How to Compare Lewis Acid Strengths in Different Solvents?
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Solvent Interaction Background and Objectives
Lewis acids, first conceptualized by Gilbert N. Lewis in 1923, represent a fundamental class of chemical species that accept electron pairs. The interaction between Lewis acids and solvents has been a critical area of study in physical organic chemistry for decades, with significant implications for catalysis, synthesis, and materials science. Understanding how solvent environments affect Lewis acid behavior is essential for predicting and controlling chemical reactions across diverse applications.
The evolution of Lewis acid chemistry has progressed from simple qualitative observations to sophisticated quantitative measurements. Early research focused primarily on gas-phase acidity measurements, which provided valuable baseline data but failed to capture the complex dynamics present in solution. The field has since expanded to incorporate advanced spectroscopic techniques, computational modeling, and thermodynamic analyses to better characterize Lewis acid-solvent interactions.
Current technological trends point toward developing more precise methods for comparing Lewis acid strengths across different solvent systems. This includes the application of NMR spectroscopy to monitor coordination events, calorimetric measurements to quantify binding energies, and computational approaches that model solvent effects at the molecular level. The integration of machine learning algorithms has further accelerated progress by enabling the prediction of Lewis acid behavior in novel solvent environments.
The primary objective of this technical investigation is to establish reliable methodologies for comparing Lewis acid strengths across diverse solvent systems. This includes developing standardized protocols that account for solvent-specific effects such as dielectric constant, donor number, and coordination ability. Additionally, we aim to identify patterns and principles that govern Lewis acid-solvent interactions, potentially leading to predictive models applicable across chemical disciplines.
Secondary goals include mapping the relationship between molecular structure and Lewis acidity in various solvents, quantifying the impact of solvent parameters on Lewis acid strength, and exploring how these interactions can be leveraged for specific applications in catalysis, separation processes, and materials development. Understanding these relationships will enable more rational design of chemical systems where Lewis acidity plays a crucial role.
The broader impact of this research extends beyond fundamental chemistry into practical applications including pharmaceutical development, green chemistry processes, and advanced materials synthesis. By establishing clear methodologies for comparing Lewis acid strengths across solvent environments, we can optimize reaction conditions, develop more efficient catalysts, and design novel materials with tailored properties.
The evolution of Lewis acid chemistry has progressed from simple qualitative observations to sophisticated quantitative measurements. Early research focused primarily on gas-phase acidity measurements, which provided valuable baseline data but failed to capture the complex dynamics present in solution. The field has since expanded to incorporate advanced spectroscopic techniques, computational modeling, and thermodynamic analyses to better characterize Lewis acid-solvent interactions.
Current technological trends point toward developing more precise methods for comparing Lewis acid strengths across different solvent systems. This includes the application of NMR spectroscopy to monitor coordination events, calorimetric measurements to quantify binding energies, and computational approaches that model solvent effects at the molecular level. The integration of machine learning algorithms has further accelerated progress by enabling the prediction of Lewis acid behavior in novel solvent environments.
The primary objective of this technical investigation is to establish reliable methodologies for comparing Lewis acid strengths across diverse solvent systems. This includes developing standardized protocols that account for solvent-specific effects such as dielectric constant, donor number, and coordination ability. Additionally, we aim to identify patterns and principles that govern Lewis acid-solvent interactions, potentially leading to predictive models applicable across chemical disciplines.
Secondary goals include mapping the relationship between molecular structure and Lewis acidity in various solvents, quantifying the impact of solvent parameters on Lewis acid strength, and exploring how these interactions can be leveraged for specific applications in catalysis, separation processes, and materials development. Understanding these relationships will enable more rational design of chemical systems where Lewis acidity plays a crucial role.
The broader impact of this research extends beyond fundamental chemistry into practical applications including pharmaceutical development, green chemistry processes, and advanced materials synthesis. By establishing clear methodologies for comparing Lewis acid strengths across solvent environments, we can optimize reaction conditions, develop more efficient catalysts, and design novel materials with tailored properties.
Market Applications of Lewis Acid Strength Measurements
The accurate measurement of Lewis acid strengths across different solvent environments has significant market applications spanning multiple industries. In the pharmaceutical sector, Lewis acid catalysts play a crucial role in drug synthesis, particularly in asymmetric reactions that produce chiral compounds. The ability to precisely compare and select appropriate Lewis acids based on their behavior in specific solvents directly impacts reaction efficiency, yield, and product purity, ultimately reducing production costs and time-to-market for new medications.
In the polymer industry, Lewis acid strength measurements enable manufacturers to optimize polymerization processes. By understanding how solvent environments affect catalyst performance, companies can develop tailored polymer materials with specific properties such as tensile strength, thermal stability, and biodegradability. This knowledge has led to innovations in sustainable packaging materials and high-performance composites used in automotive and aerospace applications.
The petrochemical industry leverages Lewis acid strength comparisons for refining processes and fuel production. Accurate measurements help in selecting optimal catalysts for isomerization, alkylation, and cracking reactions, resulting in improved fuel quality and reduced environmental impact. Companies that master this technology gain competitive advantages through enhanced process efficiency and reduced energy consumption.
Electronic materials manufacturing represents another high-value application area. The production of semiconductors, display technologies, and advanced electronic components often involves Lewis acid-mediated reactions in specialized solvent systems. Precise control of Lewis acidity enables the creation of materials with tailored electronic properties, supporting innovation in consumer electronics, medical devices, and communication technologies.
Agricultural chemical development benefits from Lewis acid strength measurements in the formulation of more effective and environmentally friendly pesticides and fertilizers. Understanding solvent effects on Lewis acidity helps in designing controlled-release formulations that minimize environmental impact while maximizing crop protection.
The fine chemicals industry utilizes this knowledge for producing specialty chemicals, flavors, fragrances, and cosmetic ingredients. Companies that can accurately measure and predict Lewis acid behavior across different solvents gain advantages in developing proprietary synthesis routes with improved selectivity and reduced waste generation.
Analytical and laboratory equipment manufacturers have developed a market niche for instruments specifically designed to measure Lewis acid strengths in various solvents, serving research institutions and industrial R&D departments with specialized tools that enable innovation across multiple sectors.
In the polymer industry, Lewis acid strength measurements enable manufacturers to optimize polymerization processes. By understanding how solvent environments affect catalyst performance, companies can develop tailored polymer materials with specific properties such as tensile strength, thermal stability, and biodegradability. This knowledge has led to innovations in sustainable packaging materials and high-performance composites used in automotive and aerospace applications.
The petrochemical industry leverages Lewis acid strength comparisons for refining processes and fuel production. Accurate measurements help in selecting optimal catalysts for isomerization, alkylation, and cracking reactions, resulting in improved fuel quality and reduced environmental impact. Companies that master this technology gain competitive advantages through enhanced process efficiency and reduced energy consumption.
Electronic materials manufacturing represents another high-value application area. The production of semiconductors, display technologies, and advanced electronic components often involves Lewis acid-mediated reactions in specialized solvent systems. Precise control of Lewis acidity enables the creation of materials with tailored electronic properties, supporting innovation in consumer electronics, medical devices, and communication technologies.
Agricultural chemical development benefits from Lewis acid strength measurements in the formulation of more effective and environmentally friendly pesticides and fertilizers. Understanding solvent effects on Lewis acidity helps in designing controlled-release formulations that minimize environmental impact while maximizing crop protection.
The fine chemicals industry utilizes this knowledge for producing specialty chemicals, flavors, fragrances, and cosmetic ingredients. Companies that can accurately measure and predict Lewis acid behavior across different solvents gain advantages in developing proprietary synthesis routes with improved selectivity and reduced waste generation.
Analytical and laboratory equipment manufacturers have developed a market niche for instruments specifically designed to measure Lewis acid strengths in various solvents, serving research institutions and industrial R&D departments with specialized tools that enable innovation across multiple sectors.
Current Methodologies and Challenges in Comparative Analysis
The comparison of Lewis acid strengths across different solvents presents significant methodological challenges due to the complex interplay between solvent properties and acid-base interactions. Current analytical approaches primarily rely on spectroscopic techniques, computational methods, and experimental reaction kinetics studies, each with distinct advantages and limitations.
Spectroscopic methods, particularly NMR spectroscopy, have emerged as powerful tools for quantifying Lewis acidity. The Gutmann-Beckett method, which measures 31P NMR chemical shifts of triethylphosphine oxide when coordinated to Lewis acids, provides a standardized approach for comparing relative acidities. However, this method faces challenges in highly polar or protic solvents where competitive coordination with the solvent molecules can significantly alter the observed measurements.
Fluoride ion affinity (FIA) calculations represent another widely adopted computational approach. These calculations estimate the enthalpy of fluoride binding to Lewis acids, offering a theoretical measure of acid strength. While valuable in solvent-free environments, FIA values require substantial correction factors when applied to solution-phase chemistry, limiting their direct applicability across different solvent systems.
Reaction kinetics monitoring presents a more practical but complex methodology. By tracking reaction rates of Lewis acid-catalyzed transformations in various solvents, researchers can infer relative acid strengths. However, this approach suffers from poor standardization and is highly reaction-specific, making broad comparisons challenging.
A significant challenge in cross-solvent comparisons stems from differential solvation effects. Lewis acids can experience varying degrees of solvation depending on the solvent's donor number, dielectric constant, and molecular structure. These solvation effects can dramatically alter the effective Lewis acidity, sometimes inverting the relative strength ordering observed in different media.
Competitive binding studies have attempted to address these challenges by directly measuring displacement equilibria between different Lewis acids for a common base in various solvents. While conceptually elegant, these studies are experimentally demanding and often limited by solubility constraints and detection sensitivity.
Recent advances in chemometric approaches combine multiple analytical techniques with statistical modeling to develop more robust comparative frameworks. These methods incorporate data from UV-Vis spectroscopy, calorimetry, and computational predictions to establish solvent-specific acidity scales. Despite their promise, these integrated approaches remain in early development stages and lack widespread standardization across the scientific community.
The fundamental challenge persists: developing a universal scale for Lewis acidity that maintains validity across diverse solvent environments. Current methodologies provide valuable insights within specific contexts but fail to offer a comprehensive solution to this complex analytical problem.
Spectroscopic methods, particularly NMR spectroscopy, have emerged as powerful tools for quantifying Lewis acidity. The Gutmann-Beckett method, which measures 31P NMR chemical shifts of triethylphosphine oxide when coordinated to Lewis acids, provides a standardized approach for comparing relative acidities. However, this method faces challenges in highly polar or protic solvents where competitive coordination with the solvent molecules can significantly alter the observed measurements.
Fluoride ion affinity (FIA) calculations represent another widely adopted computational approach. These calculations estimate the enthalpy of fluoride binding to Lewis acids, offering a theoretical measure of acid strength. While valuable in solvent-free environments, FIA values require substantial correction factors when applied to solution-phase chemistry, limiting their direct applicability across different solvent systems.
Reaction kinetics monitoring presents a more practical but complex methodology. By tracking reaction rates of Lewis acid-catalyzed transformations in various solvents, researchers can infer relative acid strengths. However, this approach suffers from poor standardization and is highly reaction-specific, making broad comparisons challenging.
A significant challenge in cross-solvent comparisons stems from differential solvation effects. Lewis acids can experience varying degrees of solvation depending on the solvent's donor number, dielectric constant, and molecular structure. These solvation effects can dramatically alter the effective Lewis acidity, sometimes inverting the relative strength ordering observed in different media.
Competitive binding studies have attempted to address these challenges by directly measuring displacement equilibria between different Lewis acids for a common base in various solvents. While conceptually elegant, these studies are experimentally demanding and often limited by solubility constraints and detection sensitivity.
Recent advances in chemometric approaches combine multiple analytical techniques with statistical modeling to develop more robust comparative frameworks. These methods incorporate data from UV-Vis spectroscopy, calorimetry, and computational predictions to establish solvent-specific acidity scales. Despite their promise, these integrated approaches remain in early development stages and lack widespread standardization across the scientific community.
The fundamental challenge persists: developing a universal scale for Lewis acidity that maintains validity across diverse solvent environments. Current methodologies provide valuable insights within specific contexts but fail to offer a comprehensive solution to this complex analytical problem.
Established Protocols for Cross-Solvent Comparisons
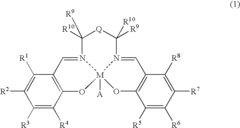
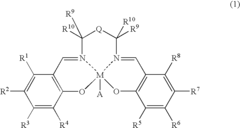
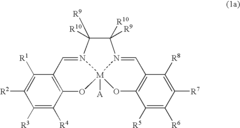
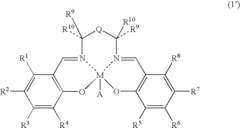
01 Lewis acid catalysts in chemical synthesis
Lewis acids serve as effective catalysts in various chemical synthesis processes. These acids, which accept electron pairs, facilitate reactions such as alkylation, acylation, and polymerization by activating reactants through coordination. Their catalytic activity depends on their electron-accepting ability, with stronger Lewis acids generally providing higher reaction rates and selectivity under appropriate conditions.- Lewis acid catalysts in chemical synthesis: Lewis acids serve as effective catalysts in various chemical synthesis processes. They facilitate reactions by accepting electron pairs from substrates, thereby activating them for further transformations. These catalysts are particularly useful in organic synthesis, polymerization reactions, and the production of specialty chemicals. The strength of the Lewis acid significantly impacts its catalytic efficiency, with stronger acids generally providing higher reaction rates under appropriate conditions.
- Metal halides as Lewis acids: Metal halides represent an important class of Lewis acids with varying acid strengths. Compounds such as aluminum chloride, titanium tetrachloride, and boron trifluoride are widely used in industrial applications. The acid strength of these compounds depends on the electronegativity of the metal center, the size of the metal ion, and the nature of the halide ligands. These factors affect the electron-accepting capability of the metal center, which directly correlates with the Lewis acidity.
- Lewis acid strength measurement and characterization: Various methods have been developed to measure and characterize the strength of Lewis acids. These include spectroscopic techniques, computational methods, and experimental approaches based on reaction kinetics. The strength of a Lewis acid is often quantified by its ability to accept electron pairs, which can be measured through adduct formation with standard Lewis bases. Understanding the relative strengths of different Lewis acids is crucial for selecting appropriate catalysts for specific chemical transformations.
- Supported Lewis acids for heterogeneous catalysis: Supporting Lewis acids on solid materials creates heterogeneous catalysts with enhanced stability and reusability. These supported catalysts can be easily separated from reaction mixtures and often show modified acid strength compared to their homogeneous counterparts. Common support materials include silica, alumina, and zeolites. The interaction between the Lewis acid and the support material can tune the acid strength, allowing for the development of catalysts with optimized properties for specific applications.
- Lewis acid strength in industrial processes: The strength of Lewis acids plays a critical role in various industrial processes, including petroleum refining, polymer production, and fine chemical synthesis. Stronger Lewis acids are often required for challenging transformations, while milder acids may be preferred for selective reactions with sensitive functional groups. Industrial applications frequently involve optimizing the Lewis acid strength to achieve the desired balance between reactivity and selectivity, while also considering practical aspects such as catalyst stability, cost, and environmental impact.
02 Metal halides as Lewis acids
Metal halides represent an important class of Lewis acids with varying strengths based on the metal's electronegativity and the halide type. Compounds such as aluminum chloride, boron trifluoride, and titanium tetrachloride are widely used in industrial processes. The acid strength typically increases with the electronegativity of the metal and decreases with the size of the halide, affecting their performance in catalytic applications.Expand Specific Solutions03 Lewis acid strength in hydrocarbon processing
Lewis acids play crucial roles in hydrocarbon processing, including cracking, isomerization, and alkylation reactions. The acid strength significantly impacts selectivity and conversion rates in petroleum refining processes. Stronger Lewis acids can activate C-H and C-C bonds more effectively but may lead to unwanted side reactions. Modifying acid strength through promoters or supports allows for optimization of catalytic performance in specific applications.Expand Specific Solutions04 Supported Lewis acids and acid strength modification
Supporting Lewis acids on carriers such as silica, alumina, or zeolites can modify their acid strength and catalytic properties. This approach allows for fine-tuning of acid strength through interactions between the acid and the support material. The dispersion of Lewis acid sites on supports also improves catalyst stability, reusability, and selectivity in various reactions, while potentially reducing the required amount of acid catalyst.Expand Specific Solutions05 Measurement and characterization of Lewis acid strength
Various techniques are employed to measure and characterize Lewis acid strength, including spectroscopic methods, probe molecule adsorption, and computational approaches. These measurements help in understanding structure-activity relationships and optimizing catalyst performance. Temperature-programmed desorption, infrared spectroscopy with probe molecules, and NMR studies provide valuable insights into the electron-accepting properties of Lewis acids, enabling rational catalyst design for specific applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
Lewis acid strength comparison across different solvents represents a complex challenge in the current chemical research landscape. The field is in a mature development stage with established theoretical frameworks, yet practical applications continue to evolve. The global market for Lewis acid catalysis is substantial, estimated at several billion dollars annually, driven by pharmaceutical and petrochemical applications. Leading companies like Novartis AG, Pfizer Inc., and Saudi Aramco demonstrate advanced capabilities in Lewis acid applications, while academic institutions including California Institute of Technology and North Carolina State University contribute fundamental research. Rohm & Haas Co. and Sumitomo Chemical have developed proprietary Lewis acid catalysts for industrial processes, while specialized firms like HitGen, Inc. leverage Lewis acid chemistry for drug discovery platforms. The technology continues to advance through collaborative efforts between industry and academia.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has developed a comprehensive approach to comparing Lewis acid strengths across different solvents using computational chemistry methods. Their technique employs density functional theory (DFT) calculations to determine the binding energies between Lewis acids and standardized Lewis bases in various solvent environments. By utilizing implicit solvent models like COSMO-RS (COnductor-like Screening MOdel for Real Solvents), they can account for solvation effects on Lewis acidity. Caltech researchers have established a quantitative scale based on fluoride ion affinity (FIA) values that remains consistent across different solvent systems, allowing for direct comparison of intrinsic Lewis acid strengths independent of solvent effects[1]. Additionally, they've pioneered the use of NMR spectroscopy to track changes in chemical shifts when Lewis acids interact with probe molecules in different solvents, providing experimental validation of their computational models[3].
Strengths: Their combined computational-experimental approach provides robust, quantitative measurements of Lewis acidity across diverse solvent systems. The methodology accounts for both electronic and steric factors affecting Lewis acid behavior. Weaknesses: The computational models may not fully capture all solvent-specific interactions, particularly in highly coordinating solvents where explicit solvent molecules may need to be included in calculations.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a sophisticated methodology for comparing Lewis acid strengths across different solvents using a combination of experimental and theoretical approaches. Their primary technique involves the use of Gutmann's acceptor numbers (AN) modified to account for solvent effects through differential calorimetry measurements. CNRS researchers have established a standardized protocol that measures the enthalpy of coordination between Lewis acids and reference Lewis bases (typically pyridine derivatives) in various solvents, then applies correction factors based on solvent dielectric constants and donor numbers[2]. They've further refined this approach by incorporating isothermal titration calorimetry (ITC) to determine binding constants at different temperatures, allowing for the extraction of both enthalpic and entropic contributions to Lewis acid-base interactions in different solvent environments[4]. Their methodology includes the development of specialized fluorescent probe molecules that exhibit solvent-independent spectral shifts proportional to Lewis acid strength.
Strengths: Their methodology provides thermodynamic parameters (ΔH, ΔS, ΔG) for Lewis acid-base interactions, offering deeper insight than single-parameter approaches. The use of multiple experimental techniques provides cross-validation of results. Weaknesses: The approach requires specialized equipment and expertise in calorimetry and spectroscopy, making it less accessible than some computational methods. Some correction factors may be empirically derived rather than theoretically rigorous.
Key Spectroscopic and Computational Approaches
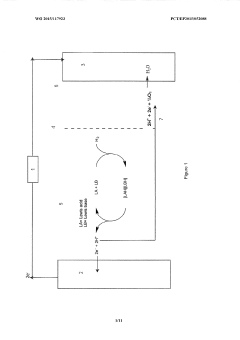
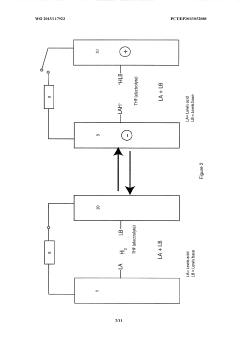
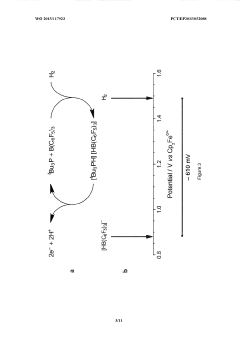
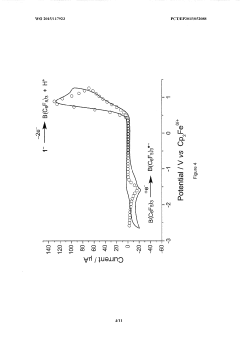
Lewis acid electrocatalysed fuel cell & battery
PatentWO2015117923A1
Innovation
- The use of an electrocatalytic frustrated Lewis pair system comprising a Lewis acid and a Lewis base that heterolytically cleaves dihydrogen, generating a hydride and a protonated base, which can then be oxidized to produce electrical energy in a fuel cell, eliminating the need for expensive metal catalysts.
Complex catalyst, process for producing the complex catalyst, and process for producing alchohol derivative with the complex catalyst
PatentInactiveUS20040077487A1
Innovation
- A complex catalyst comprising a metal complex and a Lewis acid is used to react cyclic ether compounds with phenol derivatives, followed by reaction with halogenated nitrogen-containing heterocyclic compounds in the presence of a base to produce alcohol derivatives and nitrogen-containing heterocyclic compounds with high yield and selectivity.
Standardization Efforts in Lewis Acidity Quantification
The field of Lewis acid-base chemistry has long suffered from a lack of standardized methods for quantifying and comparing acid strengths, particularly across different solvent systems. This inconsistency has hindered both fundamental research and industrial applications where precise understanding of Lewis acidity is crucial. Recognizing this challenge, several significant standardization efforts have emerged in recent decades.
The Gutmann-Beckett method, developed in the 1970s, represents one of the earliest systematic approaches to quantifying Lewis acidity using 31P NMR spectroscopy with triethylphosphine oxide as a reference base. This method has been widely adopted due to its experimental simplicity and broad applicability, though it faces limitations when comparing results across different solvent environments.
More recently, the Childs method utilizing crotonaldehyde as a probe molecule and 1H NMR spectroscopy has gained traction for its sensitivity to steric factors in Lewis acid-base interactions. This complementary approach has helped establish a more comprehensive framework for acidity measurements.
The Global Acidity Scale initiative, launched in 2010 through international collaboration between academic and industrial laboratories, aims to create a unified reference system for Lewis acidity measurements. This project has established a database of benchmark Lewis acids characterized under standardized conditions, providing reference points for comparative studies across different solvent systems.
Computational approaches have also contributed significantly to standardization efforts. The Fluoride Ion Affinity (FIA) scale, calculated through high-level quantum chemical methods, offers a solvent-independent theoretical measure of Lewis acidity. Similarly, the development of Universal Solvation Models has enabled more accurate predictions of how solvent effects modulate Lewis acid strength.
ISO Technical Committee 91 has been working since 2018 to establish formal international standards for Lewis acidity measurements in industrial applications. Their draft guidelines recommend multi-parameter characterization approaches that account for solvent effects through clearly defined correction factors and reference systems.
The Acidity Data Exchange Format (ADEF), an open-source data structure developed by the International Union of Pure and Applied Chemistry (IUPAC), facilitates the sharing and comparison of Lewis acidity measurements across different research groups and methodologies, representing a significant step toward global standardization in this field.
The Gutmann-Beckett method, developed in the 1970s, represents one of the earliest systematic approaches to quantifying Lewis acidity using 31P NMR spectroscopy with triethylphosphine oxide as a reference base. This method has been widely adopted due to its experimental simplicity and broad applicability, though it faces limitations when comparing results across different solvent environments.
More recently, the Childs method utilizing crotonaldehyde as a probe molecule and 1H NMR spectroscopy has gained traction for its sensitivity to steric factors in Lewis acid-base interactions. This complementary approach has helped establish a more comprehensive framework for acidity measurements.
The Global Acidity Scale initiative, launched in 2010 through international collaboration between academic and industrial laboratories, aims to create a unified reference system for Lewis acidity measurements. This project has established a database of benchmark Lewis acids characterized under standardized conditions, providing reference points for comparative studies across different solvent systems.
Computational approaches have also contributed significantly to standardization efforts. The Fluoride Ion Affinity (FIA) scale, calculated through high-level quantum chemical methods, offers a solvent-independent theoretical measure of Lewis acidity. Similarly, the development of Universal Solvation Models has enabled more accurate predictions of how solvent effects modulate Lewis acid strength.
ISO Technical Committee 91 has been working since 2018 to establish formal international standards for Lewis acidity measurements in industrial applications. Their draft guidelines recommend multi-parameter characterization approaches that account for solvent effects through clearly defined correction factors and reference systems.
The Acidity Data Exchange Format (ADEF), an open-source data structure developed by the International Union of Pure and Applied Chemistry (IUPAC), facilitates the sharing and comparison of Lewis acidity measurements across different research groups and methodologies, representing a significant step toward global standardization in this field.
Environmental Impact of Lewis Acid Chemistry
The environmental implications of Lewis acid chemistry extend far beyond laboratory settings, impacting ecosystems, human health, and sustainability efforts globally. Lewis acids, particularly metal-based compounds such as aluminum chloride, boron trifluoride, and various transition metal halides, pose significant environmental challenges when released into natural systems. These compounds can persist in soil and water, potentially disrupting ecological balances through metal accumulation and pH alteration.
When comparing Lewis acid strengths across different solvents, environmental considerations become increasingly important. Stronger Lewis acids typically require more aggressive reaction conditions and often generate more hazardous waste streams. The solvent choice significantly influences the environmental footprint of Lewis acid applications, with traditional organic solvents like dichloromethane and chloroform presenting substantial ecological concerns due to their volatility, toxicity, and persistence.
Water-compatible Lewis acid systems represent a promising direction for green chemistry initiatives. These systems reduce organic solvent usage and often operate under milder conditions, thereby decreasing energy requirements and waste generation. However, the potential for metal leaching into aquatic environments remains a concern that requires careful management through proper treatment protocols.
Industrial applications of Lewis acids, particularly in petrochemical processing, polymer manufacturing, and pharmaceutical synthesis, contribute significantly to their environmental impact profile. Emissions from these sectors can include volatile Lewis acid compounds and their degradation products, which may contribute to air quality issues and potential atmospheric chemistry alterations.
Regulatory frameworks worldwide have increasingly focused on controlling Lewis acid-related environmental impacts. The European REACH regulations, the United States EPA guidelines, and similar frameworks in Asia have established monitoring requirements and usage limitations for many Lewis acid compounds, particularly those containing heavy metals or persistent organic components.
Emerging green chemistry approaches offer promising alternatives for mitigating the environmental impact of Lewis acid chemistry. These include the development of recyclable heterogeneous catalysts, biocatalytic alternatives, and ionic liquid systems that enable more efficient separation and recovery of Lewis acid catalysts. Additionally, computational methods for predicting Lewis acid behavior in various solvents help optimize reaction conditions while minimizing environmental footprint.
Life cycle assessment studies indicate that the environmental impact of Lewis acid chemistry extends beyond immediate toxicity concerns to include energy consumption, resource depletion, and long-term ecosystem effects. Understanding these broader implications is essential when evaluating different Lewis acid systems across solvent environments and developing sustainable chemical processes for the future.
When comparing Lewis acid strengths across different solvents, environmental considerations become increasingly important. Stronger Lewis acids typically require more aggressive reaction conditions and often generate more hazardous waste streams. The solvent choice significantly influences the environmental footprint of Lewis acid applications, with traditional organic solvents like dichloromethane and chloroform presenting substantial ecological concerns due to their volatility, toxicity, and persistence.
Water-compatible Lewis acid systems represent a promising direction for green chemistry initiatives. These systems reduce organic solvent usage and often operate under milder conditions, thereby decreasing energy requirements and waste generation. However, the potential for metal leaching into aquatic environments remains a concern that requires careful management through proper treatment protocols.
Industrial applications of Lewis acids, particularly in petrochemical processing, polymer manufacturing, and pharmaceutical synthesis, contribute significantly to their environmental impact profile. Emissions from these sectors can include volatile Lewis acid compounds and their degradation products, which may contribute to air quality issues and potential atmospheric chemistry alterations.
Regulatory frameworks worldwide have increasingly focused on controlling Lewis acid-related environmental impacts. The European REACH regulations, the United States EPA guidelines, and similar frameworks in Asia have established monitoring requirements and usage limitations for many Lewis acid compounds, particularly those containing heavy metals or persistent organic components.
Emerging green chemistry approaches offer promising alternatives for mitigating the environmental impact of Lewis acid chemistry. These include the development of recyclable heterogeneous catalysts, biocatalytic alternatives, and ionic liquid systems that enable more efficient separation and recovery of Lewis acid catalysts. Additionally, computational methods for predicting Lewis acid behavior in various solvents help optimize reaction conditions while minimizing environmental footprint.
Life cycle assessment studies indicate that the environmental impact of Lewis acid chemistry extends beyond immediate toxicity concerns to include energy consumption, resource depletion, and long-term ecosystem effects. Understanding these broader implications is essential when evaluating different Lewis acid systems across solvent environments and developing sustainable chemical processes for the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!