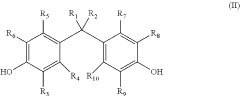
How to Select Lewis Acid for Polymerization?
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has emerged as a cornerstone in polymer chemistry since the pioneering work of Karl Ziegler and Giulio Natta in the 1950s, who discovered that certain transition metal compounds could catalyze olefin polymerization. This breakthrough revolutionized the plastics industry and earned them the Nobel Prize in Chemistry in 1963. Lewis acids, defined as electron pair acceptors, play a crucial role in various polymerization mechanisms by activating monomers through coordination with electron-rich sites.
The evolution of Lewis acid catalysis in polymerization has progressed through several distinct phases. Initially, simple metal halides such as AlCl3, TiCl4, and BF3 dominated the field. The second generation saw the development of metallocene catalysts in the 1980s, offering unprecedented control over polymer microstructure. More recently, post-metallocene single-site catalysts have further expanded the precision with which polymers can be engineered.
Current technological trends in this domain focus on developing more selective, efficient, and environmentally benign Lewis acid catalysts. There is growing interest in lanthanide and rare earth metal-based systems, which often exhibit unique reactivity patterns. Additionally, the integration of computational chemistry has accelerated catalyst design by enabling the prediction of catalyst performance before synthesis.
The primary objective of Lewis acid selection for polymerization is to achieve precise control over polymer properties including molecular weight distribution, stereochemistry, branching, and end-group functionality. This control directly translates to materials with tailored mechanical, thermal, and chemical properties for specific applications.
Another critical goal is enhancing sustainability in polymer production. This includes developing catalysts that operate under milder conditions, require lower catalyst loadings, and enable the polymerization of renewable monomers. The reduction of toxic waste and energy consumption aligns with global initiatives for greener chemical processes.
Understanding the fundamental mechanisms of Lewis acid-monomer interactions represents a key scientific objective. Despite decades of research, certain aspects of initiation, propagation, and termination steps remain incompletely understood, particularly for newer monomer classes and copolymerization systems.
The strategic selection of appropriate Lewis acids for specific polymerization processes requires balancing multiple factors including catalyst activity, selectivity, stability, cost, and environmental impact. This multifaceted decision-making process necessitates a comprehensive understanding of both the theoretical principles governing Lewis acid catalysis and the practical considerations of industrial polymer production.
The evolution of Lewis acid catalysis in polymerization has progressed through several distinct phases. Initially, simple metal halides such as AlCl3, TiCl4, and BF3 dominated the field. The second generation saw the development of metallocene catalysts in the 1980s, offering unprecedented control over polymer microstructure. More recently, post-metallocene single-site catalysts have further expanded the precision with which polymers can be engineered.
Current technological trends in this domain focus on developing more selective, efficient, and environmentally benign Lewis acid catalysts. There is growing interest in lanthanide and rare earth metal-based systems, which often exhibit unique reactivity patterns. Additionally, the integration of computational chemistry has accelerated catalyst design by enabling the prediction of catalyst performance before synthesis.
The primary objective of Lewis acid selection for polymerization is to achieve precise control over polymer properties including molecular weight distribution, stereochemistry, branching, and end-group functionality. This control directly translates to materials with tailored mechanical, thermal, and chemical properties for specific applications.
Another critical goal is enhancing sustainability in polymer production. This includes developing catalysts that operate under milder conditions, require lower catalyst loadings, and enable the polymerization of renewable monomers. The reduction of toxic waste and energy consumption aligns with global initiatives for greener chemical processes.
Understanding the fundamental mechanisms of Lewis acid-monomer interactions represents a key scientific objective. Despite decades of research, certain aspects of initiation, propagation, and termination steps remain incompletely understood, particularly for newer monomer classes and copolymerization systems.
The strategic selection of appropriate Lewis acids for specific polymerization processes requires balancing multiple factors including catalyst activity, selectivity, stability, cost, and environmental impact. This multifaceted decision-making process necessitates a comprehensive understanding of both the theoretical principles governing Lewis acid catalysis and the practical considerations of industrial polymer production.
Market Analysis of Lewis Acid Catalyzed Polymers
The global market for Lewis acid catalyzed polymers has experienced significant growth over the past decade, driven primarily by increasing demand in packaging, automotive, construction, and electronics industries. The market value reached approximately $45 billion in 2022, with a compound annual growth rate (CAGR) of 5.7% projected through 2028, potentially reaching $63 billion by that time.
Regionally, Asia-Pacific dominates the market landscape, accounting for nearly 40% of global consumption, with China being the largest single-country consumer. North America and Europe follow with market shares of 28% and 24% respectively, while Latin America and Middle East & Africa collectively represent the remaining 8%.
The packaging sector remains the largest end-use segment, consuming roughly 35% of Lewis acid catalyzed polymers, particularly in food packaging applications where these materials offer superior barrier properties and chemical resistance. The automotive industry represents the second-largest market segment at 22%, utilizing these polymers for lightweight components that contribute to fuel efficiency and reduced emissions.
Consumer demand trends increasingly favor sustainable and recyclable polymer products, creating both challenges and opportunities for manufacturers. Bio-based Lewis acid catalyzed polymers have emerged as a high-growth subsegment, currently representing only 7% of the total market but expanding at nearly triple the rate of conventional petroleum-based alternatives.
Price sensitivity varies significantly across application segments, with commodity applications demonstrating high elasticity while specialty applications in electronics and medical devices showing greater tolerance for premium pricing. The average selling price for standard-grade Lewis acid catalyzed polymers ranges between $1,800-$2,500 per metric ton, while specialty grades command prices up to $5,000 per metric ton.
Supply chain dynamics have undergone substantial transformation following global disruptions, with manufacturers increasingly pursuing regionalization strategies to mitigate risks. Raw material volatility, particularly for metal-based Lewis acids like aluminum chloride and titanium tetrachloride, has prompted development of more efficient catalytic systems that require lower loadings.
Market consolidation continues through strategic acquisitions and partnerships, with the top five producers controlling approximately 38% of global capacity. However, numerous specialized regional players maintain competitive positions through technological differentiation and customer-specific formulations.
Future market growth will likely be driven by innovations in catalyst selectivity and efficiency, enabling production of polymers with precisely tailored properties for emerging applications in 5G infrastructure, electric vehicles, and advanced medical devices.
Regionally, Asia-Pacific dominates the market landscape, accounting for nearly 40% of global consumption, with China being the largest single-country consumer. North America and Europe follow with market shares of 28% and 24% respectively, while Latin America and Middle East & Africa collectively represent the remaining 8%.
The packaging sector remains the largest end-use segment, consuming roughly 35% of Lewis acid catalyzed polymers, particularly in food packaging applications where these materials offer superior barrier properties and chemical resistance. The automotive industry represents the second-largest market segment at 22%, utilizing these polymers for lightweight components that contribute to fuel efficiency and reduced emissions.
Consumer demand trends increasingly favor sustainable and recyclable polymer products, creating both challenges and opportunities for manufacturers. Bio-based Lewis acid catalyzed polymers have emerged as a high-growth subsegment, currently representing only 7% of the total market but expanding at nearly triple the rate of conventional petroleum-based alternatives.
Price sensitivity varies significantly across application segments, with commodity applications demonstrating high elasticity while specialty applications in electronics and medical devices showing greater tolerance for premium pricing. The average selling price for standard-grade Lewis acid catalyzed polymers ranges between $1,800-$2,500 per metric ton, while specialty grades command prices up to $5,000 per metric ton.
Supply chain dynamics have undergone substantial transformation following global disruptions, with manufacturers increasingly pursuing regionalization strategies to mitigate risks. Raw material volatility, particularly for metal-based Lewis acids like aluminum chloride and titanium tetrachloride, has prompted development of more efficient catalytic systems that require lower loadings.
Market consolidation continues through strategic acquisitions and partnerships, with the top five producers controlling approximately 38% of global capacity. However, numerous specialized regional players maintain competitive positions through technological differentiation and customer-specific formulations.
Future market growth will likely be driven by innovations in catalyst selectivity and efficiency, enabling production of polymers with precisely tailored properties for emerging applications in 5G infrastructure, electric vehicles, and advanced medical devices.
Current Challenges in Lewis Acid Selection
Despite significant advancements in polymerization catalysis, selecting the appropriate Lewis acid catalyst remains a complex challenge for researchers and industrial chemists. The primary difficulty lies in predicting catalyst performance across diverse monomer systems, as Lewis acid behavior can vary dramatically depending on reaction conditions and substrate interactions.
One major challenge is the lack of standardized selection criteria that can be universally applied across different polymerization processes. While Lewis acidity strength is often considered a primary selection factor, it alone cannot predict catalytic efficiency, as factors such as steric hindrance, solubility, and coordination geometry significantly influence catalyst performance in specific polymerization systems.
Compatibility issues between Lewis acids and functional groups present in monomers or reaction media create additional complications. Many Lewis acids exhibit high sensitivity to oxygen, moisture, and certain functional groups, leading to catalyst deactivation or unwanted side reactions. This sensitivity necessitates careful handling procedures and often limits industrial scalability of promising laboratory-scale processes.
The environmental and economic sustainability of Lewis acid catalysts presents another significant challenge. Traditional Lewis acids like AlCl₃, TiCl₄, and BF₃ pose considerable environmental hazards and often require stoichiometric rather than catalytic quantities, generating substantial waste. While newer, more environmentally benign alternatives are emerging, they frequently demonstrate reduced catalytic activity or require specialized reaction conditions.
Mechanistic understanding of Lewis acid catalysis in polymerization remains incomplete, particularly regarding the dynamic interactions between catalyst, monomer, and growing polymer chains. This knowledge gap hampers rational catalyst design and often forces researchers to rely on empirical approaches and extensive screening processes.
Reproducibility issues further complicate Lewis acid selection, as trace impurities or slight variations in catalyst preparation can dramatically alter catalytic performance. This is especially problematic for moisture-sensitive Lewis acids, where consistent activity across different batches can be difficult to maintain.
The growing diversity of polymerization techniques—from traditional cationic processes to controlled radical polymerization and ring-opening methods—demands Lewis acids with increasingly specialized properties. Finding catalysts that can maintain activity while providing the desired level of reaction control represents a significant ongoing challenge in polymer chemistry.
Computational approaches to Lewis acid selection, while promising, remain limited by the complexity of modeling catalyst-monomer interactions in realistic polymerization environments. Current models often fail to account for solvent effects, aggregation phenomena, and the dynamic nature of polymerization processes.
One major challenge is the lack of standardized selection criteria that can be universally applied across different polymerization processes. While Lewis acidity strength is often considered a primary selection factor, it alone cannot predict catalytic efficiency, as factors such as steric hindrance, solubility, and coordination geometry significantly influence catalyst performance in specific polymerization systems.
Compatibility issues between Lewis acids and functional groups present in monomers or reaction media create additional complications. Many Lewis acids exhibit high sensitivity to oxygen, moisture, and certain functional groups, leading to catalyst deactivation or unwanted side reactions. This sensitivity necessitates careful handling procedures and often limits industrial scalability of promising laboratory-scale processes.
The environmental and economic sustainability of Lewis acid catalysts presents another significant challenge. Traditional Lewis acids like AlCl₃, TiCl₄, and BF₃ pose considerable environmental hazards and often require stoichiometric rather than catalytic quantities, generating substantial waste. While newer, more environmentally benign alternatives are emerging, they frequently demonstrate reduced catalytic activity or require specialized reaction conditions.
Mechanistic understanding of Lewis acid catalysis in polymerization remains incomplete, particularly regarding the dynamic interactions between catalyst, monomer, and growing polymer chains. This knowledge gap hampers rational catalyst design and often forces researchers to rely on empirical approaches and extensive screening processes.
Reproducibility issues further complicate Lewis acid selection, as trace impurities or slight variations in catalyst preparation can dramatically alter catalytic performance. This is especially problematic for moisture-sensitive Lewis acids, where consistent activity across different batches can be difficult to maintain.
The growing diversity of polymerization techniques—from traditional cationic processes to controlled radical polymerization and ring-opening methods—demands Lewis acids with increasingly specialized properties. Finding catalysts that can maintain activity while providing the desired level of reaction control represents a significant ongoing challenge in polymer chemistry.
Computational approaches to Lewis acid selection, while promising, remain limited by the complexity of modeling catalyst-monomer interactions in realistic polymerization environments. Current models often fail to account for solvent effects, aggregation phenomena, and the dynamic nature of polymerization processes.
Established Lewis Acid Selection Methodologies
01 Lewis acid catalysts in polymerization reactions
Lewis acids are widely used as catalysts in various polymerization processes. These catalysts facilitate the formation of polymers by activating monomers and promoting chain growth. They can control molecular weight distribution, stereochemistry, and reaction rates in polymerization reactions. Common Lewis acids used include metal halides and organometallic compounds that can coordinate with electron-rich species to initiate polymerization.- Lewis Acid Catalysts in Chemical Synthesis: Lewis acids serve as effective catalysts in various chemical synthesis reactions, facilitating bond formation and transformation processes. These catalysts can activate substrates by accepting electron pairs, enabling reactions that might otherwise require harsh conditions. Common applications include alkylation, acylation, and polymerization reactions where Lewis acids coordinate with reactants to lower activation energy barriers and improve reaction efficiency.
- Lewis Acid Applications in Polymerization Processes: Lewis acids play a crucial role in polymerization reactions, particularly in the production of various polymers and copolymers. They function as initiators or co-catalysts that control molecular weight distribution, stereochemistry, and reaction kinetics. These acids can activate monomers and facilitate chain growth in processes such as cationic polymerization, coordination polymerization, and ring-opening polymerization, resulting in polymers with specific properties and structures.
- Lewis Acid-Based Materials Processing and Treatment: Lewis acids are utilized in various materials processing and treatment applications, including surface modifications, etching processes, and material transformations. These compounds can modify surface properties, enhance material performance, and facilitate specific chemical transformations in solid substrates. Applications range from semiconductor processing to metal surface treatments, where Lewis acids interact with surface functional groups to achieve desired material characteristics.
- Novel Lewis Acid Structures and Compositions: Research has led to the development of novel Lewis acid structures and compositions with enhanced properties such as stability, selectivity, and activity. These innovations include supported Lewis acids, Lewis acid-functionalized materials, and hybrid catalyst systems that combine Lewis acidity with other catalytic functionalities. Such novel structures often feature improved recyclability, reduced environmental impact, and application-specific performance characteristics compared to traditional Lewis acid catalysts.
- Lewis Acid Applications in Petroleum and Hydrocarbon Processing: Lewis acids are extensively employed in petroleum refining and hydrocarbon processing industries for reactions such as isomerization, alkylation, cracking, and reforming. These catalysts facilitate the transformation of petroleum fractions into valuable products by promoting carbon-carbon bond rearrangements and other structural modifications. The selectivity and activity of Lewis acids in these applications can be tuned by modifying their structure, concentration, and the reaction conditions.
02 Lewis acids in organic synthesis and transformations
Lewis acids play a crucial role in organic synthesis by facilitating various transformations including alkylation, acylation, and cyclization reactions. They function by accepting electron pairs from substrates, thereby activating them toward nucleophilic attack. These catalysts enable selective bond formation and can improve reaction yields while operating under milder conditions than traditional methods. Their electron-accepting properties make them valuable tools for creating complex organic molecules.Expand Specific Solutions03 Lewis acid applications in petroleum refining and hydrocarbon processing
Lewis acids are employed in petroleum refining and hydrocarbon processing industries for catalyzing cracking, isomerization, and alkylation reactions. These catalysts can transform low-value hydrocarbons into higher-value products by rearranging molecular structures. They facilitate the breaking and formation of carbon-carbon bonds in complex hydrocarbon mixtures, enabling the production of fuels and petrochemical feedstocks with desired properties.Expand Specific Solutions04 Novel Lewis acid catalyst systems and structures
Research has led to the development of novel Lewis acid catalyst systems with enhanced properties. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acids with specialized ligands. Such innovations improve catalyst efficiency, selectivity, recyclability, and stability. Advanced structural designs allow for better control over catalytic activity and enable applications in environmentally friendly processes by reducing waste and energy consumption.Expand Specific Solutions05 Lewis acids in material science and specialty applications
Lewis acids have important applications in material science and specialty chemical production. They are used in the synthesis of advanced materials including semiconductors, nanomaterials, and specialty polymers. Lewis acid-mediated processes enable the creation of materials with specific electronic, optical, or mechanical properties. These catalysts also play roles in environmental applications such as waste treatment and in the production of fine chemicals for pharmaceutical and agricultural industries.Expand Specific Solutions
Major Industry Players and Research Groups
The Lewis acid polymerization technology landscape is currently in a mature growth phase, with an estimated global market size of $15-20 billion. Major industrial players like ExxonMobil Chemical, Dow Global Technologies, and LG Chem dominate commercial applications, while academic institutions such as Beijing University of Chemical Technology and Zhejiang University drive fundamental research innovations. The competitive landscape features specialized segments: petrochemical giants (China Petroleum & Chemical Corp., NIPPON STEEL Chemical) focus on large-scale polymerization processes, while specialty chemical companies (PPG Industries, Takasago International) develop niche applications. Recent technological advancements from research collaborations between industry (Samsung Electronics, Kuraray) and academia (University of Copenhagen, Centre National de la Recherche Scientifique) are accelerating commercialization of novel catalyst systems with enhanced selectivity and environmental performance.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed proprietary metallocene catalyst systems utilizing specific Lewis acids as co-catalysts for polyolefin production. Their approach involves using perfluorinated arylboranes (particularly tris(pentafluorophenyl)borane) as Lewis acid activators that interact with metallocene pre-catalysts to generate highly active cationic species. This technology enables precise control over polymer microstructure, including molecular weight distribution, comonomer incorporation, and stereochemistry. ExxonMobil's research has demonstrated that the strength of the Lewis acid significantly impacts catalyst activity, with stronger Lewis acids generally producing more active catalyst systems but potentially causing side reactions. Their patents cover specific Lewis acid selection criteria based on fluoroaryl substitution patterns that optimize polymerization efficiency while maintaining catalyst stability.
Strengths: Superior control over polymer architecture and properties; enables production of polymers with narrow molecular weight distributions; allows polymerization under milder conditions. Weaknesses: Higher production costs compared to traditional Ziegler-Natta systems; some Lewis acid co-catalysts are moisture-sensitive requiring special handling; potential for catalyst deactivation through Lewis acid-base adduct formation.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced coordination polymerization systems utilizing tailored Lewis acids for specialty polymer production. Their approach centers on rare-earth metal catalysts activated by carefully selected Lewis acids, particularly for stereoregular polymerization of dienes and polar monomers. LG Chem's technology employs modified alkylaluminum compounds (such as methylaluminoxane and alkylaluminum chlorides) with precisely controlled Lewis acidity to achieve optimal catalyst activation while minimizing side reactions. Their research has established quantitative structure-activity relationships between Lewis acid properties (measured by Gutmann acceptor numbers and computational descriptors) and polymerization outcomes including reaction rates, molecular weight control, and stereoselectivity. LG Chem has also pioneered dual Lewis acid systems where primary and secondary Lewis acids work synergistically to enhance catalyst performance and polymer property control.
Strengths: Enables production of highly stereoregular polymers with controlled microstructure; allows polymerization of challenging monomers including polar varieties; provides excellent molecular weight control. Weaknesses: Some systems require precise temperature control to maintain optimal Lewis acid-catalyst interactions; potential for catalyst poisoning by trace impurities; higher catalyst costs compared to conventional systems.
Key Mechanisms and Structure-Activity Relationships
A cross-linkable ethylene polymer composition comprising epoxy-groups and a cross-linking agent
PatentActiveUS20210221989A1
Innovation
- An ethylene polymer composition incorporating epoxy groups and a cross-linking agent comprising organo-metallic Lewis acids, amino compounds, and hydroxyl compounds, allowing for effective cross-linking at moderate temperatures and short times, thereby reducing volatile by-products and eliminating the need for high peroxide amounts.
Conductive polymers
PatentInactiveGB2579298A
Innovation
- Integration of Lewis pair side chain moieties (comprising both Lewis acid and Lewis base components) onto a conductive polymer backbone, creating a novel hybrid material with unique electronic properties.
- Strategic electrical connection of Lewis acid moieties to the conductive backbone, enabling controlled electron transfer pathways within the polymer structure.
- Incorporation of both electron pair acceptors (Lewis acid) and electron pair donors (Lewis base) within the same polymer structure, potentially enabling self-doping capabilities.
Environmental Impact and Green Chemistry Considerations
The selection of Lewis acids for polymerization processes carries significant environmental implications that must be carefully considered in modern chemical manufacturing. Traditional Lewis acid catalysts such as aluminum chloride (AlCl3), titanium tetrachloride (TiCl4), and boron trifluoride (BF3) have long been industrial staples but present substantial environmental challenges. These compounds often require large quantities, generate significant waste streams, and pose hazards through their corrosive nature and sensitivity to moisture.
Environmental risk assessment of Lewis acids must account for their potential for air and water pollution, particularly through hydrolysis reactions that can release acidic byproducts. Metal-based Lewis acids may contribute to heavy metal contamination in waste streams, creating long-term environmental persistence issues. The energy-intensive production and purification processes for conventional Lewis acids further compound their environmental footprint through increased carbon emissions.
Green chemistry principles offer promising pathways for more sustainable Lewis acid selection in polymerization. Recyclable catalytic systems represent a significant advancement, with supported Lewis acids on silica, alumina, or polymeric substrates enabling multiple reaction cycles without significant activity loss. These heterogeneous systems facilitate easier separation from reaction mixtures and reduce waste generation substantially compared to homogeneous counterparts.
Water-compatible Lewis acids have emerged as environmentally preferable alternatives, eliminating the need for anhydrous conditions and hazardous organic solvents. Lanthanide triflates, particularly ytterbium triflate, demonstrate remarkable stability in aqueous media while maintaining high catalytic activity. Similarly, metal triflimides offer excellent catalytic performance with reduced environmental impact.
Biobased and naturally derived Lewis acids represent the frontier of green catalyst development. Certain metal complexes with ligands derived from renewable resources show promising catalytic activity while reducing dependence on petrochemical feedstocks. Iron-based Lewis acids are gaining attention as less toxic alternatives to traditional heavy metal catalysts, offering comparable reactivity with significantly reduced environmental and health concerns.
Regulatory frameworks increasingly influence Lewis acid selection, with restrictions on certain metal compounds under REACH in Europe and similar regulations globally. Forward-thinking polymerization processes now incorporate life cycle assessment methodologies to evaluate the comprehensive environmental impact of catalyst choices, from raw material extraction through disposal. This holistic approach ensures that improvements in one environmental aspect don't create unintended consequences elsewhere in the product lifecycle.
Environmental risk assessment of Lewis acids must account for their potential for air and water pollution, particularly through hydrolysis reactions that can release acidic byproducts. Metal-based Lewis acids may contribute to heavy metal contamination in waste streams, creating long-term environmental persistence issues. The energy-intensive production and purification processes for conventional Lewis acids further compound their environmental footprint through increased carbon emissions.
Green chemistry principles offer promising pathways for more sustainable Lewis acid selection in polymerization. Recyclable catalytic systems represent a significant advancement, with supported Lewis acids on silica, alumina, or polymeric substrates enabling multiple reaction cycles without significant activity loss. These heterogeneous systems facilitate easier separation from reaction mixtures and reduce waste generation substantially compared to homogeneous counterparts.
Water-compatible Lewis acids have emerged as environmentally preferable alternatives, eliminating the need for anhydrous conditions and hazardous organic solvents. Lanthanide triflates, particularly ytterbium triflate, demonstrate remarkable stability in aqueous media while maintaining high catalytic activity. Similarly, metal triflimides offer excellent catalytic performance with reduced environmental impact.
Biobased and naturally derived Lewis acids represent the frontier of green catalyst development. Certain metal complexes with ligands derived from renewable resources show promising catalytic activity while reducing dependence on petrochemical feedstocks. Iron-based Lewis acids are gaining attention as less toxic alternatives to traditional heavy metal catalysts, offering comparable reactivity with significantly reduced environmental and health concerns.
Regulatory frameworks increasingly influence Lewis acid selection, with restrictions on certain metal compounds under REACH in Europe and similar regulations globally. Forward-thinking polymerization processes now incorporate life cycle assessment methodologies to evaluate the comprehensive environmental impact of catalyst choices, from raw material extraction through disposal. This holistic approach ensures that improvements in one environmental aspect don't create unintended consequences elsewhere in the product lifecycle.
Scale-up and Industrial Implementation Strategies
Scaling up Lewis acid catalyzed polymerization processes from laboratory to industrial scale requires careful consideration of multiple factors to ensure economic viability, process safety, and product consistency. The transition demands systematic engineering approaches that address the unique challenges posed by Lewis acid catalysts in large-scale operations.
Equipment selection represents a critical decision point in scale-up efforts. Reactor materials must be compatible with the often corrosive nature of Lewis acids, with glass-lined or specialized alloy reactors frequently employed. Continuous stirred-tank reactors (CSTRs) typically offer advantages for exothermic polymerizations catalyzed by Lewis acids, while tubular reactors may be preferred for processes requiring precise temperature control and residence time distribution.
Heat management strategies become increasingly important at industrial scale, particularly for highly exothermic polymerizations. Efficient cooling systems, including external jackets, internal coils, or plate heat exchangers, must be designed to handle the specific heat release profiles of Lewis acid catalyzed reactions. For particularly challenging cases, microreactor technology offers superior heat transfer capabilities despite higher capital costs.
Catalyst handling protocols require special attention due to the moisture and air sensitivity of many Lewis acids. Industrial implementation often necessitates dedicated dry rooms or inert gas handling systems. Automated dosing systems can minimize exposure risks while ensuring precise catalyst addition rates, critical for controlling molecular weight distribution in the final polymer.
Process monitoring and control systems must be adapted for Lewis acid catalyzed polymerizations. In-line spectroscopic techniques (NIR, Raman) enable real-time monitoring of conversion rates and polymer properties. Advanced process control algorithms can adjust reaction parameters based on these measurements, maintaining consistent product quality despite inevitable variations in raw materials.
Economic considerations ultimately determine industrial viability. Catalyst recovery and recycling systems can significantly reduce operational costs, particularly for expensive Lewis acids like rare earth triflates or specialized organometallic complexes. Continuous processing often presents advantages over batch operations in terms of space-time yield and energy efficiency, though implementation complexity must be weighed against these benefits.
Environmental and safety protocols must address the specific hazards associated with Lewis acids at scale. Neutralization systems for waste streams, scrubbers for potential emissions, and comprehensive emergency response procedures are essential components of responsible industrial implementation. Regulatory compliance strategies should be developed early in the scale-up process to avoid costly redesigns.
Equipment selection represents a critical decision point in scale-up efforts. Reactor materials must be compatible with the often corrosive nature of Lewis acids, with glass-lined or specialized alloy reactors frequently employed. Continuous stirred-tank reactors (CSTRs) typically offer advantages for exothermic polymerizations catalyzed by Lewis acids, while tubular reactors may be preferred for processes requiring precise temperature control and residence time distribution.
Heat management strategies become increasingly important at industrial scale, particularly for highly exothermic polymerizations. Efficient cooling systems, including external jackets, internal coils, or plate heat exchangers, must be designed to handle the specific heat release profiles of Lewis acid catalyzed reactions. For particularly challenging cases, microreactor technology offers superior heat transfer capabilities despite higher capital costs.
Catalyst handling protocols require special attention due to the moisture and air sensitivity of many Lewis acids. Industrial implementation often necessitates dedicated dry rooms or inert gas handling systems. Automated dosing systems can minimize exposure risks while ensuring precise catalyst addition rates, critical for controlling molecular weight distribution in the final polymer.
Process monitoring and control systems must be adapted for Lewis acid catalyzed polymerizations. In-line spectroscopic techniques (NIR, Raman) enable real-time monitoring of conversion rates and polymer properties. Advanced process control algorithms can adjust reaction parameters based on these measurements, maintaining consistent product quality despite inevitable variations in raw materials.
Economic considerations ultimately determine industrial viability. Catalyst recovery and recycling systems can significantly reduce operational costs, particularly for expensive Lewis acids like rare earth triflates or specialized organometallic complexes. Continuous processing often presents advantages over batch operations in terms of space-time yield and energy efficiency, though implementation complexity must be weighed against these benefits.
Environmental and safety protocols must address the specific hazards associated with Lewis acids at scale. Neutralization systems for waste streams, scrubbers for potential emissions, and comprehensive emergency response procedures are essential components of responsible industrial implementation. Regulatory compliance strategies should be developed early in the scale-up process to avoid costly redesigns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!