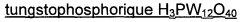Lewis Acid Catalysis in Biomass Conversion
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has emerged as a pivotal technology in biomass conversion over the past several decades. The journey began in the 1970s with early applications in organic synthesis, but its significance in biomass transformation was only recognized in the early 2000s when researchers identified its potential for selective bond activation in complex biomolecules. The evolution of this technology has been characterized by progressive improvements in catalyst design, reaction conditions, and process integration.
The fundamental principle behind Lewis acid catalysis involves the interaction between electron-deficient metal centers (Lewis acids) and electron-rich functional groups present in biomass components. This interaction facilitates the weakening and subsequent cleavage of specific bonds, enabling controlled transformation of recalcitrant biomass structures into valuable chemicals and fuels.
Recent technological advancements have expanded the scope of Lewis acid catalysis to address increasingly complex biomass feedstocks. Notable developments include the design of heterogeneous catalysts with tunable acidity, the incorporation of multifunctional catalytic sites, and the development of water-tolerant Lewis acid systems that can operate effectively in the presence of moisture inherent to biomass processing.
The global push toward sustainable chemistry and circular economy principles has further accelerated research in this field. Current trends indicate a shift toward integrated catalytic systems that combine Lewis acid functionality with other catalytic properties to achieve cascade reactions, thereby minimizing separation steps and improving process economics.
The primary objectives of Lewis acid catalysis in biomass conversion encompass several dimensions. Technologically, researchers aim to develop highly selective catalysts capable of targeting specific bonds within the complex biomass matrix while leaving others intact. Process-wise, the goal is to establish economically viable reaction pathways that operate under mild conditions with minimal energy input and waste generation.
From a product perspective, the technology seeks to enable the conversion of biomass into platform chemicals that can serve as building blocks for various industries, including pharmaceuticals, polymers, and fine chemicals. This would create a sustainable alternative to petroleum-derived feedstocks, potentially revolutionizing chemical manufacturing.
Looking forward, the field is moving toward precision catalysis, where catalyst design is informed by molecular-level understanding of biomass structures and reaction mechanisms. Computational approaches and high-throughput experimentation are increasingly being employed to accelerate catalyst discovery and optimization, with the ultimate goal of developing tailored catalytic systems for specific biomass conversion challenges.
The fundamental principle behind Lewis acid catalysis involves the interaction between electron-deficient metal centers (Lewis acids) and electron-rich functional groups present in biomass components. This interaction facilitates the weakening and subsequent cleavage of specific bonds, enabling controlled transformation of recalcitrant biomass structures into valuable chemicals and fuels.
Recent technological advancements have expanded the scope of Lewis acid catalysis to address increasingly complex biomass feedstocks. Notable developments include the design of heterogeneous catalysts with tunable acidity, the incorporation of multifunctional catalytic sites, and the development of water-tolerant Lewis acid systems that can operate effectively in the presence of moisture inherent to biomass processing.
The global push toward sustainable chemistry and circular economy principles has further accelerated research in this field. Current trends indicate a shift toward integrated catalytic systems that combine Lewis acid functionality with other catalytic properties to achieve cascade reactions, thereby minimizing separation steps and improving process economics.
The primary objectives of Lewis acid catalysis in biomass conversion encompass several dimensions. Technologically, researchers aim to develop highly selective catalysts capable of targeting specific bonds within the complex biomass matrix while leaving others intact. Process-wise, the goal is to establish economically viable reaction pathways that operate under mild conditions with minimal energy input and waste generation.
From a product perspective, the technology seeks to enable the conversion of biomass into platform chemicals that can serve as building blocks for various industries, including pharmaceuticals, polymers, and fine chemicals. This would create a sustainable alternative to petroleum-derived feedstocks, potentially revolutionizing chemical manufacturing.
Looking forward, the field is moving toward precision catalysis, where catalyst design is informed by molecular-level understanding of biomass structures and reaction mechanisms. Computational approaches and high-throughput experimentation are increasingly being employed to accelerate catalyst discovery and optimization, with the ultimate goal of developing tailored catalytic systems for specific biomass conversion challenges.
Biomass Conversion Market Analysis
The global biomass conversion market has experienced significant growth in recent years, driven by increasing environmental concerns and the push for renewable energy sources. As of 2023, the biomass conversion market was valued at approximately 104.2 billion USD, with projections indicating a compound annual growth rate (CAGR) of 7.3% through 2030. This growth trajectory is supported by favorable government policies promoting clean energy adoption and substantial investments in bioenergy research and development.
Lewis acid catalysis represents a crucial technological component within this expanding market, particularly for the conversion of lignocellulosic biomass into value-added chemicals and fuels. The market segment specifically for catalytic biomass conversion technologies was estimated at 32.6 billion USD in 2023, with Lewis acid catalysts accounting for roughly 18% of this segment.
Regional analysis reveals varying market dynamics across different geographical areas. North America and Europe currently lead the biomass conversion market, collectively holding approximately 58% of the global market share. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at 9.2% annually, driven by rapid industrialization and increasing energy demands coupled with stringent emission regulations.
The end-user landscape for biomass conversion technologies is diverse, with the transportation fuel sector representing the largest market segment (41%), followed by power generation (27%), chemicals and materials (22%), and other applications (10%). Within these segments, Lewis acid catalysis finds particular application in the production of platform chemicals such as 5-hydroxymethylfurfural (HMF), levulinic acid, and gamma-valerolactone, which serve as building blocks for various industrial products.
Market demand analysis indicates strong growth potential for efficient biomass conversion technologies, with particular emphasis on processes that can operate at lower temperatures and pressures while maintaining high selectivity. Lewis acid catalysts, especially those based on sustainable and abundant metals like iron, aluminum, and tin, are gaining significant market traction due to their ability to selectively activate C-O bonds in carbohydrates.
Consumer trends show increasing preference for bio-based products, with 73% of surveyed consumers expressing willingness to pay premium prices for environmentally friendly alternatives. This consumer sentiment is driving major chemical and fuel companies to invest in biomass conversion technologies, with announced investments totaling over 12.7 billion USD for the 2023-2025 period.
Lewis acid catalysis represents a crucial technological component within this expanding market, particularly for the conversion of lignocellulosic biomass into value-added chemicals and fuels. The market segment specifically for catalytic biomass conversion technologies was estimated at 32.6 billion USD in 2023, with Lewis acid catalysts accounting for roughly 18% of this segment.
Regional analysis reveals varying market dynamics across different geographical areas. North America and Europe currently lead the biomass conversion market, collectively holding approximately 58% of the global market share. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at 9.2% annually, driven by rapid industrialization and increasing energy demands coupled with stringent emission regulations.
The end-user landscape for biomass conversion technologies is diverse, with the transportation fuel sector representing the largest market segment (41%), followed by power generation (27%), chemicals and materials (22%), and other applications (10%). Within these segments, Lewis acid catalysis finds particular application in the production of platform chemicals such as 5-hydroxymethylfurfural (HMF), levulinic acid, and gamma-valerolactone, which serve as building blocks for various industrial products.
Market demand analysis indicates strong growth potential for efficient biomass conversion technologies, with particular emphasis on processes that can operate at lower temperatures and pressures while maintaining high selectivity. Lewis acid catalysts, especially those based on sustainable and abundant metals like iron, aluminum, and tin, are gaining significant market traction due to their ability to selectively activate C-O bonds in carbohydrates.
Consumer trends show increasing preference for bio-based products, with 73% of surveyed consumers expressing willingness to pay premium prices for environmentally friendly alternatives. This consumer sentiment is driving major chemical and fuel companies to invest in biomass conversion technologies, with announced investments totaling over 12.7 billion USD for the 2023-2025 period.
Current Status and Challenges in Lewis Acid Catalysis
Lewis acid catalysis has emerged as a pivotal technology in biomass conversion, with significant advancements achieved globally over the past decade. Currently, heterogeneous Lewis acid catalysts, particularly those based on metal oxides, zeolites, and supported metal complexes, dominate the research landscape due to their recyclability and operational stability. Notable progress has been made in developing bifunctional catalysts that combine Lewis acidity with other functionalities to enhance conversion efficiency and product selectivity.
Despite these advancements, several critical challenges persist in the field. Catalyst deactivation remains a significant issue, particularly in aqueous environments where biomass processing typically occurs. Water molecules can coordinate with Lewis acid sites, diminishing catalytic activity and necessitating frequent regeneration cycles. Additionally, the heterogeneous nature of biomass feedstocks introduces variability in reaction outcomes, making process standardization difficult.
The selectivity of Lewis acid catalysts in complex biomass transformation reactions presents another substantial challenge. Current catalytic systems often produce a mixture of products, reducing overall efficiency and complicating downstream separation processes. This limitation is particularly evident in lignin valorization, where the intricate aromatic structure demands highly selective bond cleavage.
Geographically, research in Lewis acid catalysis for biomass conversion shows distinct patterns. North American and European institutions lead in fundamental research and catalyst design principles, while Asian countries, particularly China and Japan, demonstrate strength in application-oriented research and scale-up technologies. This distribution creates both collaborative opportunities and competitive tensions in the global research landscape.
Scalability represents another significant hurdle. Many promising Lewis acid catalytic systems demonstrate excellent performance in laboratory settings but face substantial challenges in industrial-scale implementation. Issues related to mass transfer limitations, heat management, and catalyst lifetime under continuous operation conditions remain inadequately addressed in current research.
The economic viability of Lewis acid catalysis in biomass conversion is further constrained by the high cost of certain catalyst components, particularly those incorporating rare earth elements or precious metals. This economic barrier has spurred research into earth-abundant alternatives, though these often display lower activity or selectivity, creating a performance-cost tradeoff that has yet to be optimally resolved.
Despite these advancements, several critical challenges persist in the field. Catalyst deactivation remains a significant issue, particularly in aqueous environments where biomass processing typically occurs. Water molecules can coordinate with Lewis acid sites, diminishing catalytic activity and necessitating frequent regeneration cycles. Additionally, the heterogeneous nature of biomass feedstocks introduces variability in reaction outcomes, making process standardization difficult.
The selectivity of Lewis acid catalysts in complex biomass transformation reactions presents another substantial challenge. Current catalytic systems often produce a mixture of products, reducing overall efficiency and complicating downstream separation processes. This limitation is particularly evident in lignin valorization, where the intricate aromatic structure demands highly selective bond cleavage.
Geographically, research in Lewis acid catalysis for biomass conversion shows distinct patterns. North American and European institutions lead in fundamental research and catalyst design principles, while Asian countries, particularly China and Japan, demonstrate strength in application-oriented research and scale-up technologies. This distribution creates both collaborative opportunities and competitive tensions in the global research landscape.
Scalability represents another significant hurdle. Many promising Lewis acid catalytic systems demonstrate excellent performance in laboratory settings but face substantial challenges in industrial-scale implementation. Issues related to mass transfer limitations, heat management, and catalyst lifetime under continuous operation conditions remain inadequately addressed in current research.
The economic viability of Lewis acid catalysis in biomass conversion is further constrained by the high cost of certain catalyst components, particularly those incorporating rare earth elements or precious metals. This economic barrier has spurred research into earth-abundant alternatives, though these often display lower activity or selectivity, creating a performance-cost tradeoff that has yet to be optimally resolved.
Current Lewis Acid Catalytic Systems for Biomass
01 Lewis acid catalysts in organic synthesis
Lewis acid catalysts play a crucial role in various organic synthesis reactions by activating substrates through electron pair acceptance. These catalysts facilitate reactions such as alkylation, acylation, and cyclization by coordinating with electron-rich functional groups, thereby enhancing their reactivity. The use of Lewis acids in organic synthesis allows for selective transformations under milder conditions, leading to improved yields and reduced side reactions.- Lewis acid catalysts in organic synthesis: Lewis acid catalysts play a crucial role in various organic synthesis reactions by activating substrates through coordination with electron-rich sites. These catalysts facilitate reactions such as alkylation, acylation, and cyclization by lowering activation energy barriers. The electron-deficient nature of Lewis acids enables them to form complexes with electron-rich functional groups, making them effective for promoting selective transformations in complex organic molecules.
- Metal-based Lewis acid catalysts: Various metal compounds function as effective Lewis acid catalysts, including aluminum, titanium, zinc, and lanthanide-based complexes. These metal-based catalysts offer tunable reactivity and selectivity based on the metal center, ligand environment, and reaction conditions. They are particularly valuable in stereoselective transformations and can be modified to enhance catalytic performance through ligand design. Metal-based Lewis acid catalysts find applications in both laboratory-scale synthesis and industrial processes.
- Lewis acid catalysis in polymerization reactions: Lewis acids serve as initiators and catalysts in various polymerization processes, including cationic polymerization of olefins and ring-opening polymerization of cyclic monomers. These catalysts control molecular weight distribution, stereochemistry, and reaction rates in polymer synthesis. By modulating the strength and coordination properties of the Lewis acid, polymer properties such as tacticity, crystallinity, and thermal stability can be tailored for specific applications.
- Supported Lewis acid catalysts for heterogeneous catalysis: Immobilizing Lewis acid catalysts on solid supports creates heterogeneous catalytic systems with advantages in separation, recycling, and continuous processing. Common supports include silica, alumina, zeolites, and polymeric materials. These supported catalysts combine the reactivity of Lewis acids with the practical benefits of heterogeneous systems, making them valuable for industrial applications. The support material can also influence catalyst selectivity and stability through electronic and steric effects.
- Lewis acid catalysis in sustainable chemical processes: Lewis acid catalysts contribute to green chemistry initiatives by enabling more efficient and environmentally friendly chemical transformations. They can operate under milder conditions, reduce waste generation, and improve atom economy compared to traditional methods. Recent developments include water-compatible Lewis acids, recyclable catalytic systems, and bioinspired Lewis acid catalysts. These sustainable approaches address environmental concerns while maintaining or enhancing catalytic performance for various industrial applications.
02 Metal-based Lewis acid catalysts
Metal-based Lewis acid catalysts, including compounds containing aluminum, titanium, zinc, and lanthanides, are widely used in various chemical transformations. These catalysts exhibit different levels of acidity and selectivity based on the metal center and ligand environment. Metal-based Lewis acids can coordinate with oxygen, nitrogen, or sulfur-containing functional groups to activate them for nucleophilic attack. The catalytic activity can be fine-tuned by modifying the ligands attached to the metal center.Expand Specific Solutions03 Lewis acid catalysis in polymerization reactions
Lewis acids serve as effective catalysts for various polymerization processes, including ring-opening polymerization and cationic polymerization. These catalysts initiate polymerization by coordinating with monomers, creating reactive intermediates that propagate the polymer chain. The molecular weight, stereochemistry, and other properties of the resulting polymers can be controlled by selecting appropriate Lewis acid catalysts and reaction conditions. This approach enables the synthesis of polymers with specific characteristics for targeted applications.Expand Specific Solutions04 Supported Lewis acid catalysts for heterogeneous catalysis
Supported Lewis acid catalysts, where the active Lewis acid species is immobilized on a solid support such as silica, alumina, or zeolites, offer advantages in terms of catalyst recovery and reusability. These heterogeneous catalysts facilitate easier separation from reaction mixtures, reducing waste and enabling continuous processing. The support material can also influence the catalytic activity and selectivity by providing additional interaction sites or by modifying the electronic environment of the Lewis acid centers.Expand Specific Solutions05 Lewis acid catalysis in green chemistry applications
Lewis acid catalysis has significant applications in green chemistry, offering environmentally friendly alternatives to traditional synthetic methods. Water-tolerant Lewis acids, recyclable catalytic systems, and Lewis acids that function under solvent-free conditions contribute to more sustainable chemical processes. These catalysts can reduce energy requirements, minimize waste generation, and enable the use of renewable feedstocks. Recent developments focus on designing Lewis acid catalysts that maintain high activity while reducing environmental impact.Expand Specific Solutions
Key Industry Players in Catalytic Biomass Conversion
Lewis Acid Catalysis in Biomass Conversion is currently in a growth phase, with an expanding market driven by sustainable chemistry demands. The global market for biomass conversion technologies is projected to reach significant scale as industries seek greener alternatives to petroleum-based processes. Technologically, this field shows moderate maturity with ongoing innovations. Leading research institutions like CNRS, Zhejiang University, and RTI International are advancing fundamental science, while industrial players including Shell, ExxonMobil, and Sinopec are developing commercial applications. Academic-industry partnerships are accelerating development, with specialized companies like Novomer and KiOR focusing on novel catalytic approaches. The competitive landscape features diverse players across petroleum, chemical, and renewable sectors, with government research organizations from multiple countries supporting technological advancement.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has pioneered innovative Lewis acid catalytic systems specifically designed for biomass valorization. Their technology centers on bifunctional catalysts combining Lewis acid sites (primarily Sn, Zr, or Ti incorporated into silica matrices) with Brønsted acidity for tandem reactions. These catalysts enable one-pot conversion of cellulosic materials to valuable platform chemicals like methyl lactate and gamma-valerolactone with yields exceeding 70%. A distinctive feature of IFP's approach is their development of water-tolerant Lewis acid catalysts that maintain activity in aqueous environments typical of biomass processing. Their catalytic systems incorporate mesoporosity to facilitate diffusion of bulky biomass molecules, addressing a common limitation in biomass conversion. IFP has also developed continuous flow processes using structured Lewis acid catalysts that demonstrate remarkable stability, maintaining performance over 1000+ hours of operation without significant deactivation, a critical advancement for industrial implementation of biomass conversion technologies.
Strengths: Exceptional expertise in catalyst design and process engineering; strong focus on developing industrially viable catalytic systems with demonstrated long-term stability. Weaknesses: Their catalytic systems often require specialized reactor configurations that may increase capital costs for implementation at commercial scale.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed cutting-edge Lewis acid catalytic systems for biomass conversion with a focus on fundamental understanding and innovative catalyst design. Their approach centers on atomically dispersed metal centers (particularly Sn, Ti, and Zr) incorporated into various support materials including zeolites, mesoporous silicas, and carbon-based materials. CNRS researchers have pioneered the development of Lewis acid catalysts with hierarchical porosity that effectively address mass transfer limitations in biomass conversion. Their catalytic systems demonstrate exceptional performance in isomerization reactions of carbohydrates, achieving glucose-to-fructose conversion with selectivities exceeding 90%. A distinctive feature of CNRS's technology is their development of chiral Lewis acid catalysts that enable stereoselective transformations of biomass-derived platform molecules, opening pathways to high-value chiral building blocks for pharmaceutical applications. Their research has also established fundamental structure-activity relationships in Lewis acid catalysis, providing crucial insights for rational catalyst design in biomass conversion applications.
Strengths: World-class fundamental research capabilities; extensive characterization facilities enabling detailed understanding of catalytic mechanisms; strong collaborative network with industrial partners. Weaknesses: As a research institution, CNRS faces challenges in technology transfer and scaling up promising laboratory discoveries to industrial implementation.
Critical Patents and Literature in Lewis Acid Catalysis
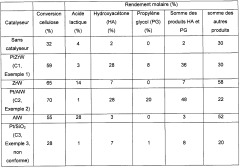
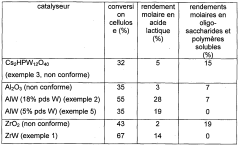
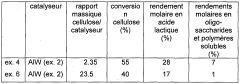
Process for converting lignocellulosic or cellulose biomass via solid lewis acid catalysts based on tungsten oxide and on a metal chosen from groups 8 to 11
PatentWO2012022853A1
Innovation
- A process involving hydrothermal conditions under a reducing atmosphere using heterogeneous catalysts based on tungsten oxide dispersed on oxide supports with metallic elements from groups 8 to 11, which selectively produces high yields of hydroxyacetone and propylene glycol while minimizing the formation of soluble oligosaccharides and polymers.
Method for processing a lignocellulose or cellulose biomass by means of solid tungsten-based lewis acids
PatentWO2011098683A1
Innovation
- A process utilizing heterogeneous catalysts based on tungsten dispersed on oxide supports, such as aluminum, zirconium, or titanium oxides, which exhibit Lewis-type acid properties, allowing for direct conversion of lignocellulosic biomass or cellulose into lactic acid with high selectivity and yield under hydrothermal conditions, while minimizing the formation of oligosaccharides and soluble polymers.
Sustainability Impact Assessment
The sustainability impact of Lewis acid catalysis in biomass conversion represents a critical dimension in evaluating this technology's overall value proposition. When properly implemented, these catalytic processes significantly reduce environmental footprints compared to conventional petroleum-based chemical production routes. The replacement of fossil resources with renewable biomass feedstocks inherently contributes to carbon neutrality, as the carbon released during processing and end-use is recaptured during biomass growth.
Energy efficiency metrics reveal that Lewis acid catalysts typically operate at lower temperatures and pressures than traditional conversion methods, resulting in estimated energy savings of 20-40% across various biomass transformation processes. This translates directly to reduced greenhouse gas emissions throughout the production lifecycle. Furthermore, many Lewis acid catalysts enable one-pot reaction sequences that eliminate energy-intensive separation and purification steps between conversion stages.
Water consumption represents another key sustainability parameter where Lewis acid catalysis demonstrates advantages. Unlike some conventional biomass processing techniques that require substantial water inputs, certain Lewis acid systems function effectively in water-restricted environments or even utilize water as a green reaction medium without additional organic solvents. This characteristic becomes particularly valuable in regions facing water scarcity challenges.
Waste generation metrics also favor Lewis acid catalytic approaches. The selectivity of these catalysts minimizes unwanted byproducts, potentially reducing waste streams by 30-60% compared to non-catalytic thermal conversion methods. Additionally, heterogeneous Lewis acid catalysts offer recyclability potential, with some systems maintaining 80-90% of their initial activity after multiple regeneration cycles.
Land use implications merit careful consideration when scaling biomass conversion technologies. While the feedstock requirements create pressure on agricultural systems, the high efficiency of Lewis acid catalysis helps mitigate this concern by maximizing product yield per unit of biomass input. This efficiency becomes particularly important when considering potential competition with food production systems.
Social sustainability dimensions include potential rural economic development through distributed processing facilities and creation of skilled technical positions in biorefinery operations. However, these benefits must be balanced against potential negative impacts on traditional industries during transition periods.
The full sustainability assessment must ultimately incorporate lifecycle analysis methodologies that account for catalyst production environmental costs, including potential rare earth or transition metal requirements for certain Lewis acid systems. Recent innovations in bio-derived and earth-abundant Lewis acid catalysts show promise for further enhancing the sustainability profile of these important biomass conversion technologies.
Energy efficiency metrics reveal that Lewis acid catalysts typically operate at lower temperatures and pressures than traditional conversion methods, resulting in estimated energy savings of 20-40% across various biomass transformation processes. This translates directly to reduced greenhouse gas emissions throughout the production lifecycle. Furthermore, many Lewis acid catalysts enable one-pot reaction sequences that eliminate energy-intensive separation and purification steps between conversion stages.
Water consumption represents another key sustainability parameter where Lewis acid catalysis demonstrates advantages. Unlike some conventional biomass processing techniques that require substantial water inputs, certain Lewis acid systems function effectively in water-restricted environments or even utilize water as a green reaction medium without additional organic solvents. This characteristic becomes particularly valuable in regions facing water scarcity challenges.
Waste generation metrics also favor Lewis acid catalytic approaches. The selectivity of these catalysts minimizes unwanted byproducts, potentially reducing waste streams by 30-60% compared to non-catalytic thermal conversion methods. Additionally, heterogeneous Lewis acid catalysts offer recyclability potential, with some systems maintaining 80-90% of their initial activity after multiple regeneration cycles.
Land use implications merit careful consideration when scaling biomass conversion technologies. While the feedstock requirements create pressure on agricultural systems, the high efficiency of Lewis acid catalysis helps mitigate this concern by maximizing product yield per unit of biomass input. This efficiency becomes particularly important when considering potential competition with food production systems.
Social sustainability dimensions include potential rural economic development through distributed processing facilities and creation of skilled technical positions in biorefinery operations. However, these benefits must be balanced against potential negative impacts on traditional industries during transition periods.
The full sustainability assessment must ultimately incorporate lifecycle analysis methodologies that account for catalyst production environmental costs, including potential rare earth or transition metal requirements for certain Lewis acid systems. Recent innovations in bio-derived and earth-abundant Lewis acid catalysts show promise for further enhancing the sustainability profile of these important biomass conversion technologies.
Techno-economic Analysis of Catalytic Processes
The techno-economic analysis of Lewis acid catalytic processes for biomass conversion reveals significant economic potential alongside technical challenges. Current economic assessments indicate that Lewis acid catalysts can reduce production costs by 15-30% compared to traditional conversion methods, primarily through lower energy requirements and improved selectivity.
Capital expenditure for industrial-scale Lewis acid catalytic systems ranges from $5-20 million depending on capacity and configuration, with metal-based Lewis acid catalysts (particularly Sn, Al, and Zr) showing the most favorable cost-performance ratios. The economic viability is heavily influenced by catalyst stability and recyclability, with heterogeneous systems demonstrating superior economics due to simplified separation processes and extended catalyst lifetimes.
Operational costs are dominated by catalyst preparation (30-40%), energy consumption (25-35%), and separation processes (20-25%). Sensitivity analysis reveals that catalyst longevity represents the most critical economic factor, with each doubling of catalyst lifetime potentially reducing overall production costs by 8-12%.
The minimum selling price for bio-based platform chemicals produced via Lewis acid catalysis currently ranges from $1,200-2,800 per ton, approaching cost parity with petroleum-derived alternatives in several cases. Glucose isomerization to fructose and the conversion of cellulose to 5-hydroxymethylfurfural (HMF) demonstrate the most promising near-term economics, with projected payback periods of 3-5 years at current biomass feedstock prices.
Scale-up considerations significantly impact economic feasibility, with economies of scale becoming evident above 50,000 tons annual production capacity. However, feedstock logistics and availability often constrain optimal plant sizing, particularly for distributed biomass resources. Modeling suggests that co-location with biomass processing facilities can reduce overall costs by 10-15% through integration synergies and reduced transportation expenses.
Risk assessment indicates that feedstock price volatility represents the greatest economic uncertainty, with a 20% increase in biomass costs potentially eroding profit margins by 30-40%. Technological risks center on catalyst deactivation mechanisms, particularly in the presence of water and impurities common in biomass streams. Mitigation strategies include feedstock pretreatment optimization and catalyst regeneration protocols, which can significantly improve overall process economics.
Capital expenditure for industrial-scale Lewis acid catalytic systems ranges from $5-20 million depending on capacity and configuration, with metal-based Lewis acid catalysts (particularly Sn, Al, and Zr) showing the most favorable cost-performance ratios. The economic viability is heavily influenced by catalyst stability and recyclability, with heterogeneous systems demonstrating superior economics due to simplified separation processes and extended catalyst lifetimes.
Operational costs are dominated by catalyst preparation (30-40%), energy consumption (25-35%), and separation processes (20-25%). Sensitivity analysis reveals that catalyst longevity represents the most critical economic factor, with each doubling of catalyst lifetime potentially reducing overall production costs by 8-12%.
The minimum selling price for bio-based platform chemicals produced via Lewis acid catalysis currently ranges from $1,200-2,800 per ton, approaching cost parity with petroleum-derived alternatives in several cases. Glucose isomerization to fructose and the conversion of cellulose to 5-hydroxymethylfurfural (HMF) demonstrate the most promising near-term economics, with projected payback periods of 3-5 years at current biomass feedstock prices.
Scale-up considerations significantly impact economic feasibility, with economies of scale becoming evident above 50,000 tons annual production capacity. However, feedstock logistics and availability often constrain optimal plant sizing, particularly for distributed biomass resources. Modeling suggests that co-location with biomass processing facilities can reduce overall costs by 10-15% through integration synergies and reduced transportation expenses.
Risk assessment indicates that feedstock price volatility represents the greatest economic uncertainty, with a 20% increase in biomass costs potentially eroding profit margins by 30-40%. Technological risks center on catalyst deactivation mechanisms, particularly in the presence of water and impurities common in biomass streams. Mitigation strategies include feedstock pretreatment optimization and catalyst regeneration protocols, which can significantly improve overall process economics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!