How to Drive Chemical Innovation with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful tool in chemical innovation. This compound, first synthesized in the 1960s, has gained significant attention in recent years due to its exceptional acidity and unique chemical properties.
The development of fluoroantimonic acid represents a milestone in the field of superacids, surpassing the acidity of conventional strong acids by several orders of magnitude. Its Hammett acidity function (H0) is estimated to be around -31.3, making it billions of times stronger than 100% sulfuric acid. This extreme acidity is attributed to the formation of the hexafluoroantimonate anion (SbF6-) and the highly acidic proton (H+) when HF and SbF5 are combined.
The primary objective of exploring fluoroantimonic acid in chemical innovation is to harness its exceptional protonating ability and catalytic properties. Researchers aim to utilize this superacid in various applications, including organic synthesis, petrochemical processing, and materials science. By leveraging its unique characteristics, scientists seek to overcome limitations in traditional acid-catalyzed reactions and develop novel synthetic pathways.
One of the key goals in fluoroantimonic acid research is to expand its applicability in industrial processes. The superacid's ability to protonate even extremely weak bases opens up possibilities for transforming unreactive compounds and catalyzing challenging reactions. This potential has sparked interest in developing more efficient and selective chemical processes, particularly in the production of high-value chemicals and advanced materials.
Another important objective is to understand and control the reactivity of fluoroantimonic acid under various conditions. This includes investigating its behavior in different solvents, at various temperatures, and in the presence of different substrates. By gaining a deeper understanding of its reaction mechanisms and kinetics, researchers aim to optimize its use and develop safer handling protocols.
The exploration of fluoroantimonic acid also extends to its potential in materials science. Scientists are investigating its role in the synthesis of novel materials, such as superhydrophobic coatings, advanced polymers, and nanostructured materials. The ability of this superacid to modify surface properties and initiate unique chemical transformations presents exciting opportunities for material innovation.
As research in this field progresses, there is a growing focus on developing more sustainable and environmentally friendly applications of fluoroantimonic acid. This includes efforts to minimize waste generation, improve recycling methods, and explore less hazardous alternatives that retain similar superacidic properties. The ultimate goal is to balance the remarkable reactivity of fluoroantimonic acid with the principles of green chemistry and sustainable development.
The development of fluoroantimonic acid represents a milestone in the field of superacids, surpassing the acidity of conventional strong acids by several orders of magnitude. Its Hammett acidity function (H0) is estimated to be around -31.3, making it billions of times stronger than 100% sulfuric acid. This extreme acidity is attributed to the formation of the hexafluoroantimonate anion (SbF6-) and the highly acidic proton (H+) when HF and SbF5 are combined.
The primary objective of exploring fluoroantimonic acid in chemical innovation is to harness its exceptional protonating ability and catalytic properties. Researchers aim to utilize this superacid in various applications, including organic synthesis, petrochemical processing, and materials science. By leveraging its unique characteristics, scientists seek to overcome limitations in traditional acid-catalyzed reactions and develop novel synthetic pathways.
One of the key goals in fluoroantimonic acid research is to expand its applicability in industrial processes. The superacid's ability to protonate even extremely weak bases opens up possibilities for transforming unreactive compounds and catalyzing challenging reactions. This potential has sparked interest in developing more efficient and selective chemical processes, particularly in the production of high-value chemicals and advanced materials.
Another important objective is to understand and control the reactivity of fluoroantimonic acid under various conditions. This includes investigating its behavior in different solvents, at various temperatures, and in the presence of different substrates. By gaining a deeper understanding of its reaction mechanisms and kinetics, researchers aim to optimize its use and develop safer handling protocols.
The exploration of fluoroantimonic acid also extends to its potential in materials science. Scientists are investigating its role in the synthesis of novel materials, such as superhydrophobic coatings, advanced polymers, and nanostructured materials. The ability of this superacid to modify surface properties and initiate unique chemical transformations presents exciting opportunities for material innovation.
As research in this field progresses, there is a growing focus on developing more sustainable and environmentally friendly applications of fluoroantimonic acid. This includes efforts to minimize waste generation, improve recycling methods, and explore less hazardous alternatives that retain similar superacidic properties. The ultimate goal is to balance the remarkable reactivity of fluoroantimonic acid with the principles of green chemistry and sustainable development.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has been experiencing significant growth and diversification in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found applications across various industries, driving innovation and creating new market opportunities.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth due to the increasing demand for cleaner-burning, high-performance fuels. The automotive industry's shift towards more efficient engines has further bolstered this market segment.
The electronics industry has also embraced fluoroantimonic acid for its ability to etch and clean semiconductor materials. As the demand for smaller, more powerful electronic devices continues to rise, the market for superacid-based etching solutions has expanded correspondingly. This trend is expected to persist with the ongoing development of advanced microchips and nanotechnology.
In materials science, fluoroantimonic acid has opened new avenues for the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that were previously impossible or impractical, leading to the development of advanced polymers, catalysts, and other high-performance materials. This has created a niche but rapidly growing market segment with applications in aerospace, defense, and advanced manufacturing.
The pharmaceutical industry has shown increasing interest in superacid applications for drug synthesis and purification processes. Fluoroantimonic acid's unique properties enable the creation of complex organic molecules, potentially accelerating drug discovery and development. While still a relatively small market segment, it shows promising growth potential as research in this area intensifies.
Environmental applications of fluoroantimonic acid, particularly in waste treatment and pollution control, represent an emerging market with significant potential. The superacid's ability to break down persistent organic pollutants and treat industrial effluents has attracted attention from environmental agencies and industrial waste management sectors.
Despite its growth, the market for fluoroantimonic acid applications faces challenges. Safety concerns and the need for specialized handling equipment limit its widespread adoption. Additionally, regulatory scrutiny regarding environmental impact and worker safety has led to increased costs for implementation in some industries.
Looking ahead, the market for superacid applications is expected to continue its growth trajectory, driven by ongoing research and development in chemical synthesis, materials science, and clean energy technologies. As new applications emerge and existing ones expand, the demand for fluoroantimonic acid and related superacid technologies is likely to increase, creating opportunities for innovation and market expansion across multiple industries.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth due to the increasing demand for cleaner-burning, high-performance fuels. The automotive industry's shift towards more efficient engines has further bolstered this market segment.
The electronics industry has also embraced fluoroantimonic acid for its ability to etch and clean semiconductor materials. As the demand for smaller, more powerful electronic devices continues to rise, the market for superacid-based etching solutions has expanded correspondingly. This trend is expected to persist with the ongoing development of advanced microchips and nanotechnology.
In materials science, fluoroantimonic acid has opened new avenues for the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that were previously impossible or impractical, leading to the development of advanced polymers, catalysts, and other high-performance materials. This has created a niche but rapidly growing market segment with applications in aerospace, defense, and advanced manufacturing.
The pharmaceutical industry has shown increasing interest in superacid applications for drug synthesis and purification processes. Fluoroantimonic acid's unique properties enable the creation of complex organic molecules, potentially accelerating drug discovery and development. While still a relatively small market segment, it shows promising growth potential as research in this area intensifies.
Environmental applications of fluoroantimonic acid, particularly in waste treatment and pollution control, represent an emerging market with significant potential. The superacid's ability to break down persistent organic pollutants and treat industrial effluents has attracted attention from environmental agencies and industrial waste management sectors.
Despite its growth, the market for fluoroantimonic acid applications faces challenges. Safety concerns and the need for specialized handling equipment limit its widespread adoption. Additionally, regulatory scrutiny regarding environmental impact and worker safety has led to increased costs for implementation in some industries.
Looking ahead, the market for superacid applications is expected to continue its growth trajectory, driven by ongoing research and development in chemical synthesis, materials science, and clean energy technologies. As new applications emerge and existing ones expand, the demand for fluoroantimonic acid and related superacid technologies is likely to increase, creating opportunities for innovation and market expansion across multiple industries.
Current Challenges in Fluoroantimonic Acid Research
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in research and application due to its extreme reactivity and corrosive nature. One of the primary obstacles is the development of suitable containment materials that can withstand its highly acidic properties. Traditional laboratory glassware and most metals are rapidly degraded by fluoroantimonic acid, necessitating the use of specialized materials such as Teflon or certain fluoropolymers.
The handling and storage of fluoroantimonic acid pose considerable safety risks, requiring stringent protocols and specialized equipment. Researchers must navigate the complexities of working with a substance that reacts violently with water and many organic compounds, limiting the range of potential applications and experimental conditions.
Another significant challenge lies in the precise control and measurement of fluoroantimonic acid's acidity. Its extreme Hammett acidity function (H0) value, estimated to be around -31.3, surpasses the capabilities of conventional pH measurement techniques. This makes it difficult to accurately quantify and standardize experimental conditions, potentially affecting the reproducibility of research results.
The synthesis and purification of fluoroantimonic acid also present technical hurdles. The acid is typically prepared by combining hydrogen fluoride and antimony pentafluoride, both of which are highly dangerous substances. Ensuring the purity and consistency of the final product while maintaining safety standards is a complex task that requires specialized equipment and expertise.
Furthermore, the environmental impact and disposal of fluoroantimonic acid and its byproducts pose significant challenges. The acid's extreme reactivity and potential for generating toxic fluorine compounds necessitate careful waste management strategies and decontamination procedures, which can be both costly and technically demanding.
In terms of practical applications, the integration of fluoroantimonic acid into industrial processes faces numerous obstacles. Its corrosive nature limits its use in conventional chemical engineering setups, requiring the development of novel reactor designs and process technologies that can harness its unique properties while mitigating its destructive effects.
Lastly, the regulatory landscape surrounding the use of fluoroantimonic acid in research and industry presents additional challenges. Stringent safety regulations and environmental concerns often restrict its availability and application, potentially hindering innovation in fields where its unique properties could be beneficial.
The handling and storage of fluoroantimonic acid pose considerable safety risks, requiring stringent protocols and specialized equipment. Researchers must navigate the complexities of working with a substance that reacts violently with water and many organic compounds, limiting the range of potential applications and experimental conditions.
Another significant challenge lies in the precise control and measurement of fluoroantimonic acid's acidity. Its extreme Hammett acidity function (H0) value, estimated to be around -31.3, surpasses the capabilities of conventional pH measurement techniques. This makes it difficult to accurately quantify and standardize experimental conditions, potentially affecting the reproducibility of research results.
The synthesis and purification of fluoroantimonic acid also present technical hurdles. The acid is typically prepared by combining hydrogen fluoride and antimony pentafluoride, both of which are highly dangerous substances. Ensuring the purity and consistency of the final product while maintaining safety standards is a complex task that requires specialized equipment and expertise.
Furthermore, the environmental impact and disposal of fluoroantimonic acid and its byproducts pose significant challenges. The acid's extreme reactivity and potential for generating toxic fluorine compounds necessitate careful waste management strategies and decontamination procedures, which can be both costly and technically demanding.
In terms of practical applications, the integration of fluoroantimonic acid into industrial processes faces numerous obstacles. Its corrosive nature limits its use in conventional chemical engineering setups, requiring the development of novel reactor designs and process technologies that can harness its unique properties while mitigating its destructive effects.
Lastly, the regulatory landscape surrounding the use of fluoroantimonic acid in research and industry presents additional challenges. Stringent safety regulations and environmental concerns often restrict its availability and application, potentially hindering innovation in fields where its unique properties could be beneficial.
Existing Applications of Fluoroantimonic Acid
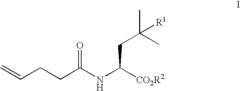
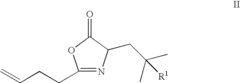
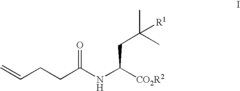
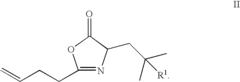
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Specialized equipment and safety measures are required due to the extreme reactivity of the acid.- Synthesis and preparation methods: Fluoroantimonic acid is a superacid that can be synthesized through various methods. These methods often involve the combination of antimony pentafluoride and hydrogen fluoride under specific conditions. The synthesis process may require careful handling due to the highly corrosive and reactive nature of the components.
- Applications in catalysis: Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions. Its strong acidity makes it effective for promoting reactions such as isomerization, alkylation, and polymerization. It is particularly useful in the petrochemical industry for processes involving hydrocarbons.
- Use in material science and surface treatments: The superacidic properties of fluoroantimonic acid make it valuable in material science applications. It can be used for surface treatments of metals and other materials, enhancing their properties or preparing them for further processing. This includes applications in etching, cleaning, and modifying surface characteristics.
- Safety and handling considerations: Due to its extremely corrosive and reactive nature, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of appropriate containment materials, personal protective equipment, and controlled environments. Proper disposal methods are crucial to prevent environmental contamination.
- Analytical and research applications: Fluoroantimonic acid finds use in various analytical and research applications. Its unique properties make it valuable in spectroscopy, as a reagent in chemical analysis, and in studying superacid chemistry. It can also be used to generate other strong acids or reactive species for research purposes.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid serves as a powerful superacid catalyst in various organic synthesis reactions. It is particularly useful in alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science for surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials such as polymers or ceramics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and strict protocols for handling and disposal. Proper training and safety precautions are essential for working with this superacid.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques are employed to study fluoroantimonic acid and its reactions. These may include spectroscopic methods, electrochemical analysis, and specialized titration procedures. Understanding the behavior and properties of this superacid is crucial for its effective use in research and industrial applications.Expand Specific Solutions
Key Players in Superacid Industry
The chemical innovation landscape driven by fluoroantimonic acid is in a nascent stage, with significant potential for growth. The market size is relatively small but expanding as researchers explore novel applications. The technology's maturity varies across sectors, with some companies leading the way. 3M Innovative Properties Co. and DuPont de Nemours, Inc. are at the forefront, leveraging their extensive R&D capabilities. Emerging players like Zhejiang Shangyu Lixing Chemical Co., Ltd and Fujian Yongjing Technology Co. Ltd. are making strides in developing practical applications. Academic institutions such as Okayama University and Hunan University are contributing to fundamental research, while industrial giants like Toyota Motor Corp. and Mitsubishi Materials Corp. are exploring potential uses in their respective fields.
3M Innovative Properties Co.
Technical Solution: 3M has developed a novel approach to utilizing fluoroantimonic acid in chemical synthesis. Their method involves encapsulating the superacid within specially designed fluoropolymer matrices, allowing for controlled release and reaction in targeted applications. This encapsulation technique not only enhances safety in handling but also enables precise dosing of the superacid catalyst. 3M's process incorporates a proprietary stabilization system that extends the shelf life of the encapsulated acid, making it more practical for industrial use. The company has also developed specialized reaction vessels lined with acid-resistant materials to further improve the handling and application of fluoroantimonic acid in various chemical processes.
Strengths: Enhanced safety, precise control, and extended shelf life. Weaknesses: Potentially higher production costs and limited to specific applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a groundbreaking approach to harnessing fluoroantimonic acid for chemical innovation. Their technology involves creating a stabilized form of the superacid using proprietary ionic liquids as solvents. This innovation allows for the acid to be used in a wider range of temperatures and pressures, significantly expanding its application potential. DuPont's method also incorporates a novel recycling system that captures and purifies the acid after use, reducing waste and improving cost-effectiveness. Additionally, they have developed specialized catalytic systems that work synergistically with fluoroantimonic acid, enabling highly selective chemical transformations that were previously challenging or impossible.
Strengths: Expanded application range, improved sustainability through recycling, and enhanced selectivity in chemical reactions. Weaknesses: Complex system requiring specialized equipment and expertise.
Breakthrough Innovations in Superacid Chemistry
P-doped surface coatings and process of preparation thereof
PatentActiveUS12122942B2
Innovation
- The development of a phosphorus-doped titania (P-doped) antimicrobial coating solution is achieved by mixing a chelating agent with titanium alkoxide and orthophosphoric acid, followed by the addition of an aqueous solution, which shifts the bandgap energy into the visible spectrum, enhances doping efficiency, and maintains the anatase phase at higher temperatures, thereby enabling activation under both UV and visible light.
Process for making fluoroleucine ethyl esters
PatentInactiveUS7238503B2
Innovation
- The process involves a fed batch reactor with a reduced enzyme to substrate ratio and increased temperature, combined with a plug flow column reactor that minimizes enzyme deactivation and uses a substrate charging strategy to enhance yield and enantiomeric excess, employing enzymes like Candida antarctica lipase B in organic solvents like MTBE.
Safety and Handling Protocols
Fluoroantimonic acid, known as the world's strongest superacid, requires exceptionally stringent safety and handling protocols due to its extreme corrosiveness and reactivity. All personnel working with this compound must undergo comprehensive training and adhere to strict guidelines to mitigate risks.
Personal protective equipment (PPE) is paramount when handling fluoroantimonic acid. This includes wearing a fully encapsulated chemical suit, multiple layers of acid-resistant gloves, and a self-contained breathing apparatus. The outer layer of PPE should be disposable to prevent contamination spread. Regular inspection and replacement of PPE are crucial to maintain its integrity.
Specialized containment systems are essential for storing and transporting fluoroantimonic acid. These systems must be constructed from materials highly resistant to corrosion, such as PTFE (Teflon) or certain fluoropolymers. Double containment is recommended to provide an additional layer of protection against leaks or spills. All storage and handling areas must be equipped with proper ventilation systems to prevent the accumulation of toxic fumes.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. A detailed evacuation plan should be in place, and all personnel must be trained in its execution. Additionally, neutralizing agents like calcium carbonate or sodium bicarbonate should be available in large quantities to address potential spills.
Proper waste disposal is critical when working with fluoroantimonic acid. All waste materials, including contaminated PPE and neutralized spills, must be treated as hazardous waste and disposed of according to strict regulatory guidelines. This often involves specialized waste management services equipped to handle extremely hazardous materials.
Continuous monitoring of the work environment is essential. This includes regular air quality checks, leak detection systems, and periodic inspections of all containment vessels and handling equipment. Implementation of a robust maintenance schedule for all equipment and facilities used in conjunction with fluoroantimonic acid is crucial to prevent degradation and potential failures.
Documentation and record-keeping play a vital role in safety protocols. Detailed logs of all handling procedures, safety checks, and incident reports must be maintained. Regular safety audits should be conducted to ensure compliance with established protocols and to identify areas for improvement in handling procedures.
Personal protective equipment (PPE) is paramount when handling fluoroantimonic acid. This includes wearing a fully encapsulated chemical suit, multiple layers of acid-resistant gloves, and a self-contained breathing apparatus. The outer layer of PPE should be disposable to prevent contamination spread. Regular inspection and replacement of PPE are crucial to maintain its integrity.
Specialized containment systems are essential for storing and transporting fluoroantimonic acid. These systems must be constructed from materials highly resistant to corrosion, such as PTFE (Teflon) or certain fluoropolymers. Double containment is recommended to provide an additional layer of protection against leaks or spills. All storage and handling areas must be equipped with proper ventilation systems to prevent the accumulation of toxic fumes.
Emergency response procedures must be well-established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. A detailed evacuation plan should be in place, and all personnel must be trained in its execution. Additionally, neutralizing agents like calcium carbonate or sodium bicarbonate should be available in large quantities to address potential spills.
Proper waste disposal is critical when working with fluoroantimonic acid. All waste materials, including contaminated PPE and neutralized spills, must be treated as hazardous waste and disposed of according to strict regulatory guidelines. This often involves specialized waste management services equipped to handle extremely hazardous materials.
Continuous monitoring of the work environment is essential. This includes regular air quality checks, leak detection systems, and periodic inspections of all containment vessels and handling equipment. Implementation of a robust maintenance schedule for all equipment and facilities used in conjunction with fluoroantimonic acid is crucial to prevent degradation and potential failures.
Documentation and record-keeping play a vital role in safety protocols. Detailed logs of all handling procedures, safety checks, and incident reports must be maintained. Regular safety audits should be conducted to ensure compliance with established protocols and to identify areas for improvement in handling procedures.
Environmental Impact Assessment
The environmental impact assessment of fluoroantimonic acid in chemical innovation is a critical consideration due to its highly corrosive and reactive nature. This superacid, composed of a mixture of hydrogen fluoride and antimony pentafluoride, poses significant risks to both human health and the environment if not properly managed.
In aquatic ecosystems, even small releases of fluoroantimonic acid can cause severe pH changes, leading to the destruction of aquatic life and long-term ecological imbalances. The acid's fluoride component can accumulate in water bodies, potentially affecting fish populations and other organisms through bioaccumulation. Soil contamination is another major concern, as the acid can alter soil chemistry, rendering it unsuitable for plant growth and potentially leaching into groundwater systems.
Air pollution is a significant risk during the production and use of fluoroantimonic acid. The release of hydrogen fluoride and antimony compounds can contribute to the formation of acid rain and particulate matter, impacting air quality over wide areas. These emissions can lead to respiratory issues in humans and animals, as well as damage to vegetation and infrastructure.
The production process of fluoroantimonic acid also raises environmental concerns. The extraction and processing of antimony and fluorine compounds often involve energy-intensive methods and can result in the generation of hazardous waste. Proper disposal and treatment of these byproducts are essential to prevent environmental contamination.
To mitigate these environmental risks, stringent safety protocols and containment measures are necessary. Closed-loop systems, advanced air filtration technologies, and specialized waste treatment facilities are crucial in minimizing the environmental footprint of fluoroantimonic acid use. Additionally, the development of less hazardous alternatives or the implementation of green chemistry principles in processes involving this superacid should be prioritized.
Regulatory compliance is another key aspect of environmental management for fluoroantimonic acid. Adherence to strict environmental regulations, including proper storage, handling, and disposal guidelines, is essential. Regular environmental monitoring and impact assessments should be conducted to ensure early detection of any potential contamination or ecological changes.
In conclusion, while fluoroantimonic acid offers significant potential for chemical innovation, its environmental impact must be carefully managed. Balancing the benefits of its use with the need for environmental protection requires ongoing research, technological advancements in safety measures, and a commitment to sustainable practices in the chemical industry.
In aquatic ecosystems, even small releases of fluoroantimonic acid can cause severe pH changes, leading to the destruction of aquatic life and long-term ecological imbalances. The acid's fluoride component can accumulate in water bodies, potentially affecting fish populations and other organisms through bioaccumulation. Soil contamination is another major concern, as the acid can alter soil chemistry, rendering it unsuitable for plant growth and potentially leaching into groundwater systems.
Air pollution is a significant risk during the production and use of fluoroantimonic acid. The release of hydrogen fluoride and antimony compounds can contribute to the formation of acid rain and particulate matter, impacting air quality over wide areas. These emissions can lead to respiratory issues in humans and animals, as well as damage to vegetation and infrastructure.
The production process of fluoroantimonic acid also raises environmental concerns. The extraction and processing of antimony and fluorine compounds often involve energy-intensive methods and can result in the generation of hazardous waste. Proper disposal and treatment of these byproducts are essential to prevent environmental contamination.
To mitigate these environmental risks, stringent safety protocols and containment measures are necessary. Closed-loop systems, advanced air filtration technologies, and specialized waste treatment facilities are crucial in minimizing the environmental footprint of fluoroantimonic acid use. Additionally, the development of less hazardous alternatives or the implementation of green chemistry principles in processes involving this superacid should be prioritized.
Regulatory compliance is another key aspect of environmental management for fluoroantimonic acid. Adherence to strict environmental regulations, including proper storage, handling, and disposal guidelines, is essential. Regular environmental monitoring and impact assessments should be conducted to ensure early detection of any potential contamination or ecological changes.
In conclusion, while fluoroantimonic acid offers significant potential for chemical innovation, its environmental impact must be carefully managed. Balancing the benefits of its use with the need for environmental protection requires ongoing research, technological advancements in safety measures, and a commitment to sustainable practices in the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!