Hydrofluoric Acid vs Hexafluorosilicic Acid: Handling Comparison
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Handling Technology Background and Objectives
The handling of corrosive acids represents a critical aspect of industrial safety and operational efficiency across multiple sectors including semiconductor manufacturing, chemical processing, and water treatment. Hydrofluoric acid (HF) and hexafluorosilicic acid (H2SiF6) are two significant compounds that have evolved distinct handling protocols over decades of industrial application. The historical development of acid handling technologies traces back to the early 20th century, with significant advancements occurring post-1950s as industrial safety standards became more stringent.
The evolution of acid handling technology has been primarily driven by three factors: increasing safety requirements, environmental regulations, and efficiency demands in industrial processes. Early handling methods focused primarily on basic containment, while modern approaches incorporate sophisticated monitoring systems, automated handling equipment, and advanced material science to develop corrosion-resistant containment solutions.
Hydrofluoric acid, discovered in the 17th century but industrialized in the late 19th century, presents unique handling challenges due to its ability to penetrate skin and cause deep tissue damage without immediate symptoms. Hexafluorosilicic acid, a byproduct of phosphate fertilizer production and commonly used in water fluoridation, presents different but equally significant handling considerations due to its high corrosivity and toxicity profile.
The technological trajectory in acid handling has moved from manual operations toward increasingly automated systems that minimize human exposure. This transition has been accelerated by significant industrial accidents involving these acids, which have served as catalysts for technological innovation and regulatory development. The Buffalo Creek incident of 1972 and the Marathon Oil refinery accident in 1987 represent watershed moments that fundamentally altered industry approaches to acid handling.
Current technological objectives in the comparison of HF and H2SiF6 handling focus on several key areas: development of more effective personal protective equipment specifically designed for each acid's unique properties; creation of advanced containment systems that account for the different corrosion profiles; implementation of real-time monitoring technologies that can detect minute leaks before they become hazardous; and establishment of standardized emergency response protocols tailored to the specific medical interventions required for each acid.
The ultimate goal of this technological evolution is to achieve zero-incident handling of both acids through engineering controls that eliminate human exposure risks, while simultaneously improving process efficiency and reducing environmental impact. This represents a complex balance between safety imperatives, economic considerations, and technological feasibility that continues to drive innovation in the field.
The evolution of acid handling technology has been primarily driven by three factors: increasing safety requirements, environmental regulations, and efficiency demands in industrial processes. Early handling methods focused primarily on basic containment, while modern approaches incorporate sophisticated monitoring systems, automated handling equipment, and advanced material science to develop corrosion-resistant containment solutions.
Hydrofluoric acid, discovered in the 17th century but industrialized in the late 19th century, presents unique handling challenges due to its ability to penetrate skin and cause deep tissue damage without immediate symptoms. Hexafluorosilicic acid, a byproduct of phosphate fertilizer production and commonly used in water fluoridation, presents different but equally significant handling considerations due to its high corrosivity and toxicity profile.
The technological trajectory in acid handling has moved from manual operations toward increasingly automated systems that minimize human exposure. This transition has been accelerated by significant industrial accidents involving these acids, which have served as catalysts for technological innovation and regulatory development. The Buffalo Creek incident of 1972 and the Marathon Oil refinery accident in 1987 represent watershed moments that fundamentally altered industry approaches to acid handling.
Current technological objectives in the comparison of HF and H2SiF6 handling focus on several key areas: development of more effective personal protective equipment specifically designed for each acid's unique properties; creation of advanced containment systems that account for the different corrosion profiles; implementation of real-time monitoring technologies that can detect minute leaks before they become hazardous; and establishment of standardized emergency response protocols tailored to the specific medical interventions required for each acid.
The ultimate goal of this technological evolution is to achieve zero-incident handling of both acids through engineering controls that eliminate human exposure risks, while simultaneously improving process efficiency and reducing environmental impact. This represents a complex balance between safety imperatives, economic considerations, and technological feasibility that continues to drive innovation in the field.
Industrial Applications and Market Demand Analysis
The global market for acids used in industrial processes continues to expand, with hydrofluoric acid (HF) and hexafluorosilicic acid (H2SiF6) playing significant roles across multiple sectors. The combined market value for these fluorine-based acids reached approximately 3 billion USD in 2022, with projections indicating growth at a compound annual rate of 4.7% through 2030.
Hydrofluoric acid maintains dominant market share due to its established applications in petroleum refining, where it serves as a catalyst in alkylation processes for high-octane fuel production. The semiconductor industry represents another major consumption sector, utilizing HF for silicon wafer etching and cleaning. The glass etching industry also relies heavily on HF for decorative glass production and surface treatment applications.
Hexafluorosilicic acid, while commanding a smaller market segment, has experienced steady growth primarily driven by water fluoridation programs worldwide. Municipal water treatment represents over 60% of H2SiF6 consumption globally. Additional applications in aluminum production, chemical manufacturing, and as a wood preservative contribute to its market presence.
Regional analysis reveals Asia-Pacific as the fastest-growing market for both acids, with China and India leading consumption growth due to rapid industrialization and expanding manufacturing sectors. North America maintains significant demand primarily through established petroleum refining operations and water treatment infrastructure. European markets show more moderate growth, influenced by stringent environmental regulations affecting industrial chemical usage.
Market demand analysis indicates shifting preferences toward hexafluorosilicic acid in certain applications due to its handling advantages. The occupational safety concerns associated with hydrofluoric acid have prompted industries to seek alternatives where technically feasible. This transition is particularly evident in water treatment facilities, where H2SiF6 offers comparable effectiveness with reduced handling hazards.
Recent market surveys indicate that approximately 30% of industrial facilities using hydrofluoric acid are evaluating potential alternatives, with handling safety cited as the primary motivation. This trend has created new market opportunities for equipment manufacturers specializing in acid handling systems designed specifically for hexafluorosilicic acid's properties.
The price differential between these acids also influences market dynamics, with hexafluorosilicic acid generally available at lower cost due to its status as a byproduct of phosphate fertilizer production. This economic advantage, combined with reduced safety infrastructure requirements, has strengthened its competitive position in cost-sensitive applications.
Hydrofluoric acid maintains dominant market share due to its established applications in petroleum refining, where it serves as a catalyst in alkylation processes for high-octane fuel production. The semiconductor industry represents another major consumption sector, utilizing HF for silicon wafer etching and cleaning. The glass etching industry also relies heavily on HF for decorative glass production and surface treatment applications.
Hexafluorosilicic acid, while commanding a smaller market segment, has experienced steady growth primarily driven by water fluoridation programs worldwide. Municipal water treatment represents over 60% of H2SiF6 consumption globally. Additional applications in aluminum production, chemical manufacturing, and as a wood preservative contribute to its market presence.
Regional analysis reveals Asia-Pacific as the fastest-growing market for both acids, with China and India leading consumption growth due to rapid industrialization and expanding manufacturing sectors. North America maintains significant demand primarily through established petroleum refining operations and water treatment infrastructure. European markets show more moderate growth, influenced by stringent environmental regulations affecting industrial chemical usage.
Market demand analysis indicates shifting preferences toward hexafluorosilicic acid in certain applications due to its handling advantages. The occupational safety concerns associated with hydrofluoric acid have prompted industries to seek alternatives where technically feasible. This transition is particularly evident in water treatment facilities, where H2SiF6 offers comparable effectiveness with reduced handling hazards.
Recent market surveys indicate that approximately 30% of industrial facilities using hydrofluoric acid are evaluating potential alternatives, with handling safety cited as the primary motivation. This trend has created new market opportunities for equipment manufacturers specializing in acid handling systems designed specifically for hexafluorosilicic acid's properties.
The price differential between these acids also influences market dynamics, with hexafluorosilicic acid generally available at lower cost due to its status as a byproduct of phosphate fertilizer production. This economic advantage, combined with reduced safety infrastructure requirements, has strengthened its competitive position in cost-sensitive applications.
Current Challenges in Corrosive Acid Management
The management of highly corrosive acids presents significant challenges across industrial applications, particularly when comparing hydrofluoric acid (HF) and hexafluorosilicic acid (H2SiF6). These challenges stem from their inherent chemical properties, handling requirements, and safety considerations that impact operational efficiency and worker safety.
Material compatibility remains a primary concern as both acids aggressively attack common containment materials. HF penetrates glass and many metals, necessitating specialized storage in polyethylene, PTFE, or specific alloys like Monel. H2SiF6, while less aggressive toward certain materials, still requires careful selection of compatible containment systems, with notable differences in corrosion rates that affect equipment longevity and maintenance schedules.
Worker safety protocols present complex implementation challenges due to the distinct hazard profiles of these acids. HF poses immediate and severe health risks through skin absorption, causing deep tissue damage and potential systemic toxicity even at low concentrations. H2SiF6, while also hazardous, typically presents different exposure risks and requires tailored safety measures, creating difficulties in standardizing protection protocols across facilities handling both chemicals.
Transportation and storage regulations introduce compliance complexities that vary by jurisdiction. HF is subject to stringent controls due to its dual classification as both a highly hazardous chemical and potential security risk. H2SiF6 faces different regulatory frameworks, creating administrative burdens for organizations managing both substances across multiple locations or international boundaries.
Environmental management presents escalating challenges as discharge regulations tighten globally. Neutralization processes differ significantly between these acids, with HF requiring calcium-based treatments while H2SiF6 often demands alternative approaches due to its silicon content. These differences complicate the development of unified waste treatment systems capable of handling both acids efficiently.
Emergency response preparedness represents a critical operational challenge, as incidents involving these acids require fundamentally different mitigation strategies. HF releases necessitate calcium gluconate treatment capabilities and specialized containment procedures, while H2SiF6 spills may require different neutralization agents and containment approaches, forcing facilities to maintain multiple parallel emergency response systems.
Cost management considerations further complicate acid selection decisions, as the total ownership cost extends beyond purchase price to include specialized handling equipment, safety systems, training requirements, and waste disposal expenses. The economic equation varies significantly between these acids depending on application requirements, facility infrastructure, and regional regulatory environments.
Material compatibility remains a primary concern as both acids aggressively attack common containment materials. HF penetrates glass and many metals, necessitating specialized storage in polyethylene, PTFE, or specific alloys like Monel. H2SiF6, while less aggressive toward certain materials, still requires careful selection of compatible containment systems, with notable differences in corrosion rates that affect equipment longevity and maintenance schedules.
Worker safety protocols present complex implementation challenges due to the distinct hazard profiles of these acids. HF poses immediate and severe health risks through skin absorption, causing deep tissue damage and potential systemic toxicity even at low concentrations. H2SiF6, while also hazardous, typically presents different exposure risks and requires tailored safety measures, creating difficulties in standardizing protection protocols across facilities handling both chemicals.
Transportation and storage regulations introduce compliance complexities that vary by jurisdiction. HF is subject to stringent controls due to its dual classification as both a highly hazardous chemical and potential security risk. H2SiF6 faces different regulatory frameworks, creating administrative burdens for organizations managing both substances across multiple locations or international boundaries.
Environmental management presents escalating challenges as discharge regulations tighten globally. Neutralization processes differ significantly between these acids, with HF requiring calcium-based treatments while H2SiF6 often demands alternative approaches due to its silicon content. These differences complicate the development of unified waste treatment systems capable of handling both acids efficiently.
Emergency response preparedness represents a critical operational challenge, as incidents involving these acids require fundamentally different mitigation strategies. HF releases necessitate calcium gluconate treatment capabilities and specialized containment procedures, while H2SiF6 spills may require different neutralization agents and containment approaches, forcing facilities to maintain multiple parallel emergency response systems.
Cost management considerations further complicate acid selection decisions, as the total ownership cost extends beyond purchase price to include specialized handling equipment, safety systems, training requirements, and waste disposal expenses. The economic equation varies significantly between these acids depending on application requirements, facility infrastructure, and regional regulatory environments.
Comparative Analysis of HF and H2SiF6 Handling Solutions
01 Protective equipment and clothing for acid handling
When handling hydrofluoric acid and hexafluorosilicic acid, appropriate protective equipment is essential to prevent exposure. This includes chemical-resistant gloves, face shields, acid-resistant clothing, and respiratory protection. The protective gear should be specifically designed to withstand these highly corrosive acids and prevent skin contact, inhalation of vapors, and eye exposure, which can cause severe burns and tissue damage.- Protective equipment and clothing for acid handling: When handling hydrofluoric acid and hexafluorosilicic acid, appropriate protective equipment is essential to prevent exposure. This includes chemical-resistant gloves, face shields, acid-resistant clothing, and respiratory protection. The protective gear should be specifically designed to withstand these highly corrosive acids and prevent skin contact, inhalation of vapors, and eye exposure. Regular inspection and maintenance of protective equipment ensures continued safety during handling operations.
- Storage and containment systems: Specialized storage and containment systems are required for hydrofluoric acid and hexafluorosilicic acid to prevent leaks and spills. These systems include acid-resistant containers, secondary containment structures, specialized ventilation systems, and monitoring equipment. Materials used for storage must be compatible with these highly corrosive acids, typically including fluoropolymer linings or specialized plastics. Proper labeling and segregation from incompatible chemicals is also essential for safe storage.
- Emergency response and first aid procedures: Specific emergency response protocols are necessary when handling hydrofluoric acid and hexafluorosilicic acid due to their unique hazards. This includes specialized first aid procedures for acid exposure, such as calcium gluconate treatment for hydrofluoric acid burns, emergency shower and eyewash stations, spill containment and neutralization methods, and evacuation procedures. Training personnel in these emergency procedures is critical as these acids can cause severe and potentially delayed injuries requiring immediate specialized treatment.
- Neutralization and waste treatment methods: Safe handling of hydrofluoric acid and hexafluorosilicic acid includes proper neutralization and waste treatment procedures. This involves specific neutralizing agents like calcium compounds for hydrofluoric acid, controlled dilution techniques, waste treatment systems designed for fluoride-containing compounds, and monitoring of effluent pH and fluoride levels. These methods ensure that acid waste is rendered safe before disposal and complies with environmental regulations.
- Engineering controls and facility design: Specialized engineering controls and facility design elements are essential for facilities handling hydrofluoric acid and hexafluorosilicic acid. These include closed handling systems to minimize exposure, dedicated ventilation systems with scrubbers to capture acid vapors, acid-resistant flooring and work surfaces, safety showers and eyewash stations positioned strategically throughout the facility, and continuous monitoring systems for detecting acid leaks or vapors. These engineering controls work together to minimize risk during routine operations.
02 Containment systems and storage solutions
Specialized containment systems are required for the safe storage and handling of hydrofluoric acid and hexafluorosilicic acid. These include corrosion-resistant tanks, double-walled containers, secondary containment structures, and specialized piping systems. Proper storage facilities should include ventilation systems, temperature control, and monitoring equipment to detect leaks or releases, minimizing the risk of accidents and environmental contamination.Expand Specific Solutions03 Neutralization and emergency response procedures
Effective emergency response procedures for hydrofluoric acid and hexafluorosilicic acid incidents include immediate neutralization protocols using calcium gluconate or other neutralizing agents. Emergency stations should be equipped with calcium gluconate gel for skin exposure, eyewash stations, and emergency showers. Detailed response plans should outline evacuation procedures, containment strategies for spills, and medical treatment protocols for exposed individuals.Expand Specific Solutions04 Handling equipment and transfer systems
Specialized equipment for the safe handling and transfer of hydrofluoric acid and hexafluorosilicic acid includes automated pumping systems, closed transfer systems, and specialized valves and fittings made from acid-resistant materials. These systems minimize direct human contact with the acids during transfer operations and reduce the risk of spills or splashes. Proper maintenance and regular inspection of handling equipment are essential to ensure continued safe operation.Expand Specific Solutions05 Training and safety protocols
Comprehensive training programs and safety protocols are crucial for personnel working with hydrofluoric acid and hexafluorosilicic acid. These include detailed handling procedures, hazard communication, regular safety drills, and certification requirements. Workers should be trained in the specific hazards of these acids, proper use of protective equipment, emergency response procedures, and first aid techniques for acid exposure. Regular refresher training and safety audits help maintain awareness and compliance with safety standards.Expand Specific Solutions
Major Industry Players and Supplier Landscape
The hydrofluoric acid and hexafluorosilicic acid handling comparison market is in a growth phase, with increasing demand driven by semiconductor manufacturing, chemical processing, and electronics industries. The market size is expanding at approximately 4-5% CAGR, particularly in Asia-Pacific regions. Regarding technical maturity, companies like Do-Fluoride New Materials and Stella Chemifa have established advanced handling protocols for hydrofluoric acid, while Jiangyin Runma Electronic Material and TOKYO OHKA KOGYO focus on ultra-pure acid formulations for semiconductor applications. BASF, Solvay, and Henkel have developed sophisticated containment systems and neutralization technologies, reflecting the industry's evolution toward safer handling practices. Emerging players like Hubei Hongyuan and Guangdong Guanghua Sci-Tech are advancing regional capabilities in specialized acid applications.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton has developed specialized handling technologies for both hydrofluoric acid and hexafluorosilicic acid used in oil field operations, particularly in well stimulation and acidizing processes. Their ACIDGUARD™ system implements distinct protocols based on the different hazard profiles of these acids. For HF, Halliburton employs proprietary high-density polyethylene containment systems with redundant barriers and automated transfer mechanisms that minimize human exposure. Their hexafluorosilicic acid handling approach focuses on the acid's unique silicon-based chemistry, utilizing specialized alloys that resist the particular corrosion patterns this acid exhibits. Halliburton's comparative analysis demonstrates that while HF requires more immediate emergency response capabilities due to its rapid tissue penetration properties (with their mobile calcium gluconate treatment stations positioned at all handling locations), H2SiF6 handling emphasizes equipment longevity through specialized material selection and more frequent integrity testing protocols. Their field data indicates that HF incidents require response within seconds, while H2SiF6 exposure allows slightly longer response windows but potentially more extensive equipment damage.
Strengths: Field-proven technologies specifically designed for harsh oilfield environments; mobile emergency response capabilities; extensive experience with high-pressure acid applications. Weaknesses: Systems optimized primarily for oilfield applications may require modification for other industries; higher operational costs; specialized training requirements for field personnel.
Baker Hughes Co.
Technical Solution: Baker Hughes has engineered comprehensive acid handling systems that address the distinct properties of hydrofluoric acid and hexafluorosilicic acid in industrial applications. Their ACIDSHIELD™ technology implements a dual approach to these chemicals based on their different hazard profiles. For hydrofluoric acid, Baker Hughes developed rapid-response containment systems featuring proprietary fluoropolymer materials with leak detection capabilities sensitive to concentrations as low as 0.3ppm. Their hexafluorosilicic acid handling technology focuses on the unique silicon-based chemistry of this acid, utilizing specialized silicon-resistant alloys and elastomers that withstand its particular corrosion patterns. Baker Hughes' comparative analysis shows that while HF requires more immediate emergency response protocols (with their integrated calcium-based neutralization systems), H2SiF6 handling emphasizes equipment integrity through specialized material selection and predictive maintenance algorithms that can detect subtle changes in equipment performance before failures occur. Their field data indicates that proper HF handling requires response times under 30 seconds for exposure incidents, while H2SiF6 allows slightly longer response windows but potentially more extensive equipment damage over time.
Strengths: Integrated safety systems with real-time monitoring capabilities; extensive field experience in high-pressure acid applications; comprehensive training programs for operational personnel. Weaknesses: Higher implementation costs compared to standard systems; complex maintenance requirements; specialized equipment may limit operational flexibility in some applications.
Key Patents and Innovations in Acid Containment
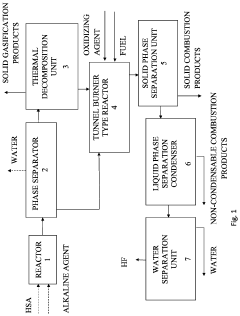
Method for producing hydrogen fluoride from hexafluorosilicic acid
PatentInactiveUS20210284532A1
Innovation
- The method involves neutralizing the HSA solution with an alkaline agent, followed by processing the resulting solid salt in a fire with a hydrogen-containing fuel and oxygen-containing oxidant to produce HF, which eliminates the need for sulfuric acid and reduces waste, and optionally includes preliminary thermal decomposition to separate fluorinated substances and silicon dioxide, simplifying the process and reducing energy consumption.
Composition for removing sidewall residues
PatentWO2004001834A1
Innovation
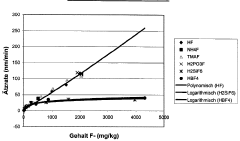
- A composition containing hexafluorosilicic acid (H2SiF6) and/or tetrafluoroboric acid (HBF4) in combination with sulfuric acid and hydrogen peroxide, used as a stripper, provides stable etching rates over a wide concentration range, reducing the need for continuous measurement and dosing systems, and offering effective removal of residual polymers without damaging the metallization layers.
Regulatory Compliance and Safety Standards
The regulatory landscape governing the handling of hydrofluoric acid (HF) and hexafluorosilicic acid (H2SiF6) is extensive and multifaceted, reflecting the severe hazards these chemicals present. In the United States, the Occupational Safety and Health Administration (OSHA) has established specific permissible exposure limits (PELs) for HF at 3 ppm as an 8-hour time-weighted average, while H2SiF6 falls under general acid mist regulations. The Environmental Protection Agency (EPA) regulates both substances under the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA), with HF receiving additional scrutiny due to its classification as an extremely hazardous substance.
European regulations are generally more stringent, with the EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) program requiring extensive documentation for both acids. The Classification, Labeling and Packaging (CLP) Regulation mandates specific hazard communication standards that differ slightly between the two acids, with HF carrying additional acute toxicity classifications.
Safety standards for handling these acids are codified in numerous industry protocols. The American National Standards Institute (ANSI) and the American Society for Testing and Materials (ASTM) have developed specific guidelines for containment systems, with HF requiring specialized materials like PTFE or specific grades of stainless steel, while H2SiF6 can often be handled with a broader range of acid-resistant materials.
Personal protective equipment (PPE) requirements represent a critical difference between the two acids. HF handling necessitates specialized PPE including fluoride-specific impermeable suits, butyl rubber gloves, and full-face respiratory protection with appropriate cartridges. H2SiF6, while still requiring comprehensive protection, generally follows standard strong acid protocols without the specialized materials needed for HF.
Emergency response protocols also differ significantly. Facilities handling HF must maintain calcium gluconate gel antidotes and develop specific emergency response plans that address HF's unique ability to penetrate tissue and cause systemic toxicity. H2SiF6 emergency responses follow more standard acid spill protocols, though still requiring specialized training.
International transportation regulations, including the UN Recommendations on the Transport of Dangerous Goods, classify both acids differently, with HF receiving more restrictive shipping requirements. This impacts packaging specifications, labeling requirements, and transportation route planning, with HF shipments often requiring additional security measures and specialized carrier certifications.
Compliance documentation requirements are more extensive for HF, with many jurisdictions requiring specific training certifications, medical surveillance programs for workers, and detailed exposure monitoring records that exceed those required for H2SiF6 handling operations.
European regulations are generally more stringent, with the EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) program requiring extensive documentation for both acids. The Classification, Labeling and Packaging (CLP) Regulation mandates specific hazard communication standards that differ slightly between the two acids, with HF carrying additional acute toxicity classifications.
Safety standards for handling these acids are codified in numerous industry protocols. The American National Standards Institute (ANSI) and the American Society for Testing and Materials (ASTM) have developed specific guidelines for containment systems, with HF requiring specialized materials like PTFE or specific grades of stainless steel, while H2SiF6 can often be handled with a broader range of acid-resistant materials.
Personal protective equipment (PPE) requirements represent a critical difference between the two acids. HF handling necessitates specialized PPE including fluoride-specific impermeable suits, butyl rubber gloves, and full-face respiratory protection with appropriate cartridges. H2SiF6, while still requiring comprehensive protection, generally follows standard strong acid protocols without the specialized materials needed for HF.
Emergency response protocols also differ significantly. Facilities handling HF must maintain calcium gluconate gel antidotes and develop specific emergency response plans that address HF's unique ability to penetrate tissue and cause systemic toxicity. H2SiF6 emergency responses follow more standard acid spill protocols, though still requiring specialized training.
International transportation regulations, including the UN Recommendations on the Transport of Dangerous Goods, classify both acids differently, with HF receiving more restrictive shipping requirements. This impacts packaging specifications, labeling requirements, and transportation route planning, with HF shipments often requiring additional security measures and specialized carrier certifications.
Compliance documentation requirements are more extensive for HF, with many jurisdictions requiring specific training certifications, medical surveillance programs for workers, and detailed exposure monitoring records that exceed those required for H2SiF6 handling operations.
Environmental Impact and Sustainable Handling Practices
The environmental impact of acid handling extends far beyond immediate safety concerns, with both hydrofluoric acid (HF) and hexafluorosilicic acid (H2SiF6) presenting distinct ecological challenges. HF releases pose severe risks to ecosystems, causing vegetation damage through fluoride accumulation and potentially contaminating groundwater systems with long-term persistence. When released into aquatic environments, HF can dramatically alter pH levels, resulting in devastating effects on aquatic life even at relatively low concentrations.
Hexafluorosilicic acid, while sharing some environmental concerns with HF, demonstrates different ecological behavior. Its silicon component introduces additional considerations for environmental management, particularly in water systems where silicon compounds can affect aquatic organism development. However, H2SiF6 typically exhibits slower environmental reactivity compared to HF, potentially allowing more response time during accidental releases.
Sustainable handling practices for both acids have evolved significantly in recent years, with closed-loop systems representing the gold standard for environmental protection. These systems minimize emissions and waste by capturing and recycling acid vapors and residues, substantially reducing environmental discharge. Advanced scrubber technologies have become increasingly sophisticated, with multi-stage systems capable of capturing over 99% of acid emissions before they reach the atmosphere.
Neutralization technologies have also advanced considerably, with specialized lime-based treatments optimized for fluoride-containing waste streams. Modern facilities increasingly implement real-time monitoring systems that track potential emissions and leaks, enabling immediate response to prevent environmental contamination. This represents a significant improvement over historical practices where detection often occurred only after environmental damage had manifested.
Industry best practices now emphasize lifecycle management approaches that consider environmental impact from production through disposal. This includes strategic facility location planning to minimize ecological risk, implementation of buffer zones around acid handling facilities, and development of comprehensive emergency response protocols specifically designed to address environmental contamination scenarios.
Regulatory frameworks worldwide have progressively tightened restrictions on both acids, with particular focus on emission limits and disposal requirements. The European Union's REACH regulations and similar frameworks in other regions have driven significant improvements in handling practices, requiring detailed environmental impact assessments and mitigation strategies as prerequisites for operational permits.
Future sustainable handling innovations show promise in several areas, including development of less environmentally persistent alternatives, advanced containment materials with improved resistance to degradation, and intelligent monitoring systems capable of predicting potential failures before they result in environmental releases.
Hexafluorosilicic acid, while sharing some environmental concerns with HF, demonstrates different ecological behavior. Its silicon component introduces additional considerations for environmental management, particularly in water systems where silicon compounds can affect aquatic organism development. However, H2SiF6 typically exhibits slower environmental reactivity compared to HF, potentially allowing more response time during accidental releases.
Sustainable handling practices for both acids have evolved significantly in recent years, with closed-loop systems representing the gold standard for environmental protection. These systems minimize emissions and waste by capturing and recycling acid vapors and residues, substantially reducing environmental discharge. Advanced scrubber technologies have become increasingly sophisticated, with multi-stage systems capable of capturing over 99% of acid emissions before they reach the atmosphere.
Neutralization technologies have also advanced considerably, with specialized lime-based treatments optimized for fluoride-containing waste streams. Modern facilities increasingly implement real-time monitoring systems that track potential emissions and leaks, enabling immediate response to prevent environmental contamination. This represents a significant improvement over historical practices where detection often occurred only after environmental damage had manifested.
Industry best practices now emphasize lifecycle management approaches that consider environmental impact from production through disposal. This includes strategic facility location planning to minimize ecological risk, implementation of buffer zones around acid handling facilities, and development of comprehensive emergency response protocols specifically designed to address environmental contamination scenarios.
Regulatory frameworks worldwide have progressively tightened restrictions on both acids, with particular focus on emission limits and disposal requirements. The European Union's REACH regulations and similar frameworks in other regions have driven significant improvements in handling practices, requiring detailed environmental impact assessments and mitigation strategies as prerequisites for operational permits.
Future sustainable handling innovations show promise in several areas, including development of less environmentally persistent alternatives, advanced containment materials with improved resistance to degradation, and intelligent monitoring systems capable of predicting potential failures before they result in environmental releases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!