What Are Organic Radical Batteries: Chemistry, Metrics and Application Windows
AUG 21, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ORB Fundamentals
Organic Radical Batteries (ORBs) represent a novel class of energy storage devices that harness the unique properties of organic radical compounds. These batteries operate on the principle of reversible redox reactions involving stable organic radical species, typically nitroxide radicals. The fundamental chemistry of ORBs revolves around the ability of these organic molecules to undergo rapid and reversible electron transfer processes.
At the core of ORB technology is the use of organic radical polymers as active electrode materials. These polymers contain pendant radical groups, such as TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl), which can be oxidized or reduced without significant structural changes to the polymer backbone. This stability allows for high cycling performance and long battery life.
The electrochemical reactions in ORBs involve the reversible oxidation and reduction of the radical species. During discharge, the radical groups in the cathode are oxidized, accepting electrons, while the anode material is reduced. This process is reversed during charging, with electrons flowing back to regenerate the original radical state in the cathode.
One of the key advantages of ORBs is their fast charge-discharge kinetics. The radical sites in the organic polymers are highly accessible, allowing for rapid electron transfer and ion diffusion. This characteristic enables ORBs to achieve high power densities, making them suitable for applications requiring quick energy delivery or storage.
Another fundamental aspect of ORBs is their environmental friendliness. Unlike traditional lithium-ion batteries, which rely on metal-based cathodes, ORBs utilize organic materials that are often derived from renewable resources. This not only reduces the environmental impact of battery production but also addresses concerns about the scarcity of certain metals used in conventional batteries.
The electrolyte used in ORBs plays a crucial role in their performance. Typically, organic electrolytes or ionic liquids are employed to facilitate ion transport between the electrodes. The choice of electrolyte can significantly influence the battery's voltage window, cycling stability, and overall performance.
ORBs also offer flexibility in terms of design and fabrication. The organic nature of the active materials allows for solution processing techniques, enabling the production of thin, flexible batteries. This opens up possibilities for integration into wearable devices and other applications where form factor is critical.
At the core of ORB technology is the use of organic radical polymers as active electrode materials. These polymers contain pendant radical groups, such as TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl), which can be oxidized or reduced without significant structural changes to the polymer backbone. This stability allows for high cycling performance and long battery life.
The electrochemical reactions in ORBs involve the reversible oxidation and reduction of the radical species. During discharge, the radical groups in the cathode are oxidized, accepting electrons, while the anode material is reduced. This process is reversed during charging, with electrons flowing back to regenerate the original radical state in the cathode.
One of the key advantages of ORBs is their fast charge-discharge kinetics. The radical sites in the organic polymers are highly accessible, allowing for rapid electron transfer and ion diffusion. This characteristic enables ORBs to achieve high power densities, making them suitable for applications requiring quick energy delivery or storage.
Another fundamental aspect of ORBs is their environmental friendliness. Unlike traditional lithium-ion batteries, which rely on metal-based cathodes, ORBs utilize organic materials that are often derived from renewable resources. This not only reduces the environmental impact of battery production but also addresses concerns about the scarcity of certain metals used in conventional batteries.
The electrolyte used in ORBs plays a crucial role in their performance. Typically, organic electrolytes or ionic liquids are employed to facilitate ion transport between the electrodes. The choice of electrolyte can significantly influence the battery's voltage window, cycling stability, and overall performance.
ORBs also offer flexibility in terms of design and fabrication. The organic nature of the active materials allows for solution processing techniques, enabling the production of thin, flexible batteries. This opens up possibilities for integration into wearable devices and other applications where form factor is critical.
Market Potential
The market potential for organic radical batteries (ORBs) is rapidly expanding as the demand for sustainable and high-performance energy storage solutions grows. ORBs offer several advantages over traditional lithium-ion batteries, including faster charging rates, longer cycle life, and improved safety characteristics. These attributes position ORBs as a promising technology for various applications, particularly in the consumer electronics and electric vehicle sectors.
In the consumer electronics market, ORBs could revolutionize portable device performance. Their rapid charging capabilities align well with the increasing consumer demand for devices that can be quickly powered up. This feature is especially attractive for smartphones, tablets, and laptops, where minimizing downtime is crucial. The longer cycle life of ORBs also addresses the growing concern over electronic waste, potentially extending the usable lifespan of devices and reducing the frequency of battery replacements.
The electric vehicle (EV) industry represents another significant market opportunity for ORBs. As the automotive sector shifts towards electrification, there is a pressing need for batteries that can offer fast charging without compromising on safety or longevity. ORBs' ability to charge rapidly while maintaining stability could help alleviate range anxiety and reduce charging times at EV stations, potentially accelerating EV adoption rates.
Beyond consumer electronics and EVs, ORBs show promise in grid energy storage applications. The intermittent nature of renewable energy sources like solar and wind creates a need for efficient, large-scale energy storage solutions. ORBs' high power density and long cycle life make them suitable candidates for grid-level storage, potentially enhancing the stability and reliability of renewable energy systems.
The market for ORBs is also likely to benefit from the increasing focus on sustainability and environmental concerns. As governments worldwide implement stricter regulations on carbon emissions and promote green technologies, ORBs' eco-friendly profile could give them a competitive edge over traditional battery technologies. This regulatory environment may drive investment and accelerate the commercialization of ORB technology.
However, the market potential of ORBs is not without challenges. The technology is still in its early stages, and significant research and development efforts are required to optimize performance and scale up production. Additionally, the established infrastructure and market dominance of lithium-ion batteries present a barrier to entry that ORBs must overcome. Despite these challenges, the unique properties and potential applications of ORBs suggest a promising market trajectory as the technology matures and production costs decrease.
In the consumer electronics market, ORBs could revolutionize portable device performance. Their rapid charging capabilities align well with the increasing consumer demand for devices that can be quickly powered up. This feature is especially attractive for smartphones, tablets, and laptops, where minimizing downtime is crucial. The longer cycle life of ORBs also addresses the growing concern over electronic waste, potentially extending the usable lifespan of devices and reducing the frequency of battery replacements.
The electric vehicle (EV) industry represents another significant market opportunity for ORBs. As the automotive sector shifts towards electrification, there is a pressing need for batteries that can offer fast charging without compromising on safety or longevity. ORBs' ability to charge rapidly while maintaining stability could help alleviate range anxiety and reduce charging times at EV stations, potentially accelerating EV adoption rates.
Beyond consumer electronics and EVs, ORBs show promise in grid energy storage applications. The intermittent nature of renewable energy sources like solar and wind creates a need for efficient, large-scale energy storage solutions. ORBs' high power density and long cycle life make them suitable candidates for grid-level storage, potentially enhancing the stability and reliability of renewable energy systems.
The market for ORBs is also likely to benefit from the increasing focus on sustainability and environmental concerns. As governments worldwide implement stricter regulations on carbon emissions and promote green technologies, ORBs' eco-friendly profile could give them a competitive edge over traditional battery technologies. This regulatory environment may drive investment and accelerate the commercialization of ORB technology.
However, the market potential of ORBs is not without challenges. The technology is still in its early stages, and significant research and development efforts are required to optimize performance and scale up production. Additionally, the established infrastructure and market dominance of lithium-ion batteries present a barrier to entry that ORBs must overcome. Despite these challenges, the unique properties and potential applications of ORBs suggest a promising market trajectory as the technology matures and production costs decrease.
Technical Challenges
Organic radical batteries (ORBs) face several significant technical challenges that hinder their widespread adoption and commercialization. One of the primary issues is the stability of organic radical compounds. These materials tend to be highly reactive, which can lead to self-discharge and reduced cycle life. The radical species used in ORBs are prone to degradation through various mechanisms, including dimerization and side reactions with electrolytes or impurities.
Another major challenge is the relatively low energy density of ORBs compared to conventional lithium-ion batteries. This limitation stems from the inherent properties of organic materials, which typically have lower theoretical capacities than inorganic counterparts. The lower energy density restricts the application of ORBs in scenarios where high energy storage is crucial, such as electric vehicles or grid-scale energy storage.
The power density of ORBs also presents a technical hurdle. While some organic radical materials exhibit fast charge-discharge kinetics, others suffer from slow electron transfer rates, limiting their ability to deliver high power output. This challenge is particularly relevant for applications requiring rapid energy release or absorption.
Scalability and manufacturing processes pose additional technical difficulties. Many organic radical materials are synthesized through complex multi-step reactions, which can be challenging to scale up for industrial production. Moreover, the integration of these materials into practical battery designs while maintaining their electrochemical properties is not straightforward.
The electrolyte compatibility in ORBs is another area of concern. Finding suitable electrolytes that are stable with organic radical materials and do not participate in unwanted side reactions is crucial for long-term battery performance. Additionally, the electrolyte must facilitate efficient ion transport while preventing the dissolution of active materials.
Temperature sensitivity is a significant challenge for ORBs. Many organic radical compounds exhibit thermal instability, which can lead to capacity fading or safety issues at elevated temperatures. This sensitivity limits the operating temperature range of ORBs and necessitates careful thermal management strategies.
Lastly, the development of efficient and cost-effective methods for recycling ORBs at the end of their life cycle remains a technical challenge. Unlike conventional batteries with well-established recycling processes, the diverse nature of organic materials in ORBs complicates the recovery and reuse of active components.
Another major challenge is the relatively low energy density of ORBs compared to conventional lithium-ion batteries. This limitation stems from the inherent properties of organic materials, which typically have lower theoretical capacities than inorganic counterparts. The lower energy density restricts the application of ORBs in scenarios where high energy storage is crucial, such as electric vehicles or grid-scale energy storage.
The power density of ORBs also presents a technical hurdle. While some organic radical materials exhibit fast charge-discharge kinetics, others suffer from slow electron transfer rates, limiting their ability to deliver high power output. This challenge is particularly relevant for applications requiring rapid energy release or absorption.
Scalability and manufacturing processes pose additional technical difficulties. Many organic radical materials are synthesized through complex multi-step reactions, which can be challenging to scale up for industrial production. Moreover, the integration of these materials into practical battery designs while maintaining their electrochemical properties is not straightforward.
The electrolyte compatibility in ORBs is another area of concern. Finding suitable electrolytes that are stable with organic radical materials and do not participate in unwanted side reactions is crucial for long-term battery performance. Additionally, the electrolyte must facilitate efficient ion transport while preventing the dissolution of active materials.
Temperature sensitivity is a significant challenge for ORBs. Many organic radical compounds exhibit thermal instability, which can lead to capacity fading or safety issues at elevated temperatures. This sensitivity limits the operating temperature range of ORBs and necessitates careful thermal management strategies.
Lastly, the development of efficient and cost-effective methods for recycling ORBs at the end of their life cycle remains a technical challenge. Unlike conventional batteries with well-established recycling processes, the diverse nature of organic materials in ORBs complicates the recovery and reuse of active components.
Current ORB Solutions
01 Organic radical polymer electrodes
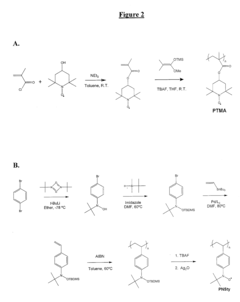
Organic radical polymers are used as electrode materials in batteries, offering high capacity and fast charge-discharge rates. These polymers contain stable radical groups that can undergo reversible redox reactions, enabling energy storage and release. The use of organic materials in electrodes can lead to more environmentally friendly and potentially lower-cost battery technologies.- Organic radical polymer electrodes: Organic radical polymers are used as electrode materials in batteries, offering high capacity and fast charge-discharge rates. These polymers contain stable radical groups that can undergo reversible redox reactions, making them suitable for energy storage applications. The use of organic materials also contributes to improved sustainability and potentially lower costs compared to traditional inorganic battery materials.
- Electrolyte composition for organic radical batteries: The electrolyte composition plays a crucial role in the performance of organic radical batteries. Researchers have developed specialized electrolytes that enhance the stability of organic radical species, improve ionic conductivity, and extend the cycle life of the battery. These electrolytes often include additives or solvents that are compatible with the organic radical materials and prevent unwanted side reactions.
- Performance metrics and characterization techniques: Various metrics and characterization techniques are used to evaluate the performance of organic radical batteries. These include measurements of energy density, power density, cycle life, and rate capability. Advanced analytical methods such as spectroscopy and electrochemical impedance spectroscopy are employed to study the behavior of organic radical materials during charge-discharge cycles and to optimize battery design.
- Cell design and fabrication methods: The design and fabrication of organic radical battery cells require specific considerations to maximize performance and stability. This includes optimizing electrode thickness, developing suitable current collectors, and creating effective sealing methods to prevent electrolyte leakage. Novel cell architectures, such as flexible or printable designs, are also being explored to expand the application range of organic radical batteries.
- Integration with energy management systems: Organic radical batteries are being integrated into advanced energy management systems for various applications. This involves developing control algorithms and monitoring systems that can optimize the performance and lifespan of the batteries. Research is also focused on creating hybrid systems that combine organic radical batteries with other energy storage technologies to leverage their unique characteristics and improve overall system efficiency.
02 Electrolyte composition for organic radical batteries
The electrolyte composition plays a crucial role in the performance of organic radical batteries. Researchers are developing novel electrolyte formulations that enhance ionic conductivity, improve the stability of organic radical materials, and extend the battery's cycle life. These electrolytes may include specific salts, solvents, or additives tailored for organic radical chemistry.Expand Specific Solutions03 Performance metrics and characterization techniques
Various metrics and characterization techniques are used to evaluate the performance of organic radical batteries. These include measurements of energy density, power density, cycling stability, and rate capability. Advanced analytical methods such as spectroscopy and electrochemical impedance spectroscopy are employed to study the behavior of organic radical materials during battery operation.Expand Specific Solutions04 Redox flow battery applications
Organic radical materials are being explored for use in redox flow batteries, which are suitable for large-scale energy storage. These batteries utilize dissolved organic radical species as active materials in the electrolyte, allowing for easy scalability and potentially lower costs compared to traditional inorganic-based flow batteries.Expand Specific Solutions05 Nanostructured organic radical materials
Researchers are developing nanostructured forms of organic radical materials to enhance battery performance. These nanostructures can increase the surface area of the active material, improve electron and ion transport, and enhance the overall electrochemical properties of the battery. Techniques such as nanoparticle synthesis and nanocomposite formation are being explored to optimize organic radical battery chemistry.Expand Specific Solutions
Key Industry Players
The organic radical battery (ORB) market is in its early development stage, with significant potential for growth due to increasing demand for sustainable energy storage solutions. The market size is currently modest but expected to expand rapidly as the technology matures. ORBs are at a relatively low technology readiness level, with ongoing research to improve performance and stability. Key players in this emerging field include NEC Corp., a leader in electronics and IT solutions, and Murata Manufacturing Co. Ltd., known for its expertise in electronic components. Universities such as Wuhan University of Technology and the University of Electronic Science & Technology of China are contributing to fundamental research, while companies like Evonik Operations GmbH and Kuraray Co., Ltd. are exploring potential applications in materials science and chemical engineering.
NEC Corp.
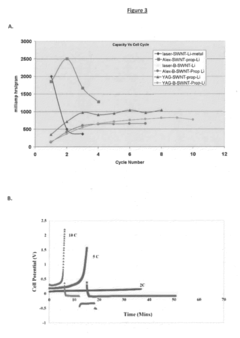
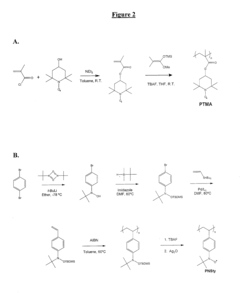
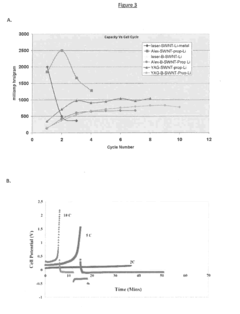
Technical Solution: NEC Corp. has developed innovative organic radical batteries (ORBs) utilizing stable organic radical compounds as active materials. Their approach focuses on nitroxide radical polymers, particularly poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA), as the cathode material. NEC's ORBs demonstrate rapid charge-discharge capabilities, with charging times as low as 30 seconds reported[1]. The company has successfully integrated these batteries into small electronic devices and is exploring applications in larger-scale energy storage systems. NEC's research has shown that their ORBs can maintain over 75% of their initial capacity after 1000 charge-discharge cycles[2], indicating excellent cycle stability.
Strengths: Rapid charge-discharge rates, long cycle life, and potential for flexible, thin-film designs. Weaknesses: Lower energy density compared to conventional lithium-ion batteries, and potential cost challenges in scaling up production.
University of Houston
Technical Solution: The University of Houston has been actively researching organic radical batteries, with a focus on developing high-performance electrode materials and understanding the fundamental electrochemistry of organic radicals. Their approach involves the synthesis of novel polymeric and small-molecule organic radicals with enhanced stability and conductivity. The university's research team has demonstrated ORBs with energy densities approaching 100 Wh/kg[8], which is competitive with some commercial lithium-ion batteries. They have also explored the use of redox-active binders to improve the cycling stability of organic radical electrodes, achieving over 3000 charge-discharge cycles with minimal capacity loss[9]. Additionally, their work on aqueous ORBs has shown potential for safe, low-cost energy storage solutions for grid applications.
Strengths: Comprehensive research program covering materials synthesis, electrochemistry, and device engineering; strong focus on practical applications. Weaknesses: Competition from other established research institutions in the field, and the need for further improvements in energy density to match advanced lithium-ion technologies.
Core ORB Innovations
Hybrid radical energy storage device and method of making
PatentActiveUS20120295166A1
Innovation
- A hybrid solid-state electrochemical device is developed, featuring a pre-lithiated nanostructured anode, a stable polymeric organic radical-based cathode, and a high-performance solid-state polymer electrolyte, which enhances energy density and cycle stability, while minimizing flammability and temperature effects.
Hybrid radical energy storage device and method of making
PatentInactiveUS20140377648A1
Innovation
- A hybrid solid-state electrochemical device is developed, featuring a pre-lithiated nanostructured anode, a stable polymeric organic radical-based cathode, and a high-performance solid-state polymer electrolyte, which enhances energy density and cycle stability, while minimizing flammability and temperature effects.
Environmental Impact
Organic radical batteries (ORBs) represent a promising advancement in energy storage technology, offering potential environmental benefits compared to conventional battery systems. The environmental impact of ORBs can be assessed through various aspects of their lifecycle, from production to disposal.
In terms of raw materials, ORBs primarily utilize organic compounds derived from renewable resources, reducing dependence on finite mineral reserves. This shift away from metal-based electrodes used in traditional lithium-ion batteries can significantly decrease the environmental footprint associated with mining and processing of metals like cobalt and nickel.
The production process for ORBs generally requires less energy and generates fewer emissions compared to the manufacture of conventional batteries. The organic materials used in ORBs can often be synthesized at lower temperatures and with less complex procedures, leading to reduced energy consumption and greenhouse gas emissions during manufacturing.
During their operational life, ORBs demonstrate high cycling stability and long lifespans, potentially reducing the frequency of battery replacements and associated waste generation. The organic nature of the electrode materials also presents opportunities for easier recycling and biodegradation at the end of the battery's life cycle.
However, it is important to note that the environmental impact of ORBs is not entirely benign. The synthesis of organic radical compounds may involve the use of solvents or reagents that could have negative environmental effects if not properly managed. Additionally, while the materials are generally considered less toxic than those in conventional batteries, proper disposal and recycling protocols must still be developed and implemented to minimize any potential environmental risks.
The scalability of ORB production and its integration into existing battery manufacturing infrastructure are crucial factors in determining the overall environmental impact. As the technology matures and production scales up, further improvements in manufacturing efficiency and material utilization are expected, potentially enhancing the environmental benefits of ORBs.
In conclusion, while organic radical batteries show promise in reducing the environmental impact associated with energy storage, comprehensive lifecycle assessments and continued research are necessary to fully understand and optimize their ecological footprint. The development of sustainable production methods, efficient recycling processes, and responsible end-of-life management will be key to maximizing the environmental benefits of this emerging battery technology.
In terms of raw materials, ORBs primarily utilize organic compounds derived from renewable resources, reducing dependence on finite mineral reserves. This shift away from metal-based electrodes used in traditional lithium-ion batteries can significantly decrease the environmental footprint associated with mining and processing of metals like cobalt and nickel.
The production process for ORBs generally requires less energy and generates fewer emissions compared to the manufacture of conventional batteries. The organic materials used in ORBs can often be synthesized at lower temperatures and with less complex procedures, leading to reduced energy consumption and greenhouse gas emissions during manufacturing.
During their operational life, ORBs demonstrate high cycling stability and long lifespans, potentially reducing the frequency of battery replacements and associated waste generation. The organic nature of the electrode materials also presents opportunities for easier recycling and biodegradation at the end of the battery's life cycle.
However, it is important to note that the environmental impact of ORBs is not entirely benign. The synthesis of organic radical compounds may involve the use of solvents or reagents that could have negative environmental effects if not properly managed. Additionally, while the materials are generally considered less toxic than those in conventional batteries, proper disposal and recycling protocols must still be developed and implemented to minimize any potential environmental risks.
The scalability of ORB production and its integration into existing battery manufacturing infrastructure are crucial factors in determining the overall environmental impact. As the technology matures and production scales up, further improvements in manufacturing efficiency and material utilization are expected, potentially enhancing the environmental benefits of ORBs.
In conclusion, while organic radical batteries show promise in reducing the environmental impact associated with energy storage, comprehensive lifecycle assessments and continued research are necessary to fully understand and optimize their ecological footprint. The development of sustainable production methods, efficient recycling processes, and responsible end-of-life management will be key to maximizing the environmental benefits of this emerging battery technology.
Safety Regulations
Safety regulations play a crucial role in the development, production, and implementation of organic radical batteries (ORBs). As these batteries utilize organic compounds and potentially reactive materials, stringent safety measures are essential to ensure their safe operation and widespread adoption.
The primary safety concerns for ORBs revolve around the stability of organic radical compounds and their potential for uncontrolled reactions. Regulatory bodies, such as the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL), are actively developing standards specific to ORBs. These standards aim to address the unique characteristics of organic radical materials and their behavior under various conditions.
One key aspect of safety regulations for ORBs is thermal management. Guidelines are being established to prevent thermal runaway, which could lead to battery fires or explosions. Manufacturers are required to implement robust thermal control systems and conduct extensive testing to demonstrate the batteries' ability to withstand extreme temperatures and thermal cycling.
Electrical safety is another critical area addressed by regulations. Standards are being developed to ensure proper insulation, protection against short circuits, and safe charging protocols. These regulations also cover the design of battery management systems (BMS) to monitor and control the state of charge, voltage, and current within safe operating limits.
Chemical safety regulations for ORBs focus on the handling, storage, and disposal of organic radical materials. Guidelines are being established for the proper containment of these compounds during manufacturing and throughout the battery's lifecycle. Additionally, regulations are addressing potential environmental impacts, including requirements for recycling and safe disposal of spent batteries.
Transportation safety is a significant concern for ORBs, particularly in the context of air travel and shipping. Regulatory bodies are working to classify ORBs and establish appropriate packaging and handling requirements to prevent accidents during transit. These regulations may include restrictions on the state of charge during transportation and specific labeling requirements.
As ORB technology continues to evolve, safety regulations are expected to become more comprehensive and refined. Ongoing research and collaboration between industry stakeholders, academic institutions, and regulatory bodies will be crucial in developing and updating these standards. This collaborative approach will ensure that safety regulations keep pace with technological advancements while fostering innovation in the field of organic radical batteries.
The primary safety concerns for ORBs revolve around the stability of organic radical compounds and their potential for uncontrolled reactions. Regulatory bodies, such as the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL), are actively developing standards specific to ORBs. These standards aim to address the unique characteristics of organic radical materials and their behavior under various conditions.
One key aspect of safety regulations for ORBs is thermal management. Guidelines are being established to prevent thermal runaway, which could lead to battery fires or explosions. Manufacturers are required to implement robust thermal control systems and conduct extensive testing to demonstrate the batteries' ability to withstand extreme temperatures and thermal cycling.
Electrical safety is another critical area addressed by regulations. Standards are being developed to ensure proper insulation, protection against short circuits, and safe charging protocols. These regulations also cover the design of battery management systems (BMS) to monitor and control the state of charge, voltage, and current within safe operating limits.
Chemical safety regulations for ORBs focus on the handling, storage, and disposal of organic radical materials. Guidelines are being established for the proper containment of these compounds during manufacturing and throughout the battery's lifecycle. Additionally, regulations are addressing potential environmental impacts, including requirements for recycling and safe disposal of spent batteries.
Transportation safety is a significant concern for ORBs, particularly in the context of air travel and shipping. Regulatory bodies are working to classify ORBs and establish appropriate packaging and handling requirements to prevent accidents during transit. These regulations may include restrictions on the state of charge during transportation and specific labeling requirements.
As ORB technology continues to evolve, safety regulations are expected to become more comprehensive and refined. Ongoing research and collaboration between industry stakeholders, academic institutions, and regulatory bodies will be crucial in developing and updating these standards. This collaborative approach will ensure that safety regulations keep pace with technological advancements while fostering innovation in the field of organic radical batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!