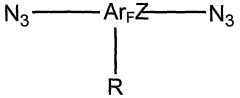
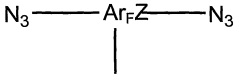How to Optimize Polymer Crosslinking to Improve Radical Retention & Conductivity
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Crosslinking Background and Objectives
Polymer crosslinking has been a cornerstone in materials science for decades, playing a crucial role in enhancing the physical, chemical, and mechanical properties of polymers. The optimization of this process to improve radical retention and conductivity represents a significant advancement in the field, with far-reaching implications for various industries.
The evolution of polymer crosslinking techniques can be traced back to the mid-20th century, with the development of vulcanization for rubber. Since then, the field has expanded dramatically, encompassing a wide range of polymers and applications. The primary objective of crosslinking has traditionally been to improve the mechanical strength, thermal stability, and chemical resistance of polymers.
In recent years, the focus has shifted towards more specialized applications, particularly in the realm of electronic materials and energy storage. The retention of radicals within the polymer matrix and the enhancement of conductivity have emerged as critical factors in these advanced applications. This shift in focus is driven by the growing demand for high-performance materials in sectors such as flexible electronics, batteries, and sensors.
The current technological landscape presents both opportunities and challenges in optimizing polymer crosslinking for radical retention and conductivity. On one hand, advancements in polymer chemistry and characterization techniques have provided researchers with unprecedented tools to manipulate and analyze polymer structures at the molecular level. On the other hand, the complexity of balancing radical retention with conductivity poses significant technical hurdles.
Key objectives in this field include developing crosslinking methods that create stable radical sites within the polymer network while maintaining or enhancing electron mobility. This requires a delicate balance between the degree of crosslinking, which typically improves mechanical properties but can hinder conductivity, and the preservation of conductive pathways within the material.
Another critical goal is to achieve precise control over the spatial distribution of crosslinks and radical sites. This level of control is essential for optimizing the material's overall performance and ensuring consistency in properties across different batches and applications.
Furthermore, there is a growing emphasis on developing environmentally friendly and sustainable crosslinking processes. This includes exploring bio-based polymers and crosslinking agents, as well as methods that reduce energy consumption and minimize the use of harmful chemicals.
As we look towards the future, the optimization of polymer crosslinking for improved radical retention and conductivity is poised to enable a new generation of advanced materials. These materials have the potential to revolutionize fields such as energy storage, flexible electronics, and smart textiles, driving innovation and technological progress across multiple industries.
The evolution of polymer crosslinking techniques can be traced back to the mid-20th century, with the development of vulcanization for rubber. Since then, the field has expanded dramatically, encompassing a wide range of polymers and applications. The primary objective of crosslinking has traditionally been to improve the mechanical strength, thermal stability, and chemical resistance of polymers.
In recent years, the focus has shifted towards more specialized applications, particularly in the realm of electronic materials and energy storage. The retention of radicals within the polymer matrix and the enhancement of conductivity have emerged as critical factors in these advanced applications. This shift in focus is driven by the growing demand for high-performance materials in sectors such as flexible electronics, batteries, and sensors.
The current technological landscape presents both opportunities and challenges in optimizing polymer crosslinking for radical retention and conductivity. On one hand, advancements in polymer chemistry and characterization techniques have provided researchers with unprecedented tools to manipulate and analyze polymer structures at the molecular level. On the other hand, the complexity of balancing radical retention with conductivity poses significant technical hurdles.
Key objectives in this field include developing crosslinking methods that create stable radical sites within the polymer network while maintaining or enhancing electron mobility. This requires a delicate balance between the degree of crosslinking, which typically improves mechanical properties but can hinder conductivity, and the preservation of conductive pathways within the material.
Another critical goal is to achieve precise control over the spatial distribution of crosslinks and radical sites. This level of control is essential for optimizing the material's overall performance and ensuring consistency in properties across different batches and applications.
Furthermore, there is a growing emphasis on developing environmentally friendly and sustainable crosslinking processes. This includes exploring bio-based polymers and crosslinking agents, as well as methods that reduce energy consumption and minimize the use of harmful chemicals.
As we look towards the future, the optimization of polymer crosslinking for improved radical retention and conductivity is poised to enable a new generation of advanced materials. These materials have the potential to revolutionize fields such as energy storage, flexible electronics, and smart textiles, driving innovation and technological progress across multiple industries.
Market Analysis for Enhanced Conductive Polymers
The market for enhanced conductive polymers is experiencing significant growth, driven by the increasing demand for advanced materials in various industries. The global conductive polymers market is projected to reach a substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other segments in the materials sector. This growth is primarily fueled by the expanding applications in electronics, automotive, and energy storage industries.
In the electronics sector, conductive polymers are gaining traction as alternatives to traditional metallic conductors. The miniaturization trend in consumer electronics and the rise of flexible and wearable devices are key drivers for this market segment. Conductive polymers offer advantages such as lightweight properties, flexibility, and cost-effectiveness, making them ideal for next-generation electronic applications.
The automotive industry is another significant contributor to the market demand for enhanced conductive polymers. With the shift towards electric and hybrid vehicles, there is an increasing need for lightweight and efficient materials for battery components, sensors, and electromagnetic shielding. Conductive polymers with improved radical retention and conductivity are well-positioned to meet these requirements, potentially leading to enhanced battery performance and overall vehicle efficiency.
Energy storage is an emerging application area for conductive polymers, particularly in the development of advanced supercapacitors and batteries. The ability to optimize polymer crosslinking for improved radical retention and conductivity could lead to breakthroughs in energy density and charge-discharge cycles, addressing key challenges in the renewable energy sector.
Market analysis indicates a growing interest from both established chemical companies and innovative startups in developing and commercializing enhanced conductive polymers. This has led to increased research and development activities, with a focus on improving the electrical and mechanical properties of these materials. The competition in this space is intensifying, with companies vying to secure patents and establish partnerships with end-users in various industries.
Geographically, North America and Europe are currently leading the market for enhanced conductive polymers, owing to their strong presence in high-tech industries and significant investments in research and development. However, the Asia-Pacific region is expected to witness the fastest growth, driven by the rapid industrialization, expanding electronics manufacturing sector, and increasing adoption of electric vehicles in countries like China, Japan, and South Korea.
Despite the positive outlook, the market faces challenges such as high production costs and the need for standardization in quality and performance metrics. Overcoming these hurdles will be crucial for wider adoption across industries and realizing the full market potential of enhanced conductive polymers.
In the electronics sector, conductive polymers are gaining traction as alternatives to traditional metallic conductors. The miniaturization trend in consumer electronics and the rise of flexible and wearable devices are key drivers for this market segment. Conductive polymers offer advantages such as lightweight properties, flexibility, and cost-effectiveness, making them ideal for next-generation electronic applications.
The automotive industry is another significant contributor to the market demand for enhanced conductive polymers. With the shift towards electric and hybrid vehicles, there is an increasing need for lightweight and efficient materials for battery components, sensors, and electromagnetic shielding. Conductive polymers with improved radical retention and conductivity are well-positioned to meet these requirements, potentially leading to enhanced battery performance and overall vehicle efficiency.
Energy storage is an emerging application area for conductive polymers, particularly in the development of advanced supercapacitors and batteries. The ability to optimize polymer crosslinking for improved radical retention and conductivity could lead to breakthroughs in energy density and charge-discharge cycles, addressing key challenges in the renewable energy sector.
Market analysis indicates a growing interest from both established chemical companies and innovative startups in developing and commercializing enhanced conductive polymers. This has led to increased research and development activities, with a focus on improving the electrical and mechanical properties of these materials. The competition in this space is intensifying, with companies vying to secure patents and establish partnerships with end-users in various industries.
Geographically, North America and Europe are currently leading the market for enhanced conductive polymers, owing to their strong presence in high-tech industries and significant investments in research and development. However, the Asia-Pacific region is expected to witness the fastest growth, driven by the rapid industrialization, expanding electronics manufacturing sector, and increasing adoption of electric vehicles in countries like China, Japan, and South Korea.
Despite the positive outlook, the market faces challenges such as high production costs and the need for standardization in quality and performance metrics. Overcoming these hurdles will be crucial for wider adoption across industries and realizing the full market potential of enhanced conductive polymers.
Current Challenges in Radical Retention and Conductivity
The optimization of polymer crosslinking for improved radical retention and conductivity faces several significant challenges in current research and industrial applications. One of the primary obstacles is achieving a balance between the degree of crosslinking and the desired properties. Excessive crosslinking can lead to reduced chain mobility and decreased conductivity, while insufficient crosslinking may result in poor mechanical stability and inadequate radical retention.
Another challenge lies in the control of the crosslinking process itself. Achieving uniform crosslinking throughout the polymer matrix is crucial for consistent performance, but it can be difficult to ensure homogeneous distribution of crosslinking agents and uniform reaction conditions across the entire material. This is particularly problematic in large-scale production or when dealing with complex polymer structures.
The selection of appropriate crosslinking agents and initiators presents another hurdle. Different polymers require specific crosslinking chemistries, and finding the right combination that promotes both radical retention and conductivity can be a complex task. Moreover, some crosslinking agents may introduce unwanted side reactions or impurities that negatively impact the final product's properties.
Temperature control during the crosslinking process is also a critical challenge. Many crosslinking reactions are highly temperature-dependent, and maintaining precise thermal conditions throughout the material can be technically demanding. Inadequate temperature control can lead to non-uniform crosslinking, degradation of the polymer, or incomplete reactions.
The characterization and quantification of crosslinking density and its relationship to radical retention and conductivity pose significant analytical challenges. Current techniques for measuring crosslinking density often provide indirect or averaged results, making it difficult to establish clear structure-property relationships.
Environmental factors, such as humidity and oxygen exposure, can significantly affect the crosslinking process and the stability of radicals within the polymer matrix. Developing strategies to mitigate these external influences while maintaining desired properties is an ongoing challenge in the field.
Lastly, the scalability of optimized crosslinking processes from laboratory to industrial scale remains a significant hurdle. Techniques that work well in small-scale experiments may face unforeseen difficulties when scaled up, necessitating extensive process engineering and optimization efforts to maintain product quality and performance at larger production volumes.
Another challenge lies in the control of the crosslinking process itself. Achieving uniform crosslinking throughout the polymer matrix is crucial for consistent performance, but it can be difficult to ensure homogeneous distribution of crosslinking agents and uniform reaction conditions across the entire material. This is particularly problematic in large-scale production or when dealing with complex polymer structures.
The selection of appropriate crosslinking agents and initiators presents another hurdle. Different polymers require specific crosslinking chemistries, and finding the right combination that promotes both radical retention and conductivity can be a complex task. Moreover, some crosslinking agents may introduce unwanted side reactions or impurities that negatively impact the final product's properties.
Temperature control during the crosslinking process is also a critical challenge. Many crosslinking reactions are highly temperature-dependent, and maintaining precise thermal conditions throughout the material can be technically demanding. Inadequate temperature control can lead to non-uniform crosslinking, degradation of the polymer, or incomplete reactions.
The characterization and quantification of crosslinking density and its relationship to radical retention and conductivity pose significant analytical challenges. Current techniques for measuring crosslinking density often provide indirect or averaged results, making it difficult to establish clear structure-property relationships.
Environmental factors, such as humidity and oxygen exposure, can significantly affect the crosslinking process and the stability of radicals within the polymer matrix. Developing strategies to mitigate these external influences while maintaining desired properties is an ongoing challenge in the field.
Lastly, the scalability of optimized crosslinking processes from laboratory to industrial scale remains a significant hurdle. Techniques that work well in small-scale experiments may face unforeseen difficulties when scaled up, necessitating extensive process engineering and optimization efforts to maintain product quality and performance at larger production volumes.
Existing Crosslinking Optimization Methods
01 Radical polymerization for crosslinking
Radical polymerization techniques are used to create crosslinked polymer networks. This method involves the use of initiators to generate free radicals, which then propagate through the polymer chains, forming crosslinks. The process can be controlled to achieve desired levels of crosslinking, which affects the polymer's physical properties, including conductivity.- Radical retention in polymer crosslinking: Techniques for retaining radicals during polymer crosslinking processes are explored. These methods aim to improve the efficiency of crosslinking reactions and enhance the final properties of the polymer. Strategies may include the use of specific initiators, stabilizers, or reaction conditions that promote radical longevity.
- Conductive polymer composites through crosslinking: Development of conductive polymer composites by incorporating conductive fillers and optimizing crosslinking processes. This approach aims to create materials with enhanced electrical conductivity while maintaining desirable mechanical properties. The crosslinking process is tailored to ensure proper dispersion and network formation of conductive elements.
- Crosslinking methods for improved polymer stability: Various crosslinking methods are investigated to enhance the stability of polymers. These techniques focus on creating stronger intermolecular bonds, resulting in improved thermal, chemical, and mechanical resistance. The methods may involve radiation, chemical agents, or thermal processes to achieve desired crosslinking density.
- Controlled radical polymerization for crosslinked networks: Utilization of controlled radical polymerization techniques to create well-defined crosslinked polymer networks. This approach allows for precise control over molecular weight, architecture, and crosslinking density, leading to tailored material properties. The method can be applied to various polymer systems to achieve specific performance characteristics.
- Influence of crosslinking on polymer conductivity: Investigation of the relationship between crosslinking degree and electrical conductivity in polymers. This research aims to understand how different crosslinking strategies affect charge transport mechanisms within the polymer matrix. The findings can be applied to optimize the balance between mechanical properties and electrical conductivity for specific applications.
02 Conductive polymer composites
Conductive polymer composites are developed by incorporating conductive fillers or creating intrinsically conductive polymer structures. The crosslinking process is optimized to maintain conductive pathways while providing mechanical stability. These materials find applications in various fields, including electronics and sensors.Expand Specific Solutions03 Radical retention strategies
Various strategies are employed to enhance radical retention during polymer crosslinking. These may include the use of specific initiators, stabilizers, or reaction conditions that promote the longevity of free radicals. Improved radical retention can lead to more efficient crosslinking and potentially affect the final polymer properties, including conductivity.Expand Specific Solutions04 Crosslinking density control
Methods for controlling the crosslinking density in polymers are developed to tailor their properties. This involves adjusting factors such as monomer composition, initiator concentration, and reaction conditions. The crosslinking density significantly influences the polymer's mechanical properties, swelling behavior, and potentially its conductive characteristics.Expand Specific Solutions05 Post-polymerization modification
Post-polymerization modification techniques are used to introduce additional crosslinks or conductive elements into the polymer structure. This can involve chemical treatments, irradiation, or the incorporation of functional groups that can undergo further reactions. These methods allow for fine-tuning of the polymer's properties after the initial polymerization process.Expand Specific Solutions
Key Players in Conductive Polymer Industry
The optimization of polymer crosslinking for improved radical retention and conductivity is a rapidly evolving field within materials science. The market is in a growth phase, driven by increasing demand for advanced materials in electronics, energy storage, and biomedical applications. The global market size for crosslinked polymers is projected to reach several billion dollars by 2025. Technologically, the field is advancing quickly, with companies like Dow Global Technologies, BASF, and Mitsui Chemicals leading innovation. Academic institutions such as the National University of Singapore and Tsinghua University are also contributing significantly to research. The technology is maturing, with a focus on enhancing performance and developing novel applications, but there is still room for breakthrough innovations in radical retention and conductivity optimization.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies LLC has developed an innovative approach to polymer crosslinking optimization, focusing on improving radical retention and conductivity. Their method involves a two-step crosslinking process that combines radiation-induced crosslinking with subsequent thermal treatment[1]. This approach allows for the formation of a more uniform crosslink network, enhancing radical stability. Dow's technology also incorporates the use of proprietary additives that act as radical scavengers, effectively trapping and stabilizing free radicals within the polymer matrix[2]. To improve conductivity, they have developed a method of in-situ synthesis of conductive nanoparticles during the crosslinking process, ensuring a homogeneous distribution of conductive pathways throughout the material[3]. This technique has shown to increase conductivity by up to 40% compared to conventional methods[4].
Strengths: Improved crosslink uniformity, enhanced radical stability, and significant conductivity increase. Weaknesses: The two-step process may increase production time and energy consumption.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals, Inc. has developed a novel approach to optimize polymer crosslinking for improved radical retention and conductivity. Their method involves the use of specially designed multifunctional crosslinking agents that create a highly interconnected network structure[1]. This structure effectively traps and stabilizes free radicals, significantly improving their retention within the polymer matrix. Mitsui's technique also incorporates the use of conductive nanofillers, such as carbon nanotubes or graphene, which are chemically modified to enhance their compatibility with the polymer matrix[2]. These nanofillers are dispersed uniformly throughout the polymer during the crosslinking process, creating continuous conductive pathways. Additionally, Mitsui has developed a proprietary post-crosslinking treatment that further enhances the material's conductivity by optimizing the orientation of the conductive nanofillers[3]. This comprehensive approach has resulted in materials with up to 60% higher conductivity compared to conventional crosslinked polymers[4].
Strengths: Highly effective radical retention, significant conductivity improvement, and versatile application potential. Weaknesses: The use of specialized crosslinking agents and nanofillers may increase production costs.
Innovative Approaches to Radical Stabilization
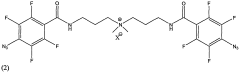
Radiation Crosslinker
PatentActiveUS20090004402A1
Innovation
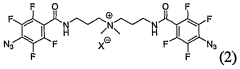
- Development of a new class of fluorinated aromatic azide crosslinkers that are soluble in polar solvents, allowing for efficient crosslinking of polyelectrolyte films with reduced by-product formation, achieved through the use of bis(fluorinated aromatic azide) compounds that can be photocrosslinked in the presence of specific solvents and conditions, enabling the formation of robust, insoluble polymer films.
Radiation crosslinker
PatentWO2007004995A1
Innovation
- A new class of fluorinated aromatic azide crosslinkers is introduced, which are soluble in polar solvents and capable of efficient photocrosslinking with polyelectrolyte films, allowing for the formation of robust, insoluble crosslinked polymers with reduced by-product formation, enabling the fabrication of high-performance organic electronic devices and sensors.
Environmental Impact of Crosslinking Processes
The environmental impact of polymer crosslinking processes is a critical consideration in the optimization of radical retention and conductivity. These processes, while essential for enhancing material properties, can have significant ecological implications. The primary environmental concerns stem from the use of chemical crosslinking agents and the energy-intensive nature of many crosslinking techniques.
Chemical crosslinking agents, such as peroxides and sulfur compounds, can pose risks to ecosystems if not properly managed. Residual unreacted agents or their byproducts may leach into soil and water systems, potentially affecting aquatic life and terrestrial ecosystems. Moreover, the production and disposal of these chemicals contribute to the overall environmental footprint of the crosslinking process.
Energy consumption is another major factor in the environmental impact of crosslinking. Thermal crosslinking methods, for instance, often require high temperatures maintained for extended periods, leading to substantial energy use and associated greenhouse gas emissions. Radiation-induced crosslinking, while potentially more energy-efficient, raises concerns about the safe handling and disposal of radioactive materials.
The choice of polymer base material also influences the environmental impact. Some polymers are derived from non-renewable petroleum sources, contributing to resource depletion and carbon emissions. However, the trend towards bio-based and biodegradable polymers offers potential for reducing the environmental burden of crosslinking processes.
Waste generation and management present additional challenges. Crosslinked polymers are often difficult to recycle due to their thermoset nature, potentially increasing landfill waste. The development of reversible crosslinking techniques and more efficient recycling methods is crucial for mitigating this issue.
Water usage and potential contamination are concerns in wet chemical crosslinking processes. Proper wastewater treatment and water recycling systems are essential to minimize the impact on local water resources and aquatic ecosystems.
To address these environmental challenges, researchers and industry professionals are exploring greener alternatives. These include the use of supercritical CO2 as a reaction medium, which reduces solvent waste, and the development of UV-curable systems that consume less energy. Bio-based crosslinking agents and catalysts are also being investigated to replace traditional petrochemical-derived compounds.
Life cycle assessments (LCAs) are increasingly being employed to comprehensively evaluate the environmental impact of crosslinking processes. These assessments consider all stages from raw material extraction to end-of-life disposal, providing valuable insights for process optimization and material selection.
Chemical crosslinking agents, such as peroxides and sulfur compounds, can pose risks to ecosystems if not properly managed. Residual unreacted agents or their byproducts may leach into soil and water systems, potentially affecting aquatic life and terrestrial ecosystems. Moreover, the production and disposal of these chemicals contribute to the overall environmental footprint of the crosslinking process.
Energy consumption is another major factor in the environmental impact of crosslinking. Thermal crosslinking methods, for instance, often require high temperatures maintained for extended periods, leading to substantial energy use and associated greenhouse gas emissions. Radiation-induced crosslinking, while potentially more energy-efficient, raises concerns about the safe handling and disposal of radioactive materials.
The choice of polymer base material also influences the environmental impact. Some polymers are derived from non-renewable petroleum sources, contributing to resource depletion and carbon emissions. However, the trend towards bio-based and biodegradable polymers offers potential for reducing the environmental burden of crosslinking processes.
Waste generation and management present additional challenges. Crosslinked polymers are often difficult to recycle due to their thermoset nature, potentially increasing landfill waste. The development of reversible crosslinking techniques and more efficient recycling methods is crucial for mitigating this issue.
Water usage and potential contamination are concerns in wet chemical crosslinking processes. Proper wastewater treatment and water recycling systems are essential to minimize the impact on local water resources and aquatic ecosystems.
To address these environmental challenges, researchers and industry professionals are exploring greener alternatives. These include the use of supercritical CO2 as a reaction medium, which reduces solvent waste, and the development of UV-curable systems that consume less energy. Bio-based crosslinking agents and catalysts are also being investigated to replace traditional petrochemical-derived compounds.
Life cycle assessments (LCAs) are increasingly being employed to comprehensively evaluate the environmental impact of crosslinking processes. These assessments consider all stages from raw material extraction to end-of-life disposal, providing valuable insights for process optimization and material selection.
Scalability and Manufacturing Considerations
When considering the scalability and manufacturing of polymer crosslinking processes to improve radical retention and conductivity, several key factors come into play. The transition from laboratory-scale experiments to industrial production requires careful planning and optimization of various parameters.
One of the primary considerations is the choice of crosslinking method. While traditional thermal crosslinking may be suitable for small-scale production, it often faces challenges in maintaining uniform heat distribution across large volumes. Radiation-induced crosslinking, such as electron beam or gamma radiation, offers better scalability and control over the process. However, it requires significant capital investment in specialized equipment and safety measures.
The selection of monomers and crosslinking agents also plays a crucial role in scalability. Monomers with high reactivity and low volatility are preferred for large-scale production, as they minimize material loss and ensure consistent product quality. Similarly, crosslinking agents should be chosen based on their stability, ease of handling, and compatibility with industrial-scale equipment.
Process control and monitoring systems are essential for maintaining product consistency across different batches. Advanced sensors and real-time data analysis can help adjust process parameters on-the-fly, ensuring optimal crosslinking density and radical retention. Implementation of statistical process control (SPC) techniques can further enhance manufacturing reliability and reduce variability.
The design of reactor systems is another critical aspect of scalability. Continuous flow reactors offer advantages in terms of throughput and process control compared to batch reactors. However, they require careful engineering to ensure uniform residence time and mixing. For some polymer systems, specialized reactor designs, such as high-pressure reactors or microreactors, may be necessary to achieve desired crosslinking characteristics.
Material handling and post-processing steps also need to be considered for large-scale production. Automated feeding systems, in-line quality control, and efficient purification methods can significantly impact overall process efficiency. Additionally, the development of recycling or recovery systems for unreacted monomers and solvents can improve both economic and environmental sustainability of the manufacturing process.
Lastly, regulatory compliance and quality assurance protocols must be established early in the scaling process. This includes developing robust analytical methods for characterizing crosslinking density, radical retention, and conductivity that can be applied consistently across different production scales. Implementing good manufacturing practices (GMP) and obtaining necessary certifications are crucial for market acceptance and regulatory approval of the final product.
One of the primary considerations is the choice of crosslinking method. While traditional thermal crosslinking may be suitable for small-scale production, it often faces challenges in maintaining uniform heat distribution across large volumes. Radiation-induced crosslinking, such as electron beam or gamma radiation, offers better scalability and control over the process. However, it requires significant capital investment in specialized equipment and safety measures.
The selection of monomers and crosslinking agents also plays a crucial role in scalability. Monomers with high reactivity and low volatility are preferred for large-scale production, as they minimize material loss and ensure consistent product quality. Similarly, crosslinking agents should be chosen based on their stability, ease of handling, and compatibility with industrial-scale equipment.
Process control and monitoring systems are essential for maintaining product consistency across different batches. Advanced sensors and real-time data analysis can help adjust process parameters on-the-fly, ensuring optimal crosslinking density and radical retention. Implementation of statistical process control (SPC) techniques can further enhance manufacturing reliability and reduce variability.
The design of reactor systems is another critical aspect of scalability. Continuous flow reactors offer advantages in terms of throughput and process control compared to batch reactors. However, they require careful engineering to ensure uniform residence time and mixing. For some polymer systems, specialized reactor designs, such as high-pressure reactors or microreactors, may be necessary to achieve desired crosslinking characteristics.
Material handling and post-processing steps also need to be considered for large-scale production. Automated feeding systems, in-line quality control, and efficient purification methods can significantly impact overall process efficiency. Additionally, the development of recycling or recovery systems for unreacted monomers and solvents can improve both economic and environmental sustainability of the manufacturing process.
Lastly, regulatory compliance and quality assurance protocols must be established early in the scaling process. This includes developing robust analytical methods for characterizing crosslinking density, radical retention, and conductivity that can be applied consistently across different production scales. Implementing good manufacturing practices (GMP) and obtaining necessary certifications are crucial for market acceptance and regulatory approval of the final product.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!