How to Use In-situ Spectroscopy to Monitor Radical Stability during Cycling
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Radical Stability Monitoring Goals
In-situ spectroscopy for monitoring radical stability during cycling is a critical technological goal in the field of energy storage and conversion. This technique aims to provide real-time insights into the behavior of radical species within electrochemical systems, particularly in batteries and fuel cells. The primary objective is to enhance our understanding of the degradation mechanisms that occur during charge-discharge cycles, ultimately leading to improved device performance and longevity.
One of the key goals of radical stability monitoring is to identify and characterize the formation, transformation, and decay of radical intermediates during electrochemical processes. This information is crucial for optimizing electrode materials and electrolyte compositions, as well as for developing strategies to mitigate unwanted side reactions that can lead to capacity fade and reduced cycle life.
Another important aspect of this technology is the ability to track the spatial distribution of radicals within the electrode structure. This can provide valuable information about local reaction kinetics and mass transport phenomena, which are essential for designing more efficient and durable energy storage devices. By mapping the radical concentration gradients across the electrode-electrolyte interface, researchers can gain insights into the factors that influence charge transfer and ion diffusion processes.
The development of in-situ spectroscopic techniques for radical stability monitoring also aims to overcome the limitations of ex-situ analysis methods. Traditional post-mortem studies often fail to capture the dynamic nature of radical species during cycling, as many of these intermediates are short-lived and sensitive to environmental changes. In-situ monitoring allows for the observation of transient species and rapid chemical transformations that would otherwise be missed in conventional analytical approaches.
Furthermore, the integration of in-situ spectroscopy with other characterization techniques, such as electrochemical impedance spectroscopy and X-ray diffraction, is a key goal in creating a comprehensive understanding of the complex processes occurring within energy storage devices. This multi-modal approach can provide correlations between radical stability, structural changes, and electrochemical performance, enabling more accurate predictions of device behavior and failure modes.
Ultimately, the overarching goal of using in-situ spectroscopy for radical stability monitoring is to accelerate the development of next-generation energy storage technologies. By providing detailed mechanistic insights, this technique can guide the rational design of advanced materials and cell architectures, leading to batteries and fuel cells with higher energy density, improved cycling stability, and enhanced safety characteristics.
One of the key goals of radical stability monitoring is to identify and characterize the formation, transformation, and decay of radical intermediates during electrochemical processes. This information is crucial for optimizing electrode materials and electrolyte compositions, as well as for developing strategies to mitigate unwanted side reactions that can lead to capacity fade and reduced cycle life.
Another important aspect of this technology is the ability to track the spatial distribution of radicals within the electrode structure. This can provide valuable information about local reaction kinetics and mass transport phenomena, which are essential for designing more efficient and durable energy storage devices. By mapping the radical concentration gradients across the electrode-electrolyte interface, researchers can gain insights into the factors that influence charge transfer and ion diffusion processes.
The development of in-situ spectroscopic techniques for radical stability monitoring also aims to overcome the limitations of ex-situ analysis methods. Traditional post-mortem studies often fail to capture the dynamic nature of radical species during cycling, as many of these intermediates are short-lived and sensitive to environmental changes. In-situ monitoring allows for the observation of transient species and rapid chemical transformations that would otherwise be missed in conventional analytical approaches.
Furthermore, the integration of in-situ spectroscopy with other characterization techniques, such as electrochemical impedance spectroscopy and X-ray diffraction, is a key goal in creating a comprehensive understanding of the complex processes occurring within energy storage devices. This multi-modal approach can provide correlations between radical stability, structural changes, and electrochemical performance, enabling more accurate predictions of device behavior and failure modes.
Ultimately, the overarching goal of using in-situ spectroscopy for radical stability monitoring is to accelerate the development of next-generation energy storage technologies. By providing detailed mechanistic insights, this technique can guide the rational design of advanced materials and cell architectures, leading to batteries and fuel cells with higher energy density, improved cycling stability, and enhanced safety characteristics.
Market Demand for In-situ Spectroscopy
The market demand for in-situ spectroscopy in monitoring radical stability during cycling has been steadily growing, driven by the increasing need for advanced analytical techniques in various industries. This technology offers real-time, non-destructive analysis of chemical reactions and material properties, making it particularly valuable in research and development, quality control, and process optimization.
In the field of energy storage and battery technology, in-situ spectroscopy has gained significant traction. As the global push for renewable energy and electric vehicles intensifies, the demand for more efficient and durable battery systems has surged. In-situ spectroscopy provides crucial insights into the behavior of radicals and other reactive species during battery cycling, enabling researchers and manufacturers to develop more stable and long-lasting energy storage solutions.
The pharmaceutical industry has also shown a strong interest in this technology. With the increasing complexity of drug formulations and the need for precise quality control, in-situ spectroscopy offers a powerful tool for monitoring chemical stability and reaction kinetics. This is particularly important in the development of new drugs and the optimization of manufacturing processes, where understanding radical stability can be critical to product efficacy and safety.
In the polymer and materials science sectors, the market for in-situ spectroscopy has been expanding rapidly. As industries seek to develop advanced materials with specific properties, the ability to monitor radical stability during synthesis and processing becomes crucial. This technology enables researchers to fine-tune material properties and improve product performance, driving innovation in areas such as aerospace, automotive, and consumer electronics.
The semiconductor industry has also recognized the value of in-situ spectroscopy for monitoring radical stability. As chip manufacturers strive for smaller, more efficient devices, understanding and controlling the behavior of radicals during fabrication processes becomes increasingly important. This technology helps in optimizing etching processes, improving yield rates, and developing new materials for next-generation semiconductors.
Environmental monitoring and green chemistry applications represent another growing market for in-situ spectroscopy. As regulations on emissions and chemical processes become more stringent, industries are turning to this technology to monitor and control radical species in real-time, ensuring compliance and minimizing environmental impact.
The market trend indicates a shift towards more compact, user-friendly, and integrated spectroscopic systems. This is driven by the need for on-site analysis in various industries and the growing adoption of Industry 4.0 principles. As a result, manufacturers are developing portable and automated in-situ spectroscopy solutions that can be easily incorporated into existing production lines and research facilities.
In the field of energy storage and battery technology, in-situ spectroscopy has gained significant traction. As the global push for renewable energy and electric vehicles intensifies, the demand for more efficient and durable battery systems has surged. In-situ spectroscopy provides crucial insights into the behavior of radicals and other reactive species during battery cycling, enabling researchers and manufacturers to develop more stable and long-lasting energy storage solutions.
The pharmaceutical industry has also shown a strong interest in this technology. With the increasing complexity of drug formulations and the need for precise quality control, in-situ spectroscopy offers a powerful tool for monitoring chemical stability and reaction kinetics. This is particularly important in the development of new drugs and the optimization of manufacturing processes, where understanding radical stability can be critical to product efficacy and safety.
In the polymer and materials science sectors, the market for in-situ spectroscopy has been expanding rapidly. As industries seek to develop advanced materials with specific properties, the ability to monitor radical stability during synthesis and processing becomes crucial. This technology enables researchers to fine-tune material properties and improve product performance, driving innovation in areas such as aerospace, automotive, and consumer electronics.
The semiconductor industry has also recognized the value of in-situ spectroscopy for monitoring radical stability. As chip manufacturers strive for smaller, more efficient devices, understanding and controlling the behavior of radicals during fabrication processes becomes increasingly important. This technology helps in optimizing etching processes, improving yield rates, and developing new materials for next-generation semiconductors.
Environmental monitoring and green chemistry applications represent another growing market for in-situ spectroscopy. As regulations on emissions and chemical processes become more stringent, industries are turning to this technology to monitor and control radical species in real-time, ensuring compliance and minimizing environmental impact.
The market trend indicates a shift towards more compact, user-friendly, and integrated spectroscopic systems. This is driven by the need for on-site analysis in various industries and the growing adoption of Industry 4.0 principles. As a result, manufacturers are developing portable and automated in-situ spectroscopy solutions that can be easily incorporated into existing production lines and research facilities.
Challenges in Radical Stability Analysis
Analyzing radical stability during cycling presents several significant challenges that researchers and engineers must overcome. The dynamic nature of radical species, coupled with the complex electrochemical environment in batteries, makes in-situ monitoring particularly demanding.
One of the primary challenges is the short-lived nature of radical intermediates. These species often have lifetimes in the microsecond to millisecond range, requiring extremely fast detection methods. Traditional spectroscopic techniques may struggle to capture these fleeting species, necessitating the development of ultrafast spectroscopy methods with high temporal resolution.
The heterogeneous nature of electrode surfaces further complicates radical stability analysis. Radicals can form and decay at different rates depending on their location within the electrode structure. This spatial variability demands spectroscopic techniques with high spatial resolution, capable of probing specific regions of the electrode-electrolyte interface.
Interference from other electrochemical processes poses another significant challenge. During cycling, numerous reactions occur simultaneously, producing various spectral signatures that can overlap with those of the radicals of interest. Distinguishing the specific radical signals from this complex background requires sophisticated data processing and analysis techniques.
The harsh chemical environment within batteries also presents difficulties for in-situ spectroscopy. Highly reactive electrolytes and degradation products can interfere with spectroscopic measurements or damage sensitive equipment. Developing robust, chemically resistant spectroscopic probes and cells is crucial for long-term stability monitoring.
Quantification of radical concentrations remains a formidable challenge. While spectroscopic techniques can often detect the presence of radicals, accurately determining their concentrations in a dynamic, heterogeneous environment is far more complex. This requires careful calibration and the development of advanced mathematical models to interpret spectral data.
The need for operando measurements adds another layer of complexity. Spectroscopic techniques must be compatible with the physical constraints of functioning battery cells, often requiring specially designed cells or the integration of spectroscopic windows. Balancing the need for optical access with maintaining cell performance and safety is a delicate engineering challenge.
Finally, the multidisciplinary nature of this research area presents its own set of challenges. Effective radical stability analysis requires expertise in electrochemistry, spectroscopy, materials science, and data analysis. Bridging these diverse fields and fostering collaboration between specialists is essential for advancing the state of the art in radical stability monitoring during battery cycling.
One of the primary challenges is the short-lived nature of radical intermediates. These species often have lifetimes in the microsecond to millisecond range, requiring extremely fast detection methods. Traditional spectroscopic techniques may struggle to capture these fleeting species, necessitating the development of ultrafast spectroscopy methods with high temporal resolution.
The heterogeneous nature of electrode surfaces further complicates radical stability analysis. Radicals can form and decay at different rates depending on their location within the electrode structure. This spatial variability demands spectroscopic techniques with high spatial resolution, capable of probing specific regions of the electrode-electrolyte interface.
Interference from other electrochemical processes poses another significant challenge. During cycling, numerous reactions occur simultaneously, producing various spectral signatures that can overlap with those of the radicals of interest. Distinguishing the specific radical signals from this complex background requires sophisticated data processing and analysis techniques.
The harsh chemical environment within batteries also presents difficulties for in-situ spectroscopy. Highly reactive electrolytes and degradation products can interfere with spectroscopic measurements or damage sensitive equipment. Developing robust, chemically resistant spectroscopic probes and cells is crucial for long-term stability monitoring.
Quantification of radical concentrations remains a formidable challenge. While spectroscopic techniques can often detect the presence of radicals, accurately determining their concentrations in a dynamic, heterogeneous environment is far more complex. This requires careful calibration and the development of advanced mathematical models to interpret spectral data.
The need for operando measurements adds another layer of complexity. Spectroscopic techniques must be compatible with the physical constraints of functioning battery cells, often requiring specially designed cells or the integration of spectroscopic windows. Balancing the need for optical access with maintaining cell performance and safety is a delicate engineering challenge.
Finally, the multidisciplinary nature of this research area presents its own set of challenges. Effective radical stability analysis requires expertise in electrochemistry, spectroscopy, materials science, and data analysis. Bridging these diverse fields and fostering collaboration between specialists is essential for advancing the state of the art in radical stability monitoring during battery cycling.
Current In-situ Spectroscopy Methods
01 In-situ spectroscopy techniques for radical stability analysis
Various in-situ spectroscopy methods are employed to study radical stability in real-time. These techniques allow for the observation of radical formation, decay, and interactions under different conditions, providing valuable insights into reaction mechanisms and kinetics. Advanced spectroscopic tools enable researchers to monitor radical behavior in complex chemical environments, enhancing understanding of radical-mediated processes.- In-situ spectroscopy techniques for radical stability analysis: Various in-situ spectroscopy methods are employed to study radical stability in real-time. These techniques allow for the observation of radical formation, decay, and interactions under different conditions, providing valuable insights into reaction mechanisms and kinetics. Advanced spectroscopic tools enable researchers to monitor radical behavior in complex chemical environments, enhancing understanding of radical-mediated processes.
- Electron Paramagnetic Resonance (EPR) for radical detection: EPR spectroscopy is a powerful technique for detecting and characterizing free radicals. It offers high sensitivity and specificity in identifying unpaired electrons, making it ideal for studying radical stability. In-situ EPR measurements can provide real-time information on radical formation, concentration, and lifetime in various chemical and biological systems.
- Optical spectroscopy methods for radical analysis: Optical spectroscopy techniques, including UV-Vis, fluorescence, and Raman spectroscopy, are utilized for in-situ radical stability studies. These methods offer non-invasive, real-time monitoring of radical species in solution and solid-state samples. By analyzing spectral changes, researchers can track radical formation, transformation, and decay, providing insights into reaction mechanisms and kinetics.
- Mass spectrometry for radical identification and quantification: In-situ mass spectrometry techniques are employed to identify and quantify radical species in complex mixtures. These methods offer high sensitivity and the ability to detect short-lived radical intermediates. By coupling mass spectrometry with other spectroscopic techniques, researchers can obtain comprehensive information on radical stability and reactivity in various chemical processes.
- Advanced data analysis and modeling for radical stability studies: Sophisticated data analysis techniques and computational modeling are used to interpret spectroscopic data and predict radical stability. Machine learning algorithms and quantum chemical calculations are employed to process complex spectral information, enabling researchers to extract meaningful insights into radical behavior and stability under various conditions. These advanced analytical approaches enhance the accuracy and predictive power of in-situ radical stability studies.
02 Electron Paramagnetic Resonance (EPR) for radical detection
EPR spectroscopy is a powerful technique for detecting and characterizing free radicals. It provides information about the electronic structure and environment of unpaired electrons, making it particularly useful for studying radical stability. In-situ EPR measurements allow for real-time monitoring of radical formation and decay, offering insights into reaction kinetics and mechanisms in various chemical and biological systems.Expand Specific Solutions03 Optical spectroscopy methods for radical analysis
Various optical spectroscopy techniques, including UV-Vis, fluorescence, and Raman spectroscopy, are utilized for in-situ radical stability studies. These methods offer non-invasive, real-time monitoring of radical species in different media. By analyzing spectral changes, researchers can track radical formation, transformation, and decay, providing valuable information about reaction progress and stability of radical intermediates.Expand Specific Solutions04 Mass spectrometry for radical identification and quantification
In-situ mass spectrometry techniques are employed to identify and quantify radical species in complex mixtures. These methods allow for high sensitivity and selectivity in radical detection, enabling researchers to track the formation and disappearance of specific radical species over time. Advanced mass spectrometry approaches provide detailed information about radical structures and their transformations during chemical reactions.Expand Specific Solutions05 Combination of spectroscopic techniques for comprehensive radical analysis
Researchers often combine multiple spectroscopic techniques to gain a more comprehensive understanding of radical stability and behavior. By integrating data from different spectroscopic methods, such as EPR, NMR, and optical spectroscopy, scientists can obtain complementary information about radical structure, dynamics, and interactions. This multi-technique approach enhances the accuracy and reliability of radical stability assessments in complex chemical systems.Expand Specific Solutions
Key Players in Spectroscopy Industry
The in-situ spectroscopy for monitoring radical stability during cycling is in an early development stage, with a growing market driven by the increasing demand for advanced battery technologies. The market size is expanding as more industries recognize the importance of real-time monitoring in battery performance optimization. Technologically, it's still evolving, with companies like Applied Materials, Inc. and Robert Bosch GmbH leading in innovation. Research institutions such as the Centre National de la Recherche Scientifique and Nanjing University are contributing significantly to advancing the technology. While not yet fully mature, the field is rapidly progressing, with collaborations between industry and academia accelerating development and practical applications.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed an advanced in-situ spectroscopy system for monitoring radical stability during cycling in semiconductor manufacturing processes. Their solution integrates real-time optical emission spectroscopy (OES) with plasma etch chambers, allowing for continuous monitoring of radical species concentrations[1]. The system employs a high-resolution spectrometer coupled with sophisticated data analysis algorithms to detect subtle changes in radical stability. This enables precise control of etch processes and early detection of process drift, ultimately improving yield and reducing downtime[2]. The company has also implemented machine learning techniques to enhance the interpretation of spectral data, enabling predictive maintenance and optimization of process parameters[3].
Strengths: Highly integrated with existing semiconductor manufacturing equipment, providing seamless monitoring. Advanced data analysis capabilities for real-time process control. Weaknesses: May require significant initial investment and specialized training for operators.
Robert Bosch GmbH
Technical Solution: Bosch has developed an innovative in-situ spectroscopy system for monitoring radical stability in automotive fuel cells and batteries. Their approach combines Raman spectroscopy with electrochemical impedance spectroscopy (EIS) to provide comprehensive insights into the chemical and electrical behavior of energy storage systems during cycling[4]. The system utilizes fiber-optic probes for non-invasive measurements, allowing for real-time monitoring without disrupting the cell operation. Bosch's solution incorporates advanced signal processing algorithms to filter out noise and extract meaningful data on radical formation and degradation[5]. This technology enables more accurate prediction of battery and fuel cell lifetimes, as well as optimization of charging and discharging strategies to maximize performance and longevity[6].
Strengths: Dual-mode spectroscopy provides comprehensive analysis of both chemical and electrical properties. Non-invasive monitoring suitable for various energy storage systems. Weaknesses: May be more complex to implement in existing production lines compared to single-mode spectroscopy systems.
Innovations in Radical Detection
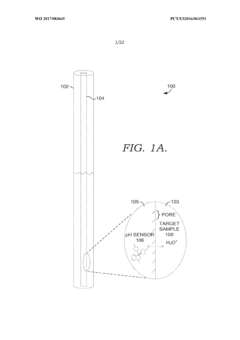
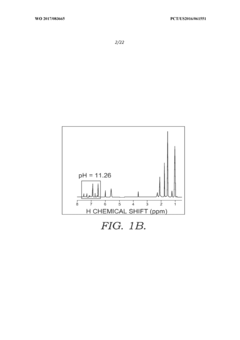
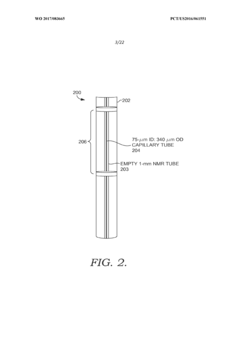
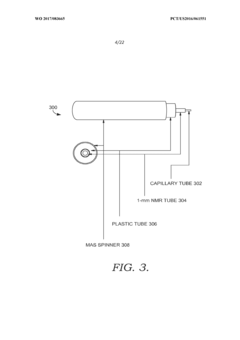
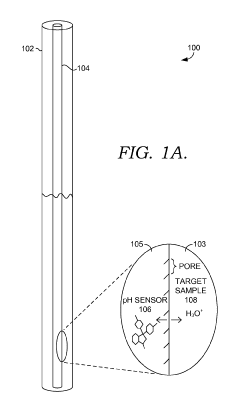
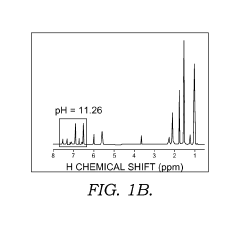
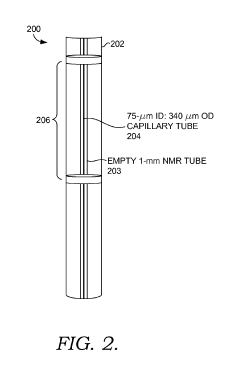
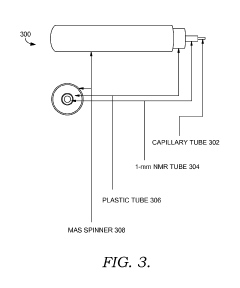
Solid state NMR spectroscopy/imaging in SITU measuring devices
PatentWO2017083665A1
Innovation
- In situ measuring devices for NMR spectroscopy that include pH sensors with NMR spectral changes and sealed capillary tubes with reference materials, allowing for continuous monitoring of pH and temperature within the NMR probe, eliminating the need for sample removal and providing accurate, integral data.
In situ NMR parameter monitoring systems and methods for measuring pH and temperature
PatentActiveUS10295487B2
Innovation
- The development of in situ pH and temperature monitoring devices using NMR spectroscopy, which include a sample housing member with a pH sensor containment member that allows diffusion of hydronium and hydroxide ions, and a capillary tube with a reference material sealed inside an NMR sample tube, allowing for continuous monitoring without removing the sample from the NMR probe.
Safety Protocols for Radical Handling
When handling radicals in spectroscopic experiments, strict safety protocols must be implemented to protect researchers and maintain the integrity of the experimental setup. Proper personal protective equipment (PPE) is essential, including chemical-resistant gloves, lab coats, and safety goggles. Researchers should work in well-ventilated areas, preferably under a fume hood, to minimize exposure to potentially harmful vapors or gases.
All radical-containing solutions and materials should be clearly labeled and stored in appropriate containers. Glassware and other equipment used in radical experiments must be thoroughly cleaned and inspected for damage before each use. Any cracked or chipped glassware should be immediately discarded to prevent potential leaks or accidents.
Radical stability monitoring often involves the use of specialized spectroscopic equipment. This equipment should be regularly calibrated and maintained according to manufacturer specifications. Researchers must be trained in the proper operation of these instruments and understand the potential hazards associated with their use.
Emergency response procedures should be established and clearly communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and fire extinguishers. A spill kit specifically designed for handling radical-containing materials should be readily available in the laboratory.
When conducting in-situ spectroscopy experiments, additional precautions may be necessary. The experimental setup should be designed to minimize the risk of sample leakage or equipment failure during cycling. Regular inspections of seals, connections, and containment systems should be performed to ensure their integrity throughout the experiment.
Proper disposal of radical-containing waste is crucial. Dedicated waste containers should be used, and disposal should follow institutional and regulatory guidelines. Neutralization or deactivation procedures may be required before disposal, depending on the specific radicals involved.
Training and education are fundamental aspects of radical handling safety protocols. All researchers working with radicals should receive comprehensive training on proper handling techniques, potential hazards, and emergency procedures. Regular safety refresher courses and updates on new safety protocols should be provided to ensure ongoing compliance and awareness.
All radical-containing solutions and materials should be clearly labeled and stored in appropriate containers. Glassware and other equipment used in radical experiments must be thoroughly cleaned and inspected for damage before each use. Any cracked or chipped glassware should be immediately discarded to prevent potential leaks or accidents.
Radical stability monitoring often involves the use of specialized spectroscopic equipment. This equipment should be regularly calibrated and maintained according to manufacturer specifications. Researchers must be trained in the proper operation of these instruments and understand the potential hazards associated with their use.
Emergency response procedures should be established and clearly communicated to all laboratory personnel. This includes the location of safety showers, eyewash stations, and fire extinguishers. A spill kit specifically designed for handling radical-containing materials should be readily available in the laboratory.
When conducting in-situ spectroscopy experiments, additional precautions may be necessary. The experimental setup should be designed to minimize the risk of sample leakage or equipment failure during cycling. Regular inspections of seals, connections, and containment systems should be performed to ensure their integrity throughout the experiment.
Proper disposal of radical-containing waste is crucial. Dedicated waste containers should be used, and disposal should follow institutional and regulatory guidelines. Neutralization or deactivation procedures may be required before disposal, depending on the specific radicals involved.
Training and education are fundamental aspects of radical handling safety protocols. All researchers working with radicals should receive comprehensive training on proper handling techniques, potential hazards, and emergency procedures. Regular safety refresher courses and updates on new safety protocols should be provided to ensure ongoing compliance and awareness.
Data Analysis and Interpretation Techniques
Data analysis and interpretation techniques are crucial for effectively utilizing in-situ spectroscopy to monitor radical stability during cycling. These techniques involve several key steps and considerations to extract meaningful insights from the collected spectroscopic data.
Preprocessing of raw spectral data is the first essential step. This includes baseline correction, noise reduction, and spectral normalization. Advanced algorithms, such as Savitzky-Golay filtering or wavelet transforms, can be employed to enhance signal quality while preserving important spectral features. Proper calibration of the spectroscopic instrument is also critical to ensure accurate and reproducible measurements.
Feature extraction is the next important phase in data analysis. This involves identifying and isolating specific spectral features that correspond to radical species of interest. Techniques such as peak fitting, principal component analysis (PCA), or partial least squares (PLS) regression can be used to extract relevant information from complex spectral data. These methods help in distinguishing between different radical species and quantifying their relative concentrations.
Multivariate analysis techniques are often employed to handle the high-dimensional nature of spectroscopic data. Methods like hierarchical cluster analysis (HCA) or discriminant analysis can be used to classify spectra and identify patterns or groupings related to radical stability. These techniques are particularly useful when dealing with large datasets collected over multiple cycling events.
Time-resolved analysis is crucial for monitoring radical stability during cycling. This involves tracking spectral changes over time and correlating them with cycling parameters. Techniques such as two-dimensional correlation spectroscopy (2D-COS) or chemometric methods can be applied to reveal dynamic changes in radical species and their interactions during the cycling process.
Quantitative analysis of radical concentrations is often a primary goal. This can be achieved through the development of calibration models using reference standards or chemometric techniques like partial least squares regression (PLSR). These models allow for the accurate determination of radical concentrations from spectral data, enabling the assessment of radical stability over time.
Data visualization plays a vital role in interpreting complex spectroscopic data. Techniques such as contour plots, 3D surface plots, or heat maps can be used to represent multidimensional data in an intuitive manner. These visual representations help in identifying trends, patterns, and anomalies in radical stability across different cycling conditions.
Integration of spectroscopic data with other experimental parameters, such as electrochemical measurements or temperature profiles, can provide a more comprehensive understanding of radical stability. Data fusion techniques or machine learning algorithms can be employed to correlate spectroscopic data with other relevant variables, leading to more robust interpretations and predictive models.
Preprocessing of raw spectral data is the first essential step. This includes baseline correction, noise reduction, and spectral normalization. Advanced algorithms, such as Savitzky-Golay filtering or wavelet transforms, can be employed to enhance signal quality while preserving important spectral features. Proper calibration of the spectroscopic instrument is also critical to ensure accurate and reproducible measurements.
Feature extraction is the next important phase in data analysis. This involves identifying and isolating specific spectral features that correspond to radical species of interest. Techniques such as peak fitting, principal component analysis (PCA), or partial least squares (PLS) regression can be used to extract relevant information from complex spectral data. These methods help in distinguishing between different radical species and quantifying their relative concentrations.
Multivariate analysis techniques are often employed to handle the high-dimensional nature of spectroscopic data. Methods like hierarchical cluster analysis (HCA) or discriminant analysis can be used to classify spectra and identify patterns or groupings related to radical stability. These techniques are particularly useful when dealing with large datasets collected over multiple cycling events.
Time-resolved analysis is crucial for monitoring radical stability during cycling. This involves tracking spectral changes over time and correlating them with cycling parameters. Techniques such as two-dimensional correlation spectroscopy (2D-COS) or chemometric methods can be applied to reveal dynamic changes in radical species and their interactions during the cycling process.
Quantitative analysis of radical concentrations is often a primary goal. This can be achieved through the development of calibration models using reference standards or chemometric techniques like partial least squares regression (PLSR). These models allow for the accurate determination of radical concentrations from spectral data, enabling the assessment of radical stability over time.
Data visualization plays a vital role in interpreting complex spectroscopic data. Techniques such as contour plots, 3D surface plots, or heat maps can be used to represent multidimensional data in an intuitive manner. These visual representations help in identifying trends, patterns, and anomalies in radical stability across different cycling conditions.
Integration of spectroscopic data with other experimental parameters, such as electrochemical measurements or temperature profiles, can provide a more comprehensive understanding of radical stability. Data fusion techniques or machine learning algorithms can be employed to correlate spectroscopic data with other relevant variables, leading to more robust interpretations and predictive models.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!