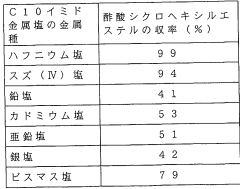
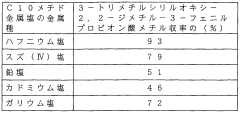
Lewis Acid Challenges in Homogeneous Catalysis
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acids have played a pivotal role in the evolution of homogeneous catalysis since the early 20th century. The concept of Lewis acidity, first proposed by Gilbert N. Lewis in 1923, defines these compounds as electron pair acceptors, creating a foundation for understanding numerous chemical transformations. The historical trajectory of Lewis acid catalysis reveals a progression from simple metal halides to sophisticated organometallic complexes with tunable electronic and steric properties.
The field experienced significant advancement in the 1950s and 1960s with the discovery of Ziegler-Natta catalysts, demonstrating the potential of Lewis acidic metal centers in polymerization reactions. This breakthrough opened new avenues for industrial applications and sparked intensive research into Lewis acid catalysis mechanisms. By the 1980s, the introduction of chiral Lewis acids revolutionized asymmetric synthesis, enabling unprecedented levels of stereoselectivity in organic transformations.
Recent decades have witnessed an exponential growth in the development of novel Lewis acid catalysts, driven by the demands for more sustainable chemical processes, higher selectivity, and expanded reaction scope. The integration of computational chemistry has accelerated this progress by providing deeper insights into reaction mechanisms and catalyst design principles. Modern Lewis acid catalysis has evolved beyond traditional metal-based systems to include main group elements, lanthanides, and even organic molecules exhibiting Lewis acidic behavior.
Despite these advances, homogeneous catalysis using Lewis acids faces persistent challenges that limit broader industrial implementation. These include catalyst deactivation through water sensitivity, limited substrate scope, high catalyst loadings, and difficulties in catalyst recovery. The technical objectives of current research focus on addressing these limitations through innovative approaches to catalyst design and reaction engineering.
The primary goals in this field include developing water-tolerant Lewis acid catalysts that maintain activity in the presence of protic solvents or substrates, creating systems with broader functional group compatibility, and designing catalysts capable of operating at lower loadings while maintaining high turnover numbers. Additionally, there is significant interest in heterogenizing homogeneous Lewis acid catalysts to facilitate recovery and recycling without sacrificing activity or selectivity.
Emerging research aims to harness the potential of dual catalytic systems where Lewis acids work synergistically with other catalytic modalities, such as photoredox catalysis or enzymatic processes. The ultimate objective is to develop next-generation Lewis acid catalysts that combine high activity, selectivity, and stability with environmental sustainability and economic viability, potentially revolutionizing chemical manufacturing processes across pharmaceutical, materials, and fine chemical industries.
The field experienced significant advancement in the 1950s and 1960s with the discovery of Ziegler-Natta catalysts, demonstrating the potential of Lewis acidic metal centers in polymerization reactions. This breakthrough opened new avenues for industrial applications and sparked intensive research into Lewis acid catalysis mechanisms. By the 1980s, the introduction of chiral Lewis acids revolutionized asymmetric synthesis, enabling unprecedented levels of stereoselectivity in organic transformations.
Recent decades have witnessed an exponential growth in the development of novel Lewis acid catalysts, driven by the demands for more sustainable chemical processes, higher selectivity, and expanded reaction scope. The integration of computational chemistry has accelerated this progress by providing deeper insights into reaction mechanisms and catalyst design principles. Modern Lewis acid catalysis has evolved beyond traditional metal-based systems to include main group elements, lanthanides, and even organic molecules exhibiting Lewis acidic behavior.
Despite these advances, homogeneous catalysis using Lewis acids faces persistent challenges that limit broader industrial implementation. These include catalyst deactivation through water sensitivity, limited substrate scope, high catalyst loadings, and difficulties in catalyst recovery. The technical objectives of current research focus on addressing these limitations through innovative approaches to catalyst design and reaction engineering.
The primary goals in this field include developing water-tolerant Lewis acid catalysts that maintain activity in the presence of protic solvents or substrates, creating systems with broader functional group compatibility, and designing catalysts capable of operating at lower loadings while maintaining high turnover numbers. Additionally, there is significant interest in heterogenizing homogeneous Lewis acid catalysts to facilitate recovery and recycling without sacrificing activity or selectivity.
Emerging research aims to harness the potential of dual catalytic systems where Lewis acids work synergistically with other catalytic modalities, such as photoredox catalysis or enzymatic processes. The ultimate objective is to develop next-generation Lewis acid catalysts that combine high activity, selectivity, and stability with environmental sustainability and economic viability, potentially revolutionizing chemical manufacturing processes across pharmaceutical, materials, and fine chemical industries.
Market Analysis of Homogeneous Catalysis Applications
The global homogeneous catalysis market demonstrates robust growth, valued at approximately $5.2 billion in 2022 and projected to reach $7.8 billion by 2027, representing a compound annual growth rate of 8.4%. This expansion is primarily driven by increasing demand across pharmaceutical, fine chemical, and petrochemical industries, where precise molecular transformations are essential for high-value product development.
Lewis acid catalysis represents a significant segment within this market, accounting for roughly 22% of homogeneous catalysis applications. The pharmaceutical sector remains the largest end-user, consuming nearly 40% of Lewis acid catalysts for asymmetric synthesis, C-C bond formation, and complex molecule assembly. This dominance stems from the industry's need for enantioselective reactions that produce single-isomer drug compounds with enhanced efficacy and reduced side effects.
The fine chemicals sector follows closely, utilizing Lewis acid catalysis for specialty chemical production, particularly in flavor and fragrance compounds, agrochemicals, and electronic materials. This segment has shown accelerated growth at 9.7% annually, outpacing the overall market as manufacturers seek more efficient and selective synthetic routes.
Regionally, North America and Europe collectively hold approximately 58% market share, primarily due to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific represents the fastest-growing region with 11.2% annual growth, driven by rapid industrialization in China and India, coupled with increasing investment in advanced chemical manufacturing capabilities.
A notable market trend is the shift toward more environmentally benign Lewis acid catalysts, with water-compatible and recyclable systems gaining significant traction. This segment has grown by 15.3% annually over the past three years, reflecting broader sustainability initiatives across chemical industries and stricter environmental regulations in developed markets.
Customer demand increasingly focuses on catalyst systems that overcome traditional Lewis acid limitations, particularly moisture sensitivity and catalyst recovery challenges. Market research indicates that 76% of industrial users identify these factors as critical pain points affecting operational efficiency and cost-effectiveness. Consequently, premium pricing of 30-45% is achievable for novel catalyst systems that effectively address these challenges.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers, with recent market consolidation through strategic acquisitions aimed at expanding technology portfolios. Venture capital investment in novel homogeneous catalysis startups has reached $420 million in 2022, a 35% increase from the previous year, signaling strong market confidence in technological innovation within this space.
Lewis acid catalysis represents a significant segment within this market, accounting for roughly 22% of homogeneous catalysis applications. The pharmaceutical sector remains the largest end-user, consuming nearly 40% of Lewis acid catalysts for asymmetric synthesis, C-C bond formation, and complex molecule assembly. This dominance stems from the industry's need for enantioselective reactions that produce single-isomer drug compounds with enhanced efficacy and reduced side effects.
The fine chemicals sector follows closely, utilizing Lewis acid catalysis for specialty chemical production, particularly in flavor and fragrance compounds, agrochemicals, and electronic materials. This segment has shown accelerated growth at 9.7% annually, outpacing the overall market as manufacturers seek more efficient and selective synthetic routes.
Regionally, North America and Europe collectively hold approximately 58% market share, primarily due to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific represents the fastest-growing region with 11.2% annual growth, driven by rapid industrialization in China and India, coupled with increasing investment in advanced chemical manufacturing capabilities.
A notable market trend is the shift toward more environmentally benign Lewis acid catalysts, with water-compatible and recyclable systems gaining significant traction. This segment has grown by 15.3% annually over the past three years, reflecting broader sustainability initiatives across chemical industries and stricter environmental regulations in developed markets.
Customer demand increasingly focuses on catalyst systems that overcome traditional Lewis acid limitations, particularly moisture sensitivity and catalyst recovery challenges. Market research indicates that 76% of industrial users identify these factors as critical pain points affecting operational efficiency and cost-effectiveness. Consequently, premium pricing of 30-45% is achievable for novel catalyst systems that effectively address these challenges.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers, with recent market consolidation through strategic acquisitions aimed at expanding technology portfolios. Venture capital investment in novel homogeneous catalysis startups has reached $420 million in 2022, a 35% increase from the previous year, signaling strong market confidence in technological innovation within this space.
Current Challenges in Lewis Acid Homogeneous Catalysis
Despite significant advancements in Lewis acid catalysis, several persistent challenges continue to impede progress in homogeneous catalysis applications. Water and oxygen sensitivity remains a primary obstacle, as many Lewis acids rapidly decompose or become deactivated upon exposure to moisture or air. This necessitates stringent reaction conditions including inert atmospheres and anhydrous solvents, substantially increasing operational complexity and cost while limiting industrial scalability.
Selectivity issues present another significant challenge, particularly in reactions involving multiple functional groups. Lewis acids often exhibit poor chemoselectivity, regioselectivity, or stereoselectivity, leading to complex product mixtures that require extensive purification. This problem becomes especially pronounced when working with complex substrates containing multiple Lewis basic sites that can coordinate with the catalyst.
Catalyst recovery and recyclability continue to be problematic aspects of homogeneous Lewis acid catalysis. The homogeneous nature of these catalysts inherently complicates their separation from reaction mixtures, resulting in metal contamination of products and significant catalyst loss. While immobilization strategies on solid supports have been explored, they frequently lead to diminished catalytic activity or altered selectivity profiles.
The limited substrate scope represents another critical challenge. Many Lewis acid catalysts demonstrate high efficiency only with specific substrate classes, restricting their broader applicability. This limitation stems from varying coordination strengths between different functional groups and Lewis acids, as well as potential side reactions that can occur with certain substrate types.
Mechanistic understanding gaps further complicate catalyst development. Despite extensive research, the precise reaction mechanisms and transition states in many Lewis acid-catalyzed transformations remain poorly understood. This knowledge deficit hampers rational catalyst design and optimization efforts, often necessitating empirical approaches to catalyst development.
Thermal stability issues also plague many Lewis acid catalysts, particularly at elevated temperatures required for certain transformations. Decomposition, ligand dissociation, or structural rearrangements can occur, leading to catalyst deactivation or formation of undesired species that promote side reactions.
Finally, compatibility with green chemistry principles presents an ongoing challenge. Many traditional Lewis acids involve toxic or environmentally problematic metals and require hazardous solvents, contradicting sustainability goals. The development of benign alternatives that maintain high catalytic performance remains an active research frontier in the field.
Selectivity issues present another significant challenge, particularly in reactions involving multiple functional groups. Lewis acids often exhibit poor chemoselectivity, regioselectivity, or stereoselectivity, leading to complex product mixtures that require extensive purification. This problem becomes especially pronounced when working with complex substrates containing multiple Lewis basic sites that can coordinate with the catalyst.
Catalyst recovery and recyclability continue to be problematic aspects of homogeneous Lewis acid catalysis. The homogeneous nature of these catalysts inherently complicates their separation from reaction mixtures, resulting in metal contamination of products and significant catalyst loss. While immobilization strategies on solid supports have been explored, they frequently lead to diminished catalytic activity or altered selectivity profiles.
The limited substrate scope represents another critical challenge. Many Lewis acid catalysts demonstrate high efficiency only with specific substrate classes, restricting their broader applicability. This limitation stems from varying coordination strengths between different functional groups and Lewis acids, as well as potential side reactions that can occur with certain substrate types.
Mechanistic understanding gaps further complicate catalyst development. Despite extensive research, the precise reaction mechanisms and transition states in many Lewis acid-catalyzed transformations remain poorly understood. This knowledge deficit hampers rational catalyst design and optimization efforts, often necessitating empirical approaches to catalyst development.
Thermal stability issues also plague many Lewis acid catalysts, particularly at elevated temperatures required for certain transformations. Decomposition, ligand dissociation, or structural rearrangements can occur, leading to catalyst deactivation or formation of undesired species that promote side reactions.
Finally, compatibility with green chemistry principles presents an ongoing challenge. Many traditional Lewis acids involve toxic or environmentally problematic metals and require hazardous solvents, contradicting sustainability goals. The development of benign alternatives that maintain high catalytic performance remains an active research frontier in the field.
Contemporary Lewis Acid Catalyst Solutions
01 Lewis Acids in Catalytic Reactions
Lewis acids are widely used as catalysts in various chemical reactions, particularly in organic synthesis. They facilitate reactions by accepting electron pairs from substrates, thereby activating them for further transformations. Common Lewis acid catalysts include metal halides, metal triflates, and organometallic compounds. These catalysts enhance reaction rates and selectivity in processes such as alkylation, acylation, and polymerization reactions.- Lewis Acids as Catalysts in Chemical Synthesis: Lewis acids function as effective catalysts in various chemical synthesis processes by accepting electron pairs from reactants. They facilitate reactions such as alkylation, acylation, and polymerization by activating substrates through coordination. Common Lewis acid catalysts include metal halides like aluminum chloride, boron trifluoride, and titanium tetrachloride, which enhance reaction rates and selectivity in organic transformations.
- Lewis Acids in Polymerization Processes: Lewis acids play a crucial role in polymerization reactions, particularly in cationic and coordination polymerization. They initiate polymerization by generating active species through interaction with monomers or co-catalysts. These acids control molecular weight distribution, stereochemistry, and reaction kinetics in polymer production. Metal-based Lewis acids are commonly employed in industrial polymerization processes to produce various polymers with specific properties.
- Lewis Acids for Material Processing and Treatment: Lewis acids are utilized in various material processing applications, including surface treatment, etching, and modification of materials. They can alter the physical and chemical properties of surfaces through coordination with functional groups. These acids are employed in semiconductor processing, metal surface treatments, and in the preparation of specialized materials with enhanced properties such as improved adhesion, conductivity, or chemical resistance.
- Lewis Acids in Separation and Purification Technologies: Lewis acids are employed in separation and purification processes due to their ability to form complexes with specific compounds. They can selectively bind to target molecules, enabling their separation from mixtures. Applications include extraction of valuable compounds, removal of contaminants, and chromatographic separations. Lewis acid-based materials are also used as adsorbents in gas purification systems and as selective complexing agents in chemical processing.
- Novel Lewis Acid Structures and Formulations: Research has led to the development of novel Lewis acid structures with enhanced properties such as increased stability, selectivity, and activity. These include supported Lewis acids, Lewis acid-surfactant combined structures, and Lewis acids with specialized ligands. Innovations in Lewis acid design focus on recyclability, reduced environmental impact, and application-specific performance characteristics. These novel formulations expand the utility of Lewis acids across various industrial and research applications.
02 Lewis Acids in Polymer Chemistry
Lewis acids play a crucial role in polymer chemistry, particularly in the initiation and control of polymerization processes. They can activate monomers for polymerization, control molecular weight distribution, and influence the stereochemistry of the resulting polymers. Lewis acid catalysts are especially important in cationic polymerization and ring-opening polymerization of cyclic monomers, leading to the production of various industrial polymers with tailored properties.Expand Specific Solutions03 Lewis Acids in Material Science Applications
Lewis acids are utilized in various material science applications, including the development of advanced materials, coatings, and composites. They can modify surface properties, enhance adhesion between different materials, and improve the performance characteristics of materials. Lewis acids are also employed in the synthesis of nanoparticles, the preparation of porous materials, and the modification of inorganic substrates for specialized applications.Expand Specific Solutions04 Lewis Acids in Pharmaceutical Synthesis
Lewis acids are essential tools in pharmaceutical synthesis, enabling the creation of complex drug molecules through selective transformations. They facilitate stereoselective reactions, functional group modifications, and carbon-carbon bond formations that are crucial in building pharmaceutical compounds. Lewis acid-mediated reactions often provide higher yields, greater selectivity, and milder reaction conditions compared to alternative methods, making them valuable in the development of new therapeutic agents.Expand Specific Solutions05 Novel Lewis Acid Structures and Formulations
Research into novel Lewis acid structures and formulations focuses on developing more efficient, selective, and environmentally friendly catalysts. These innovations include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acids with tunable acidity. Novel formulations often aim to address limitations of traditional Lewis acids, such as sensitivity to moisture, difficulty in recovery, and environmental concerns, while maintaining or enhancing catalytic activity for specific applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid catalysis market is currently in a growth phase, with increasing applications in fine chemicals, pharmaceuticals, and materials science. The competitive landscape is characterized by a mix of established chemical giants like BASF, Asahi Kasei, and ExxonMobil Chemical, alongside specialized research institutions such as Dalian Institute of Chemical Physics and Noguchi Institute. Market size is expanding due to growing demand for efficient and selective catalytic processes. Technologically, companies are at varying maturity levels - BASF, Johnson Matthey, and Haldor Topsøe lead with commercial applications, while academic-industrial partnerships from institutions like Tianjin University and Zhejiang University are advancing fundamental research. Recent innovations focus on addressing traditional Lewis acid challenges including water sensitivity, catalyst recovery, and selectivity improvement.
BASF Corp.
Technical Solution: BASF has developed innovative Lewis acid catalysts for homogeneous catalysis, focusing on metal-organic frameworks (MOFs) with tunable Lewis acidity. Their approach involves incorporating various metal centers (Al, Fe, Zn) into MOF structures to create highly selective catalysts for industrial processes. BASF's technology enables precise control of Lewis acid strength through metal center selection and ligand modification, allowing optimization for specific reactions. Their catalysts demonstrate exceptional performance in Diels-Alder reactions, Friedel-Crafts alkylations, and carbonyl transformations with reduced side reactions. BASF has also pioneered recyclable Lewis acid systems that maintain activity through multiple reaction cycles, addressing traditional recovery challenges. Their latest generation catalysts feature enhanced stability against moisture and air, overcoming common deactivation pathways that plague conventional Lewis acids.
Strengths: Superior selectivity and activity compared to traditional Lewis acids; excellent recyclability reducing catalyst costs; industrial scalability with established manufacturing processes. Weaknesses: Some systems still show sensitivity to extreme moisture conditions; higher production costs compared to conventional catalysts; limited effectiveness for certain substrate classes.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed proprietary Lewis acid catalyst systems specifically designed for hydrocarbon transformation processes in petroleum refining and petrochemical production. Their technology centers on perfluorinated aryl borane catalysts that exhibit exceptional Lewis acidity while maintaining stability in hydrocarbon media. These catalysts feature modified ligand structures that prevent common deactivation pathways through steric protection of the active metal center. ExxonMobil's systems demonstrate remarkable activity for olefin polymerization, alkylation, and isomerization reactions under industrially relevant conditions. Their catalysts incorporate specially designed co-catalyst systems that enhance activity while allowing precise control over reaction selectivity. ExxonMobil has also pioneered supported versions of these catalysts that maintain homogeneous-like activity while enabling easier separation and recovery, addressing a key challenge in homogeneous Lewis acid catalysis.
Strengths: Exceptional stability in hydrocarbon environments; highly active for petroleum-relevant transformations; scalable production methods aligned with industrial requirements. Weaknesses: Limited application outside hydrocarbon chemistry; higher production costs compared to traditional acid catalysts; some systems require specialized handling due to air/moisture sensitivity.
Key Patents and Breakthroughs in Lewis Acid Chemistry
Lewis acid catalyst composition
PatentWO2003051511A1
Innovation
- A Lewis acid catalyst composition utilizing a mixed medium of fluorinated and non-fluorinated compounds, where the catalyst has specific fluorinated substituents, enhancing solubility and allowing for easy separation and reuse, and enabling continuous reaction processes with high phase separation rates.
Lewis acid catalyst composition
PatentInactiveUS7084088B2
Innovation
- A Lewis acid catalyst composition using a mixed medium of fluorinated and non-fluorinated compounds, where the catalyst is a compound with specific perfluorinated and partially substituted hydrocarbon groups, enhancing solubility and allowing for rapid phase separation of the reaction mixture, facilitating easy recovery and continuous reaction processes.
Green Chemistry Implications of Lewis Acid Catalysis
The integration of Lewis acid catalysis with green chemistry principles represents a significant opportunity for sustainable chemical processes. Lewis acids have traditionally been associated with environmental concerns due to their corrosive nature, toxicity, and waste generation. However, recent advancements are transforming these catalysts into environmentally benign alternatives that align with the twelve principles of green chemistry.
Water-compatible Lewis acid catalysts have emerged as a major breakthrough, enabling reactions in aqueous media rather than hazardous organic solvents. This development significantly reduces volatile organic compound (VOC) emissions and minimizes the environmental footprint of catalytic processes. Notable examples include scandium triflate and lanthanide-based catalysts that maintain high activity in water while offering simplified product isolation and catalyst recovery.
Recyclability has become a central focus in modern Lewis acid catalyst design. Heterogeneous systems and supported Lewis acids facilitate catalyst separation and reuse through simple filtration processes. Magnetic nanoparticle-supported Lewis acids represent an innovative approach, allowing magnetic recovery and maintaining catalytic activity over multiple cycles, thereby reducing waste generation and resource consumption.
Energy efficiency improvements in Lewis acid catalysis contribute substantially to green chemistry objectives. Room temperature reactions catalyzed by designer Lewis acids eliminate the need for energy-intensive heating, while microwave-assisted Lewis acid catalysis dramatically reduces reaction times and energy requirements. These approaches directly address the energy efficiency principle of green chemistry.
Atom economy has been enhanced through the development of multicomponent reactions (MCRs) catalyzed by Lewis acids. These reactions incorporate multiple reagents into the final product with minimal waste generation. The Biginelli, Mannich, and Ugi reactions exemplify this approach, achieving complex molecular structures with exceptional atom efficiency under Lewis acid catalysis.
Biodegradable and non-toxic Lewis acid alternatives derived from natural sources represent the frontier of green catalysis. Iron and aluminum-based Lewis acids offer reduced environmental impact compared to traditional heavy metal catalysts. Additionally, bio-derived Lewis acids from waste materials demonstrate the potential for circular economy approaches in catalysis.
The economic benefits of green Lewis acid catalysis extend beyond environmental considerations. Reduced waste treatment costs, lower energy consumption, and simplified purification processes translate to significant cost savings in industrial applications. These economic incentives are driving increased industrial adoption of environmentally responsible Lewis acid catalysis technologies.
Water-compatible Lewis acid catalysts have emerged as a major breakthrough, enabling reactions in aqueous media rather than hazardous organic solvents. This development significantly reduces volatile organic compound (VOC) emissions and minimizes the environmental footprint of catalytic processes. Notable examples include scandium triflate and lanthanide-based catalysts that maintain high activity in water while offering simplified product isolation and catalyst recovery.
Recyclability has become a central focus in modern Lewis acid catalyst design. Heterogeneous systems and supported Lewis acids facilitate catalyst separation and reuse through simple filtration processes. Magnetic nanoparticle-supported Lewis acids represent an innovative approach, allowing magnetic recovery and maintaining catalytic activity over multiple cycles, thereby reducing waste generation and resource consumption.
Energy efficiency improvements in Lewis acid catalysis contribute substantially to green chemistry objectives. Room temperature reactions catalyzed by designer Lewis acids eliminate the need for energy-intensive heating, while microwave-assisted Lewis acid catalysis dramatically reduces reaction times and energy requirements. These approaches directly address the energy efficiency principle of green chemistry.
Atom economy has been enhanced through the development of multicomponent reactions (MCRs) catalyzed by Lewis acids. These reactions incorporate multiple reagents into the final product with minimal waste generation. The Biginelli, Mannich, and Ugi reactions exemplify this approach, achieving complex molecular structures with exceptional atom efficiency under Lewis acid catalysis.
Biodegradable and non-toxic Lewis acid alternatives derived from natural sources represent the frontier of green catalysis. Iron and aluminum-based Lewis acids offer reduced environmental impact compared to traditional heavy metal catalysts. Additionally, bio-derived Lewis acids from waste materials demonstrate the potential for circular economy approaches in catalysis.
The economic benefits of green Lewis acid catalysis extend beyond environmental considerations. Reduced waste treatment costs, lower energy consumption, and simplified purification processes translate to significant cost savings in industrial applications. These economic incentives are driving increased industrial adoption of environmentally responsible Lewis acid catalysis technologies.
Scale-up and Industrial Implementation Considerations
The transition from laboratory-scale experiments to industrial implementation presents significant challenges for Lewis acid catalysis systems. Process engineers must carefully consider reactor design modifications to accommodate the sensitivity of Lewis acids to moisture and oxygen. Specialized materials such as hastelloy or glass-lined reactors are often required to prevent catalyst degradation through unwanted side reactions with reactor surfaces, substantially increasing capital expenditure for industrial adoption.
Temperature and pressure control systems demand greater precision in industrial settings, as Lewis acid catalysts frequently exhibit narrow operational windows. The implementation of advanced monitoring technologies, including in-line spectroscopic methods, becomes essential for real-time reaction monitoring and quality control, particularly when scaling to multi-ton production volumes.
Solvent recovery and recycling systems represent another critical consideration. Many Lewis acid catalyzed processes employ anhydrous, aprotic solvents that require sophisticated recovery systems to maintain economic viability. The environmental impact of these specialized solvents necessitates comprehensive lifecycle assessments and potential regulatory compliance measures before industrial implementation.
Catalyst recovery presents perhaps the most significant economic hurdle. Homogeneous Lewis acid catalysts, particularly those containing precious metals or specialized ligands, require efficient separation and recycling protocols. Membrane filtration, selective precipitation, and supported catalyst technologies are being explored to address this challenge, though each approach introduces additional process complexity and potential yield losses.
Safety protocols must be substantially enhanced when scaling Lewis acid processes. Many Lewis acids are highly reactive, potentially pyrophoric, or generate hazardous byproducts. Comprehensive risk assessments, specialized handling procedures, and dedicated containment systems are necessary prerequisites for industrial implementation, adding significant operational complexity and training requirements.
Economic feasibility ultimately determines industrial adoption. The higher catalyst costs, specialized equipment requirements, and potentially lower space-time yields compared to traditional processes must be offset by product value or process advantages. Industries are increasingly developing hybrid approaches that maintain catalytic efficiency while addressing scale-up limitations, such as continuous flow processing with immobilized Lewis acid catalysts or biphasic systems that facilitate catalyst separation.
Temperature and pressure control systems demand greater precision in industrial settings, as Lewis acid catalysts frequently exhibit narrow operational windows. The implementation of advanced monitoring technologies, including in-line spectroscopic methods, becomes essential for real-time reaction monitoring and quality control, particularly when scaling to multi-ton production volumes.
Solvent recovery and recycling systems represent another critical consideration. Many Lewis acid catalyzed processes employ anhydrous, aprotic solvents that require sophisticated recovery systems to maintain economic viability. The environmental impact of these specialized solvents necessitates comprehensive lifecycle assessments and potential regulatory compliance measures before industrial implementation.
Catalyst recovery presents perhaps the most significant economic hurdle. Homogeneous Lewis acid catalysts, particularly those containing precious metals or specialized ligands, require efficient separation and recycling protocols. Membrane filtration, selective precipitation, and supported catalyst technologies are being explored to address this challenge, though each approach introduces additional process complexity and potential yield losses.
Safety protocols must be substantially enhanced when scaling Lewis acid processes. Many Lewis acids are highly reactive, potentially pyrophoric, or generate hazardous byproducts. Comprehensive risk assessments, specialized handling procedures, and dedicated containment systems are necessary prerequisites for industrial implementation, adding significant operational complexity and training requirements.
Economic feasibility ultimately determines industrial adoption. The higher catalyst costs, specialized equipment requirements, and potentially lower space-time yields compared to traditional processes must be offset by product value or process advantages. Industries are increasingly developing hybrid approaches that maintain catalytic efficiency while addressing scale-up limitations, such as continuous flow processing with immobilized Lewis acid catalysts or biphasic systems that facilitate catalyst separation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!