specific Solutions04 Recovery and recycling of hydrofluoric acid
Systems and methods for recovering and recycling hydrofluoric acid from industrial processes help reduce waste and environmental impact. These include absorption techniques, membrane separation, distillation processes, and chemical conversion methods that allow for the capture and reuse of hydrofluoric acid from waste streams. Such recycling technologies are particularly important in semiconductor manufacturing where large volumes of the acid are used in cleaning and etching operations.Expand Specific Solutions05 Hydrofluoric acid in chemical reactions and synthesis
Hydrofluoric acid serves as an important reagent in various chemical reactions and synthesis processes, particularly for introducing fluorine into organic and inorganic compounds. It functions as a catalyst, fluorinating agent, and reaction medium in the production of fluorochemicals, pharmaceuticals, and specialty materials. Modified forms of hydrofluoric acid, including buffered solutions and complexes with other chemicals, provide controlled reactivity for specific applications while reducing hazards associated with the pure acid.Expand Specific Solutions
Leading Organizations in HF Research and Production
The hydrofluoric acid (HF) market demonstrates a mature yet evolving competitive landscape, with established players like Honeywell International, Daikin Industries, and The Chemours Co. dominating industrial applications. The market is characterized by segmentation between high-purity electronic-grade HF (led by Do-Fluoride New Materials and Jiangyin Runma) and industrial-grade applications (where Halliburton and Schlumberger are prominent in oil services). Technical maturity varies significantly across applications, with semiconductor etching and electronics manufacturing showing the highest sophistication. Research institutions like Shandong University and Chinese Academy of Science are advancing novel applications, while specialty chemical companies such as Arkema and DuPont are developing proprietary HF-based processes for advanced materials. The global market is projected to reach $2.7 billion by 2027, growing at 5.3% CAGR, driven by semiconductor and pharmaceutical research applications.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed advanced HF handling systems incorporating proprietary fluoropolymer materials resistant to HF corrosion. Their technology includes automated dispensing systems with real-time monitoring capabilities that maintain precise concentration levels during chemical processes. Honeywell's approach integrates safety protocols with efficiency, using specialized containment vessels lined with their patented materials that extend equipment lifespan while reducing contamination risks. Their systems feature remote operation capabilities, allowing researchers to manipulate HF reactions from safe distances, and incorporate neutralization technologies that can rapidly respond to potential leaks or spills. Honeywell's integrated approach connects HF handling to broader laboratory management systems, enabling comprehensive data collection on usage patterns and safety metrics.
Strengths: Superior safety features with remote handling capabilities minimize researcher exposure; integrated monitoring systems provide comprehensive data for process optimization. Weaknesses: Higher implementation costs compared to standard systems; requires specialized training and maintenance protocols that may increase operational complexity.
Do-Fluoride New Materials Co., Ltd.
Technical Solution: Do-Fluoride has pioneered a closed-loop HF recycling system specifically designed for semiconductor manufacturing and materials research. Their technology captures, purifies, and reuses HF from etching processes, achieving recovery rates exceeding 95%. The system employs a multi-stage purification process including specialized membrane filtration and proprietary adsorption materials that remove metal contaminants down to ppb levels. Do-Fluoride's approach integrates with existing manufacturing lines through modular units that can be scaled according to production volume. Their technology also includes real-time purity monitoring using spectroscopic methods that ensure consistent quality of recovered HF. The company has developed specialized storage solutions using silicon carbide-lined vessels that minimize contamination risks while extending storage stability of ultra-pure HF for sensitive applications.
Strengths: Exceptional recovery efficiency significantly reduces waste disposal costs and environmental impact; modular design allows for flexible implementation across different research scales. Weaknesses: Initial capital investment is substantial; system requires regular maintenance of specialized filtration components to maintain high recovery rates.
Key Patents and Innovations in HF Chemistry
Method for producing diluted hydrofluoric acid
PatentActiveUS20180148332A1
Innovation
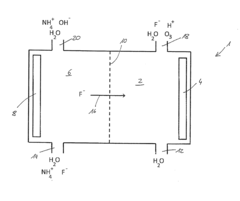
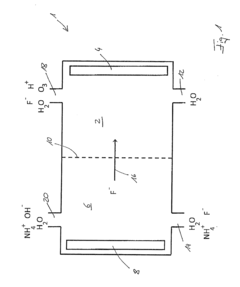
- A method using an electrode arrangement with an anode and cathode chambers separated by an anion exchange membrane, where pure water and an electrolyte solution containing fluoride ions are electrolyzed to produce dilute hydrofluoric acid with precise concentration control, and ozone can be simultaneously produced, with the ability to adjust concentrations independently using electrical current and electrolyte concentration.
Method of recovering hydrofluoric acid
PatentInactiveUS20100280292A1
Innovation
- A method involving the compression of a hydrofluoric acid gas stream, initially cooled to between −20 and 100°C, followed by a separation step via distillation at pressures between 5 and 20 bar absolute, allowing for efficient recovery of hydrofluoric acid with reduced cooling requirements.
Safety Protocols and Risk Management
Working with hydrofluoric acid (HF) in advanced chemical research requires rigorous safety protocols and comprehensive risk management strategies due to its extreme hazards. All laboratory personnel must undergo specialized training before handling HF, covering proper handling techniques, emergency response procedures, and first aid measures. This training should be refreshed annually and documented in institutional records.
Personal protective equipment (PPE) requirements for HF handling are more stringent than for most chemicals. Researchers must wear HF-resistant gloves (typically neoprene or butyl rubber), face shields with chemical splash goggles, chemical-resistant lab coats, and closed-toe shoes. For higher concentration work, full-face respirators with appropriate cartridges and acid-resistant aprons or full-body protection may be necessary.
Laboratory infrastructure must include dedicated fume hoods with proper ventilation systems specifically designed for HF work. Emergency equipment including calcium gluconate gel (the specific antidote for HF exposure), eyewash stations, and safety showers must be readily accessible within 10 seconds of any HF handling area. All containers must be clearly labeled with HF-specific hazard warnings and stored in acid-resistant secondary containment systems.
Standard operating procedures (SOPs) for HF work should detail step-by-step protocols for each procedure, including specific safety measures, waste handling instructions, and emergency response guidelines. These SOPs must be reviewed and approved by safety officers and updated regularly to incorporate new safety information or procedural improvements.
Risk assessment frameworks specific to HF applications should be implemented, evaluating factors such as concentration, volume, temperature, pressure, and potential for aerosolization. Each research protocol involving HF requires documented risk assessment before approval, with higher-risk procedures demanding additional safety reviews and controls.
Emergency response planning must address HF-specific scenarios, including skin or eye exposure, inhalation, ingestion, and spills. All researchers should be familiar with the unique medical treatment requirements for HF exposure, particularly the need for immediate calcium gluconate application and specialized medical intervention. Institutional coordination with local emergency services ensures that medical facilities are prepared to handle HF casualties.
Regular safety audits and compliance checks should be conducted to verify adherence to established protocols. Near-miss incidents and safety concerns must be reported through formal channels to facilitate continuous improvement of safety measures and prevent future accidents in this high-risk research area.
Personal protective equipment (PPE) requirements for HF handling are more stringent than for most chemicals. Researchers must wear HF-resistant gloves (typically neoprene or butyl rubber), face shields with chemical splash goggles, chemical-resistant lab coats, and closed-toe shoes. For higher concentration work, full-face respirators with appropriate cartridges and acid-resistant aprons or full-body protection may be necessary.
Laboratory infrastructure must include dedicated fume hoods with proper ventilation systems specifically designed for HF work. Emergency equipment including calcium gluconate gel (the specific antidote for HF exposure), eyewash stations, and safety showers must be readily accessible within 10 seconds of any HF handling area. All containers must be clearly labeled with HF-specific hazard warnings and stored in acid-resistant secondary containment systems.
Standard operating procedures (SOPs) for HF work should detail step-by-step protocols for each procedure, including specific safety measures, waste handling instructions, and emergency response guidelines. These SOPs must be reviewed and approved by safety officers and updated regularly to incorporate new safety information or procedural improvements.
Risk assessment frameworks specific to HF applications should be implemented, evaluating factors such as concentration, volume, temperature, pressure, and potential for aerosolization. Each research protocol involving HF requires documented risk assessment before approval, with higher-risk procedures demanding additional safety reviews and controls.
Emergency response planning must address HF-specific scenarios, including skin or eye exposure, inhalation, ingestion, and spills. All researchers should be familiar with the unique medical treatment requirements for HF exposure, particularly the need for immediate calcium gluconate application and specialized medical intervention. Institutional coordination with local emergency services ensures that medical facilities are prepared to handle HF casualties.
Regular safety audits and compliance checks should be conducted to verify adherence to established protocols. Near-miss incidents and safety concerns must be reported through formal channels to facilitate continuous improvement of safety measures and prevent future accidents in this high-risk research area.
Environmental Impact and Sustainable Alternatives
The utilization of hydrofluoric acid (HF) in advanced chemical research presents significant environmental challenges that cannot be overlooked. HF is classified as a highly hazardous substance with severe environmental implications, including potential contamination of water sources, soil degradation, and harmful effects on aquatic ecosystems when improperly managed. Research facilities using HF generate waste streams that require specialized treatment protocols to neutralize the acid before disposal, adding considerable complexity to waste management systems.
Atmospheric emissions containing HF can contribute to acid rain formation, which damages vegetation, aquatic life, and infrastructure. The production process of HF itself is energy-intensive and generates substantial greenhouse gas emissions, contributing to the overall carbon footprint of research operations. These environmental concerns have prompted regulatory bodies worldwide to implement stringent guidelines for HF handling, storage, and disposal, necessitating comprehensive environmental management strategies for research institutions.
In response to these challenges, the scientific community has been actively developing sustainable alternatives to reduce dependence on HF in advanced chemical applications. Green chemistry principles are increasingly being applied to redesign processes that traditionally relied on HF. For instance, ionic liquids have emerged as promising substitutes in certain catalytic reactions and metal surface treatments, offering similar effectiveness with significantly reduced environmental impact and toxicity.
Supercritical carbon dioxide represents another innovative alternative, particularly in extraction processes where HF was previously considered indispensable. This technology utilizes CO2 in its supercritical state to achieve similar results without the associated environmental hazards of HF. Additionally, enzymatic processes and biocatalysts are gaining traction as environmentally friendly alternatives for specific reactions that conventionally required strong acids like HF.
The development of closed-loop systems for HF usage represents a significant advancement in sustainable research practices. These systems enable the recovery and reuse of HF, substantially reducing waste generation and environmental exposure. Complementing these technological innovations, comprehensive risk assessment frameworks have been established to evaluate the environmental impact of HF-based processes against potential alternatives, facilitating informed decision-making in research design.
While complete elimination of HF from all chemical research applications remains challenging due to its unique properties, the integration of these sustainable alternatives and improved management practices demonstrates promising progress toward minimizing environmental impact while maintaining scientific advancement. The continued investment in green chemistry research and sustainable technology development will be crucial for further reducing the environmental footprint of advanced chemical research.
Atmospheric emissions containing HF can contribute to acid rain formation, which damages vegetation, aquatic life, and infrastructure. The production process of HF itself is energy-intensive and generates substantial greenhouse gas emissions, contributing to the overall carbon footprint of research operations. These environmental concerns have prompted regulatory bodies worldwide to implement stringent guidelines for HF handling, storage, and disposal, necessitating comprehensive environmental management strategies for research institutions.
In response to these challenges, the scientific community has been actively developing sustainable alternatives to reduce dependence on HF in advanced chemical applications. Green chemistry principles are increasingly being applied to redesign processes that traditionally relied on HF. For instance, ionic liquids have emerged as promising substitutes in certain catalytic reactions and metal surface treatments, offering similar effectiveness with significantly reduced environmental impact and toxicity.
Supercritical carbon dioxide represents another innovative alternative, particularly in extraction processes where HF was previously considered indispensable. This technology utilizes CO2 in its supercritical state to achieve similar results without the associated environmental hazards of HF. Additionally, enzymatic processes and biocatalysts are gaining traction as environmentally friendly alternatives for specific reactions that conventionally required strong acids like HF.
The development of closed-loop systems for HF usage represents a significant advancement in sustainable research practices. These systems enable the recovery and reuse of HF, substantially reducing waste generation and environmental exposure. Complementing these technological innovations, comprehensive risk assessment frameworks have been established to evaluate the environmental impact of HF-based processes against potential alternatives, facilitating informed decision-making in research design.
While complete elimination of HF from all chemical research applications remains challenging due to its unique properties, the integration of these sustainable alternatives and improved management practices demonstrates promising progress toward minimizing environmental impact while maintaining scientific advancement. The continued investment in green chemistry research and sustainable technology development will be crucial for further reducing the environmental footprint of advanced chemical research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!