Hydrofluoric Acid vs Perchloric Acid: Reactivity and Stability
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Chemistry Background and Research Objectives
Acid chemistry has evolved significantly since the identification of acids as a distinct class of compounds in the 17th century. The understanding of acid-base interactions progressed from Arrhenius's theory focusing on hydrogen ions to Brønsted-Lowry's proton donor-acceptor concept, and finally to Lewis's electron pair theory. This evolution has enabled precise characterization of acids based on their strength, reactivity patterns, and stability under various conditions.
Hydrofluoric acid (HF) and perchloric acid (HClO4) represent two distinct categories within strong acid chemistry, each with unique properties that have shaped their applications in industrial processes and scientific research. HF, despite its relatively weak acid strength in aqueous solutions (pKa ≈ 3.17), demonstrates exceptional reactivity with silicates and glass, making it invaluable in semiconductor manufacturing and glass etching. Perchloric acid, conversely, stands as one of the strongest mineral acids (pKa ≈ -10) with powerful oxidizing capabilities.
The historical development of these acids has been closely tied to industrial needs. HF production was refined in the early 20th century to support aluminum manufacturing and later the nuclear industry, while perchloric acid's development was accelerated by its applications in analytical chemistry and as an oxidizer in rocket propellants. Their divergent properties have created distinct technological trajectories despite both being classified as strong acids.
Current technological trends indicate growing importance for both acids in emerging fields. HF continues to be critical in the semiconductor industry as device miniaturization demands increasingly precise etching processes. Meanwhile, perchloric acid's role in advanced materials synthesis and high-precision analytical chemistry is expanding with the development of new composite materials and battery technologies.
This research aims to conduct a comprehensive comparative analysis of hydrofluoric and perchloric acids, focusing specifically on their reactivity mechanisms and stability characteristics across various conditions. The primary objectives include: establishing quantitative parameters for comparing their reactivity with different substrate classes; determining stability thresholds under varying temperature, pressure, and concentration conditions; and identifying potential synergistic or antagonistic effects when these acids interact with common industrial catalysts and solvents.
Additionally, this investigation seeks to develop predictive models for reaction outcomes when these acids are employed in novel applications, particularly in advanced materials processing and green chemistry initiatives. The findings will inform safer handling protocols and potentially reveal new applications where the unique properties of either acid could enable breakthrough technologies in semiconductor manufacturing, energy storage systems, and specialized materials synthesis.
Hydrofluoric acid (HF) and perchloric acid (HClO4) represent two distinct categories within strong acid chemistry, each with unique properties that have shaped their applications in industrial processes and scientific research. HF, despite its relatively weak acid strength in aqueous solutions (pKa ≈ 3.17), demonstrates exceptional reactivity with silicates and glass, making it invaluable in semiconductor manufacturing and glass etching. Perchloric acid, conversely, stands as one of the strongest mineral acids (pKa ≈ -10) with powerful oxidizing capabilities.
The historical development of these acids has been closely tied to industrial needs. HF production was refined in the early 20th century to support aluminum manufacturing and later the nuclear industry, while perchloric acid's development was accelerated by its applications in analytical chemistry and as an oxidizer in rocket propellants. Their divergent properties have created distinct technological trajectories despite both being classified as strong acids.
Current technological trends indicate growing importance for both acids in emerging fields. HF continues to be critical in the semiconductor industry as device miniaturization demands increasingly precise etching processes. Meanwhile, perchloric acid's role in advanced materials synthesis and high-precision analytical chemistry is expanding with the development of new composite materials and battery technologies.
This research aims to conduct a comprehensive comparative analysis of hydrofluoric and perchloric acids, focusing specifically on their reactivity mechanisms and stability characteristics across various conditions. The primary objectives include: establishing quantitative parameters for comparing their reactivity with different substrate classes; determining stability thresholds under varying temperature, pressure, and concentration conditions; and identifying potential synergistic or antagonistic effects when these acids interact with common industrial catalysts and solvents.
Additionally, this investigation seeks to develop predictive models for reaction outcomes when these acids are employed in novel applications, particularly in advanced materials processing and green chemistry initiatives. The findings will inform safer handling protocols and potentially reveal new applications where the unique properties of either acid could enable breakthrough technologies in semiconductor manufacturing, energy storage systems, and specialized materials synthesis.
Market Applications and Industry Demand Analysis
The global market for hydrofluoric acid (HF) and perchloric acid (HClO4) continues to expand, driven by their unique chemical properties and diverse applications across multiple industries. HF maintains a dominant position with an estimated market value exceeding $2.5 billion, growing at approximately 5% annually. This growth is primarily fueled by its essential role in semiconductor manufacturing, where it serves as a critical etching agent for silicon wafers and glass components.
The electronics industry represents the largest consumer of high-purity HF, accounting for nearly 40% of total demand. The rapid expansion of consumer electronics, coupled with the ongoing miniaturization of electronic components, has significantly increased the need for precise etching capabilities that only HF can provide. Additionally, the fluorochemicals sector utilizes HF as a key precursor in the production of refrigerants, fluoropolymers, and various fluorine-containing compounds.
Perchloric acid, while commanding a smaller market share valued at approximately $350 million, exhibits stronger growth rates of 6-7% annually. This accelerated growth stems from its increasing adoption in specialized analytical chemistry applications and advanced materials processing. The pharmaceutical and life sciences sectors have emerged as significant consumers of perchloric acid, particularly for high-precision analytical procedures and catalyst systems.
The automotive industry represents an expanding market for both acids. HF is utilized in aluminum wheel brightening and metal surface treatment, while perchloric acid finds application in specialized battery technologies and sensors. The renewable energy sector has also begun incorporating these acids in manufacturing processes for solar panels and certain types of fuel cells, creating new demand streams.
Regional analysis reveals Asia-Pacific as the dominant market for both acids, accounting for over 50% of global consumption. This concentration aligns with the region's manufacturing strength in electronics, semiconductors, and chemical processing. North America and Europe follow with approximately 25% and 20% market share respectively, primarily driven by pharmaceutical, aerospace, and specialty chemical applications.
Industry forecasts indicate continued growth for both acids, with particular emphasis on high-purity grades. The semiconductor industry's expansion, coupled with emerging applications in energy storage and advanced materials, suggests sustained demand growth. However, regulatory pressures regarding environmental impact and worker safety are prompting research into alternatives or closed-loop systems that minimize exposure and emissions while maintaining the unique reactivity profiles these acids offer.
The electronics industry represents the largest consumer of high-purity HF, accounting for nearly 40% of total demand. The rapid expansion of consumer electronics, coupled with the ongoing miniaturization of electronic components, has significantly increased the need for precise etching capabilities that only HF can provide. Additionally, the fluorochemicals sector utilizes HF as a key precursor in the production of refrigerants, fluoropolymers, and various fluorine-containing compounds.
Perchloric acid, while commanding a smaller market share valued at approximately $350 million, exhibits stronger growth rates of 6-7% annually. This accelerated growth stems from its increasing adoption in specialized analytical chemistry applications and advanced materials processing. The pharmaceutical and life sciences sectors have emerged as significant consumers of perchloric acid, particularly for high-precision analytical procedures and catalyst systems.
The automotive industry represents an expanding market for both acids. HF is utilized in aluminum wheel brightening and metal surface treatment, while perchloric acid finds application in specialized battery technologies and sensors. The renewable energy sector has also begun incorporating these acids in manufacturing processes for solar panels and certain types of fuel cells, creating new demand streams.
Regional analysis reveals Asia-Pacific as the dominant market for both acids, accounting for over 50% of global consumption. This concentration aligns with the region's manufacturing strength in electronics, semiconductors, and chemical processing. North America and Europe follow with approximately 25% and 20% market share respectively, primarily driven by pharmaceutical, aerospace, and specialty chemical applications.
Industry forecasts indicate continued growth for both acids, with particular emphasis on high-purity grades. The semiconductor industry's expansion, coupled with emerging applications in energy storage and advanced materials, suggests sustained demand growth. However, regulatory pressures regarding environmental impact and worker safety are prompting research into alternatives or closed-loop systems that minimize exposure and emissions while maintaining the unique reactivity profiles these acids offer.
Current Technical Challenges in Acid Handling
The handling of highly corrosive acids such as hydrofluoric acid (HF) and perchloric acid (HClO4) presents significant technical challenges that require specialized protocols and equipment. These acids possess unique chemical properties that make their management particularly demanding in laboratory and industrial settings.
Hydrofluoric acid poses exceptional handling difficulties due to its ability to penetrate skin and tissues rapidly, causing deep tissue damage and potential systemic toxicity through calcium sequestration. Current containment systems struggle with HF's ability to etch glass and attack many common materials, necessitating specialized storage in polyethylene, PTFE, or certain grades of stainless steel containers.
Perchloric acid presents different but equally serious challenges, primarily related to its strong oxidizing properties and potential to form explosive perchlorates when in contact with organic materials or metals. The acid becomes increasingly unstable at concentrations above 72%, particularly when heated, creating significant explosion risks in laboratory environments.
Temperature control represents a critical challenge for both acids. HF releases toxic fumes at room temperature, requiring specialized ventilation systems with scrubbers. Perchloric acid demands even more stringent temperature management, as heating can lead to spontaneous decomposition and potential detonation, especially in the presence of organic contaminants.
Material compatibility issues continue to challenge engineers developing acid handling equipment. HF's corrosivity to silica-based materials limits the use of conventional glass labware, while perchloric acid's oxidizing nature restricts the use of many metals and organic materials. This necessitates ongoing research into advanced fluoropolymers and specialized alloys capable of withstanding these extreme chemical environments.
Waste neutralization and disposal present substantial technical hurdles. HF requires specialized neutralization protocols using calcium compounds to prevent fluoride toxicity, while perchloric acid waste must be carefully treated to prevent the formation of explosive perchlorates during concentration or drying processes.
Detection and monitoring technologies for both acids remain inadequate in many settings. Current sensors often lack the sensitivity needed for early leak detection, particularly for HF, which can cause harm at very low concentrations. Real-time monitoring systems that can function reliably in the presence of these highly corrosive substances represent an ongoing development challenge.
Emergency response protocols face limitations with current decontamination technologies. HF exposures require immediate application of calcium gluconate, but delivery systems that can rapidly and effectively neutralize larger spills while protecting first responders remain underdeveloped. Similarly, perchloric acid spills demand specialized non-organic absorbents and neutralization agents that are not universally available in emergency response kits.
Hydrofluoric acid poses exceptional handling difficulties due to its ability to penetrate skin and tissues rapidly, causing deep tissue damage and potential systemic toxicity through calcium sequestration. Current containment systems struggle with HF's ability to etch glass and attack many common materials, necessitating specialized storage in polyethylene, PTFE, or certain grades of stainless steel containers.
Perchloric acid presents different but equally serious challenges, primarily related to its strong oxidizing properties and potential to form explosive perchlorates when in contact with organic materials or metals. The acid becomes increasingly unstable at concentrations above 72%, particularly when heated, creating significant explosion risks in laboratory environments.
Temperature control represents a critical challenge for both acids. HF releases toxic fumes at room temperature, requiring specialized ventilation systems with scrubbers. Perchloric acid demands even more stringent temperature management, as heating can lead to spontaneous decomposition and potential detonation, especially in the presence of organic contaminants.
Material compatibility issues continue to challenge engineers developing acid handling equipment. HF's corrosivity to silica-based materials limits the use of conventional glass labware, while perchloric acid's oxidizing nature restricts the use of many metals and organic materials. This necessitates ongoing research into advanced fluoropolymers and specialized alloys capable of withstanding these extreme chemical environments.
Waste neutralization and disposal present substantial technical hurdles. HF requires specialized neutralization protocols using calcium compounds to prevent fluoride toxicity, while perchloric acid waste must be carefully treated to prevent the formation of explosive perchlorates during concentration or drying processes.
Detection and monitoring technologies for both acids remain inadequate in many settings. Current sensors often lack the sensitivity needed for early leak detection, particularly for HF, which can cause harm at very low concentrations. Real-time monitoring systems that can function reliably in the presence of these highly corrosive substances represent an ongoing development challenge.
Emergency response protocols face limitations with current decontamination technologies. HF exposures require immediate application of calcium gluconate, but delivery systems that can rapidly and effectively neutralize larger spills while protecting first responders remain underdeveloped. Similarly, perchloric acid spills demand specialized non-organic absorbents and neutralization agents that are not universally available in emergency response kits.
Comparative Analysis of HF and HClO4 Properties
01 Chemical reactivity of hydrofluoric acid and perchloric acid
Hydrofluoric acid and perchloric acid exhibit high reactivity due to their strong acidic properties. These acids can react vigorously with various materials, particularly metals and organic compounds. The combination of these acids can lead to enhanced oxidizing capabilities, making them useful in specific chemical processes but also increasing their hazardous nature. Their reactivity is influenced by concentration, temperature, and the presence of other chemicals.- Reactivity characteristics of hydrofluoric and perchloric acids: Hydrofluoric acid and perchloric acid exhibit distinct reactivity patterns. Hydrofluoric acid is known for its ability to dissolve silica and silicates, while perchloric acid is a strong oxidizing agent. When used together or separately, these acids can react with various materials, causing corrosion and potential hazards. Their reactivity is influenced by concentration, temperature, and the presence of other chemicals. Understanding these characteristics is crucial for safe handling and application in industrial processes.
- Stability considerations and storage requirements: The stability of hydrofluoric and perchloric acids depends on several factors including temperature, concentration, and storage conditions. Both acids require specialized containment materials resistant to their corrosive properties. Perchloric acid can form explosive compounds when in contact with organic materials or metals, while hydrofluoric acid can deteriorate certain container materials over time. Proper storage involves temperature control, appropriate container materials, and isolation from incompatible substances to prevent dangerous reactions or decomposition.
- Safety protocols and handling procedures: Handling hydrofluoric and perchloric acids requires strict safety protocols due to their hazardous nature. Personal protective equipment including acid-resistant gloves, face shields, and appropriate respiratory protection is essential. Emergency procedures must be established for spills or exposure incidents. Specialized training for personnel working with these acids is necessary, focusing on proper dilution techniques, transfer methods, and emergency response. Ventilation systems and safety equipment such as eyewash stations and safety showers must be readily available in areas where these acids are used.
- Applications in semiconductor and electronics manufacturing: Hydrofluoric and perchloric acids play crucial roles in semiconductor and electronics manufacturing processes. They are used for etching silicon wafers, cleaning electronic components, and removing contaminants from surfaces. The precise control of acid concentration, temperature, and exposure time is essential for achieving desired results without damaging sensitive components. Modified formulations containing these acids with stabilizers or buffers are developed to enhance performance while minimizing risks in high-precision manufacturing environments.
- Neutralization and waste treatment methods: Proper neutralization and waste treatment of hydrofluoric and perchloric acids are essential for environmental protection and safety. Neutralization typically involves careful addition of alkaline substances under controlled conditions to prevent violent reactions. Specialized waste treatment processes may include precipitation of fluoride ions, oxidation-reduction reactions, and dilution followed by neutralization. Advanced treatment technologies have been developed to handle these acids safely, including ion exchange systems, chemical precipitation methods, and specialized filtration techniques that minimize environmental impact while ensuring complete neutralization.
02 Stability considerations and storage requirements
Both hydrofluoric acid and perchloric acid require special storage conditions to maintain stability. Perchloric acid can form unstable perchlorates when in contact with organic materials, while hydrofluoric acid can corrode glass and many common container materials. Temperature control is critical, as elevated temperatures can accelerate decomposition and increase pressure in closed containers. Specialized containers made of fluoropolymers or certain grades of stainless steel are often required for safe storage.Expand Specific Solutions03 Safety protocols and handling procedures
Handling hydrofluoric acid and perchloric acid requires strict safety protocols due to their corrosive and toxic nature. Personal protective equipment including acid-resistant gloves, face shields, and appropriate respiratory protection is essential. Emergency response procedures must be established for spills or exposure incidents. Specialized training for personnel working with these acids is necessary, along with proper ventilation systems and safety equipment such as eyewash stations and safety showers in the immediate vicinity.Expand Specific Solutions04 Applications in semiconductor and electronics manufacturing
Hydrofluoric acid and perchloric acid are widely used in semiconductor and electronics manufacturing processes. They are employed for etching silicon wafers, cleaning electronic components, and removing metal contaminants from surfaces. The controlled reactivity of these acids makes them valuable for precise etching processes and surface modifications. Various formulations and concentrations are used depending on the specific application requirements and materials being processed.Expand Specific Solutions05 Neutralization and waste treatment methods
Proper neutralization and waste treatment of hydrofluoric acid and perchloric acid are essential for environmental protection and safety. Neutralization typically involves carefully controlled addition of alkaline substances such as calcium hydroxide or sodium carbonate. Specialized waste treatment processes may include precipitation of fluorides or perchlorates, followed by filtration and proper disposal. Monitoring of pH levels and residual acid concentrations is necessary throughout the treatment process to ensure complete neutralization before discharge.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The hydrofluoric acid and perchloric acid market is currently in a mature growth phase, with an estimated global market size of $1.2-1.5 billion. Technical maturity varies significantly between applications, with established industrial processes coexisting alongside emerging high-tech applications. Leading players demonstrate different specialization patterns: Honeywell and Daikin focus on fluorochemical innovations with enhanced safety profiles, while Merck and Shin-Etsu Chemical emphasize high-purity acid formulations for semiconductor applications. Zhejiang Quhua and Shandong Huaan are rapidly expanding production capacity in Asia, while Solvay and Kemira lead in developing environmentally sustainable acid handling technologies. The competitive landscape is characterized by increasing consolidation among major chemical manufacturers and growing emphasis on safety innovations due to the hazardous nature of these acids.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed advanced handling and containment systems for both hydrofluoric acid (HF) and perchloric acid, focusing on their distinct reactivity profiles. Their technology incorporates specialized fluoropolymer-lined equipment for HF applications that resist the acid's unique ability to penetrate glass and attack silicates. For perchloric acid, Honeywell has engineered dedicated washdown systems that prevent the accumulation of potentially explosive perchlorates formed when the acid contacts organic materials or dehydrates. Their integrated safety monitoring systems utilize real-time sensors that can detect sub-ppm levels of acid vapors, triggering automated neutralization protocols. Honeywell's approach emphasizes the fundamental chemical differences: HF's moderate strength but extreme toxicity and tissue penetration versus perchloric acid's powerful oxidizing properties and explosion risks when concentrated or heated.
Strengths: Comprehensive safety systems integration with industrial process controls; extensive experience with fluorine chemistry applications across multiple industries. Weaknesses: Higher implementation costs compared to basic containment solutions; systems require specialized maintenance protocols and trained personnel.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has pioneered specialized fluoropolymer materials specifically engineered to handle the unique reactivity profiles of both hydrofluoric acid and perchloric acid in industrial applications. Their ETFE (ethylene tetrafluoroethylene) and PFA (perfluoroalkoxy) lining systems provide exceptional resistance to HF's penetrating properties while maintaining structural integrity. For perchloric acid applications, DAIKIN has developed modified PTFE composites that resist the strong oxidizing effects even at elevated temperatures. Their dual-containment technology incorporates sacrificial fluoropolymer layers that provide early warning of potential breaches before structural failure occurs. DAIKIN's research has focused on the fundamental differences in acid behavior: HF's ability to form hydrogen bonds and penetrate tissues versus perchloric acid's strong oxidizing nature and tendency to form explosive compounds when dehydrated or in contact with organics. Their materials science approach addresses both the immediate reactivity concerns and long-term stability issues in storage and transport systems.
Strengths: Industry-leading expertise in fluoropolymer development specifically designed for extreme chemical environments; materials engineered for extended service life under harsh conditions. Weaknesses: Premium pricing compared to conventional materials; requires specialized welding and fabrication techniques for installation and repair.
Key Patents and Scientific Literature Review
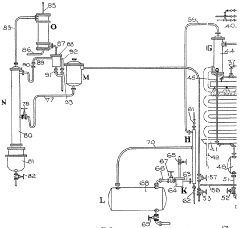
Improvements in or relating to the manufacture of hydrofluoric acid
PatentInactiveGB462131A
Innovation
- A continuous process under superatmospheric pressure reacts a fluoride with an acid, followed by preliminary cooling and condensation to separate dilute and concentrated hydrofluoric acid, using a reactor and condensers to achieve high yields and purity.
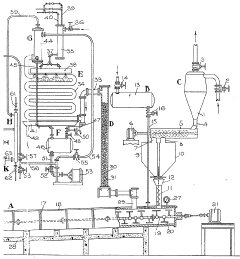
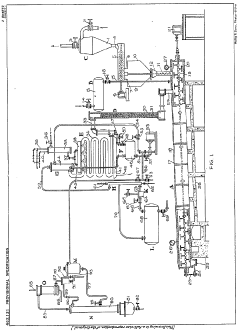
Method of recovering hydrofluoric acid
PatentInactiveUS20100280292A1
Innovation
- A method involving the compression of a hydrofluoric acid gas stream, initially cooled to between −20 and 100°C, followed by a separation step via distillation at pressures between 5 and 20 bar absolute, allowing for efficient recovery of hydrofluoric acid with reduced cooling requirements.
Safety Protocols and Risk Management Strategies
Working with hydrofluoric acid (HF) and perchloric acid (HClO4) requires comprehensive safety protocols due to their extreme hazards. For HF, immediate access to calcium gluconate gel is mandatory as it counteracts fluoride ion penetration into tissues. Facilities must install specialized emergency showers and eyewash stations with calcium gluconate solutions. Personnel handling HF must wear complete chemical protection including acid-resistant suits, face shields, and specialized gloves made of materials like neoprene or butyl rubber.
Perchloric acid demands different safety measures focusing on explosion prevention. Work areas must incorporate non-combustible materials, with wooden surfaces strictly prohibited. Dedicated fume hoods with wash-down systems prevent perchlorate salt accumulation, which can form explosive compounds. Temperature control systems must maintain perchloric acid below 150°C to prevent decomposition and potential detonation.
Risk management for both acids begins with thorough hazard assessments. Organizations should implement the hierarchy of controls: elimination, substitution with less hazardous alternatives, engineering controls, administrative controls, and personal protective equipment. Engineering controls include closed systems, remote handling equipment, and continuous monitoring systems that detect acid vapors at parts-per-billion levels.
Administrative controls encompass detailed standard operating procedures, regular safety drills, and comprehensive training programs. These programs must cover acid-specific emergency response, first aid procedures, and proper waste neutralization techniques. Documented exposure control plans should detail maximum exposure limits and monitoring protocols.
Emergency response planning requires specialized considerations for each acid. HF incidents necessitate immediate medical intervention protocols with calcium gluconate treatment. Perchloric acid spills require non-organic neutralizing agents and specialized cleanup procedures to prevent formation of explosive perchlorates during remediation.
Waste management presents significant challenges, particularly for perchloric acid, which cannot be disposed of through conventional chemical waste streams. Specialized neutralization procedures must be followed, with detailed documentation throughout the disposal process. Regular safety audits should evaluate compliance with established protocols, with findings incorporated into continuous improvement cycles.
International standards including OSHA regulations, NIOSH guidelines, and industry best practices should form the foundation of safety programs. Organizations handling these acids must maintain comprehensive incident reporting systems and conduct regular mock emergency scenarios to ensure preparedness for potential exposure events.
Perchloric acid demands different safety measures focusing on explosion prevention. Work areas must incorporate non-combustible materials, with wooden surfaces strictly prohibited. Dedicated fume hoods with wash-down systems prevent perchlorate salt accumulation, which can form explosive compounds. Temperature control systems must maintain perchloric acid below 150°C to prevent decomposition and potential detonation.
Risk management for both acids begins with thorough hazard assessments. Organizations should implement the hierarchy of controls: elimination, substitution with less hazardous alternatives, engineering controls, administrative controls, and personal protective equipment. Engineering controls include closed systems, remote handling equipment, and continuous monitoring systems that detect acid vapors at parts-per-billion levels.
Administrative controls encompass detailed standard operating procedures, regular safety drills, and comprehensive training programs. These programs must cover acid-specific emergency response, first aid procedures, and proper waste neutralization techniques. Documented exposure control plans should detail maximum exposure limits and monitoring protocols.
Emergency response planning requires specialized considerations for each acid. HF incidents necessitate immediate medical intervention protocols with calcium gluconate treatment. Perchloric acid spills require non-organic neutralizing agents and specialized cleanup procedures to prevent formation of explosive perchlorates during remediation.
Waste management presents significant challenges, particularly for perchloric acid, which cannot be disposed of through conventional chemical waste streams. Specialized neutralization procedures must be followed, with detailed documentation throughout the disposal process. Regular safety audits should evaluate compliance with established protocols, with findings incorporated into continuous improvement cycles.
International standards including OSHA regulations, NIOSH guidelines, and industry best practices should form the foundation of safety programs. Organizations handling these acids must maintain comprehensive incident reporting systems and conduct regular mock emergency scenarios to ensure preparedness for potential exposure events.
Environmental Impact and Regulatory Compliance
The environmental impact of hydrofluoric acid (HF) and perchloric acid (HClO4) represents a significant concern for industrial applications and laboratory settings. HF poses severe environmental hazards due to its high mobility in soil and groundwater systems, potentially contaminating water sources at considerable distances from release points. When released into aquatic environments, HF can dramatically alter pH levels, causing devastating effects on aquatic ecosystems and biodiversity. Additionally, HF vapor emissions contribute to air pollution and can cause vegetation damage through direct contact or acid rain formation.
Perchloric acid presents different but equally serious environmental challenges. As a strong oxidizer, accidental releases can trigger reactions with organic materials in the environment, potentially causing fires or explosions. Perchlorate contamination in groundwater has become a recognized environmental issue, with documented cases of widespread contamination near industrial facilities using perchloric acid. These perchlorates are highly persistent in the environment, with limited natural degradation pathways.
Regulatory frameworks governing these acids vary globally but generally trend toward increasing stringency. In the United States, HF is regulated under multiple frameworks including the Clean Air Act, where it is listed as a Hazardous Air Pollutant, and CERCLA (Superfund), which mandates reporting of releases exceeding reportable quantities. OSHA has established strict exposure limits for HF, currently set at 3 ppm for an 8-hour time-weighted average.
Perchloric acid faces similarly rigorous regulation, with additional requirements focused on its oxidizing properties and explosion hazards. The EPA has established drinking water advisory levels for perchlorates, reflecting concerns about their environmental persistence. International regulations, particularly in the European Union under REACH legislation, impose strict controls on both acids, requiring extensive documentation of handling procedures, exposure scenarios, and risk management measures.
Compliance requirements for facilities handling these acids include comprehensive chemical management systems, specialized storage facilities, detailed emergency response plans, and regular environmental monitoring programs. Waste management presents particular challenges, as neither acid can be disposed of through conventional waste streams. Neutralization protocols must be meticulously followed, with specialized disposal services typically required for larger quantities.
Recent regulatory trends indicate movement toward even stricter controls, particularly regarding perchlorate contamination in drinking water sources. Several jurisdictions have implemented or proposed more stringent discharge limits and monitoring requirements. Companies utilizing these acids must maintain robust compliance programs and stay abreast of evolving regulatory landscapes to ensure continued operational viability and environmental responsibility.
Perchloric acid presents different but equally serious environmental challenges. As a strong oxidizer, accidental releases can trigger reactions with organic materials in the environment, potentially causing fires or explosions. Perchlorate contamination in groundwater has become a recognized environmental issue, with documented cases of widespread contamination near industrial facilities using perchloric acid. These perchlorates are highly persistent in the environment, with limited natural degradation pathways.
Regulatory frameworks governing these acids vary globally but generally trend toward increasing stringency. In the United States, HF is regulated under multiple frameworks including the Clean Air Act, where it is listed as a Hazardous Air Pollutant, and CERCLA (Superfund), which mandates reporting of releases exceeding reportable quantities. OSHA has established strict exposure limits for HF, currently set at 3 ppm for an 8-hour time-weighted average.
Perchloric acid faces similarly rigorous regulation, with additional requirements focused on its oxidizing properties and explosion hazards. The EPA has established drinking water advisory levels for perchlorates, reflecting concerns about their environmental persistence. International regulations, particularly in the European Union under REACH legislation, impose strict controls on both acids, requiring extensive documentation of handling procedures, exposure scenarios, and risk management measures.
Compliance requirements for facilities handling these acids include comprehensive chemical management systems, specialized storage facilities, detailed emergency response plans, and regular environmental monitoring programs. Waste management presents particular challenges, as neither acid can be disposed of through conventional waste streams. Neutralization protocols must be meticulously followed, with specialized disposal services typically required for larger quantities.
Recent regulatory trends indicate movement toward even stricter controls, particularly regarding perchlorate contamination in drinking water sources. Several jurisdictions have implemented or proposed more stringent discharge limits and monitoring requirements. Companies utilizing these acids must maintain robust compliance programs and stay abreast of evolving regulatory landscapes to ensure continued operational viability and environmental responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!