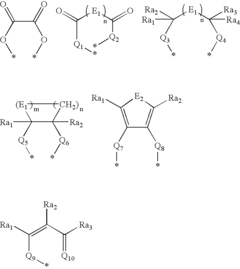
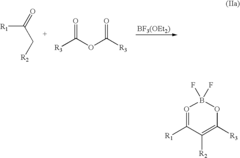
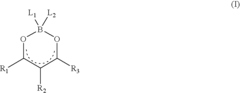Lewis Acid vs Proton Acid: Effect on Reaction Pathway
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis vs Proton Acid Background and Research Objectives
The field of acid catalysis has evolved significantly over the past century, with Lewis acids and Brønsted-Lowry (proton) acids representing two fundamental paradigms in chemical reactivity. Lewis acids, first defined by G.N. Lewis in 1923, function as electron pair acceptors, while proton acids donate hydrogen ions. This distinction, though seemingly straightforward, leads to profoundly different reaction mechanisms and product distributions across organic, inorganic, and materials chemistry.
Historical development of acid catalysis reveals a progression from empirical observations to sophisticated mechanistic understanding. Early industrial applications, such as petroleum refining and polymerization reactions, utilized acid catalysis without fully comprehending the underlying principles. The mid-20th century witnessed significant theoretical advances, particularly in understanding how these different acid types interact with substrates at the molecular level.
Recent technological developments have enabled unprecedented precision in studying reaction pathways. Advanced computational methods, in-situ spectroscopic techniques, and time-resolved analyses now allow chemists to observe and model the distinct transition states and intermediates formed under Lewis versus proton acid catalysis. This has transformed acid selection from an art to a science, with predictable outcomes based on mechanistic understanding.
The global chemical industry's increasing focus on sustainability and efficiency has heightened interest in selective acid catalysis. Reactions that previously required harsh conditions or generated substantial waste can now be optimized through precise acid selection. This represents not merely an incremental improvement but a paradigm shift in reaction design strategy.
Our technical research objectives are multifaceted. First, we aim to systematically map reaction pathway divergences when identical substrates are exposed to Lewis versus proton acids across a spectrum of reaction classes. Second, we seek to develop predictive models that can anticipate these divergences based on substrate structure and electronic properties. Third, we intend to identify novel synthetic opportunities where pathway divergence can be exploited for accessing previously challenging transformations.
Additionally, we will investigate hybrid catalytic systems that leverage both acid types synergistically, potentially unlocking reaction pathways inaccessible to either acid type alone. The research will also explore how solvent effects, temperature, and pressure differentially impact Lewis versus proton acid pathways, providing a comprehensive framework for reaction optimization.
The ultimate goal is to establish a unified theoretical framework that explains and predicts acid-type effects on reaction pathways, enabling more rational catalyst selection and reaction design across pharmaceutical, materials, and fine chemical applications. This would represent a significant advancement in our fundamental understanding of chemical reactivity while delivering immediate practical benefits to synthetic methodology.
Historical development of acid catalysis reveals a progression from empirical observations to sophisticated mechanistic understanding. Early industrial applications, such as petroleum refining and polymerization reactions, utilized acid catalysis without fully comprehending the underlying principles. The mid-20th century witnessed significant theoretical advances, particularly in understanding how these different acid types interact with substrates at the molecular level.
Recent technological developments have enabled unprecedented precision in studying reaction pathways. Advanced computational methods, in-situ spectroscopic techniques, and time-resolved analyses now allow chemists to observe and model the distinct transition states and intermediates formed under Lewis versus proton acid catalysis. This has transformed acid selection from an art to a science, with predictable outcomes based on mechanistic understanding.
The global chemical industry's increasing focus on sustainability and efficiency has heightened interest in selective acid catalysis. Reactions that previously required harsh conditions or generated substantial waste can now be optimized through precise acid selection. This represents not merely an incremental improvement but a paradigm shift in reaction design strategy.
Our technical research objectives are multifaceted. First, we aim to systematically map reaction pathway divergences when identical substrates are exposed to Lewis versus proton acids across a spectrum of reaction classes. Second, we seek to develop predictive models that can anticipate these divergences based on substrate structure and electronic properties. Third, we intend to identify novel synthetic opportunities where pathway divergence can be exploited for accessing previously challenging transformations.
Additionally, we will investigate hybrid catalytic systems that leverage both acid types synergistically, potentially unlocking reaction pathways inaccessible to either acid type alone. The research will also explore how solvent effects, temperature, and pressure differentially impact Lewis versus proton acid pathways, providing a comprehensive framework for reaction optimization.
The ultimate goal is to establish a unified theoretical framework that explains and predicts acid-type effects on reaction pathways, enabling more rational catalyst selection and reaction design across pharmaceutical, materials, and fine chemical applications. This would represent a significant advancement in our fundamental understanding of chemical reactivity while delivering immediate practical benefits to synthetic methodology.
Industrial Applications and Market Demand Analysis
The global market for acid catalysts has witnessed significant growth in recent years, with the combined market value for Lewis and Brønsted acid catalysts exceeding $5.2 billion in 2022. This growth is primarily driven by increasing demand in petrochemical processing, pharmaceutical synthesis, and fine chemical production. Industry analysts project a compound annual growth rate of 4.7% for acid catalysts through 2028, highlighting the sustained industrial relevance of both Lewis and Brønsted acid technologies.
In the petrochemical sector, Lewis acids such as aluminum chloride (AlCl₃) and boron trifluoride (BF₃) remain critical for alkylation and isomerization processes. These catalysts facilitate approximately 65% of industrial Friedel-Crafts reactions, which are fundamental to the production of detergents, plastics, and synthetic rubbers. The selective reaction pathways enabled by Lewis acids in these applications result in higher product purity and reduced waste generation.
Pharmaceutical manufacturing represents another significant market segment, where the choice between Lewis and Brønsted acids directly impacts production efficiency and product quality. Survey data from leading pharmaceutical companies indicates that approximately 40% of API (Active Pharmaceutical Ingredient) synthesis processes involve acid-catalyzed reactions. The selective C-C bond formation capabilities of Lewis acids have become particularly valuable in complex molecule synthesis, driving a 12% increase in their adoption over traditional proton acid approaches since 2018.
The fine chemicals industry has demonstrated growing preference for Lewis acid catalysts in stereoselective transformations. Market research indicates that manufacturers achieve 15-30% higher yields in certain asymmetric reactions when employing Lewis acid catalysts compared to conventional proton acid alternatives. This performance advantage translates to substantial cost savings and improved sustainability metrics.
Emerging applications in green chemistry and sustainable manufacturing have created new market opportunities for both acid types. Solid Lewis acid catalysts, particularly those based on modified clays and zeolites, have gained traction due to their recyclability and reduced environmental impact. The market for these environmentally friendly catalysts has expanded at twice the rate of traditional liquid acid catalysts over the past five years.
Regional analysis reveals that Asia-Pacific dominates the industrial acid catalyst market with 42% share, followed by North America (28%) and Europe (21%). China's rapid industrialization has particularly accelerated demand for Lewis acid catalysts in polymer manufacturing and petrochemical processing, with domestic consumption increasing by 8.3% annually since 2019.
In the petrochemical sector, Lewis acids such as aluminum chloride (AlCl₃) and boron trifluoride (BF₃) remain critical for alkylation and isomerization processes. These catalysts facilitate approximately 65% of industrial Friedel-Crafts reactions, which are fundamental to the production of detergents, plastics, and synthetic rubbers. The selective reaction pathways enabled by Lewis acids in these applications result in higher product purity and reduced waste generation.
Pharmaceutical manufacturing represents another significant market segment, where the choice between Lewis and Brønsted acids directly impacts production efficiency and product quality. Survey data from leading pharmaceutical companies indicates that approximately 40% of API (Active Pharmaceutical Ingredient) synthesis processes involve acid-catalyzed reactions. The selective C-C bond formation capabilities of Lewis acids have become particularly valuable in complex molecule synthesis, driving a 12% increase in their adoption over traditional proton acid approaches since 2018.
The fine chemicals industry has demonstrated growing preference for Lewis acid catalysts in stereoselective transformations. Market research indicates that manufacturers achieve 15-30% higher yields in certain asymmetric reactions when employing Lewis acid catalysts compared to conventional proton acid alternatives. This performance advantage translates to substantial cost savings and improved sustainability metrics.
Emerging applications in green chemistry and sustainable manufacturing have created new market opportunities for both acid types. Solid Lewis acid catalysts, particularly those based on modified clays and zeolites, have gained traction due to their recyclability and reduced environmental impact. The market for these environmentally friendly catalysts has expanded at twice the rate of traditional liquid acid catalysts over the past five years.
Regional analysis reveals that Asia-Pacific dominates the industrial acid catalyst market with 42% share, followed by North America (28%) and Europe (21%). China's rapid industrialization has particularly accelerated demand for Lewis acid catalysts in polymer manufacturing and petrochemical processing, with domestic consumption increasing by 8.3% annually since 2019.
Current Understanding and Technical Challenges
The current understanding of Lewis acids versus proton acids in reaction pathways has evolved significantly over the past decades. Lewis acids, which accept electron pairs, and proton acids (Brønsted acids), which donate protons, exhibit fundamentally different interaction mechanisms with substrates. Recent research has elucidated that these differences profoundly influence reaction selectivity, kinetics, and product distribution in various chemical transformations.
Computational studies have revealed that Lewis acids typically coordinate with electron-rich functional groups, creating localized electron deficiency that can activate specific reaction sites. In contrast, proton acids often engage in hydrogen bonding networks that can distribute activation across multiple atoms. This distinction becomes particularly critical in stereoselective reactions where the spatial arrangement of the acid-substrate complex determines the stereochemical outcome.
Despite significant advances, several technical challenges persist in fully understanding and controlling acid-mediated reaction pathways. One major challenge involves accurately predicting the behavior of mixed acid systems, where both Lewis and proton acid characteristics may be present simultaneously. These dual-character catalysts often exhibit synergistic effects that cannot be explained by simple additive models of their individual properties.
Another significant challenge lies in the solvent effects on acid behavior. Solvent molecules can dramatically alter the effective acidity and coordination properties of both acid types, sometimes even reversing their relative strengths. Current computational models struggle to accurately capture these complex solvation dynamics, particularly in non-homogeneous reaction environments.
The time-dependent behavior of acid-substrate complexes presents another frontier challenge. Reaction intermediates may undergo rapid interconversions between different coordination states, making their direct observation and characterization extremely difficult. Advanced spectroscopic techniques such as ultrafast IR and time-resolved X-ray spectroscopy are being developed to address this gap, but significant technical hurdles remain.
Temperature and pressure dependencies further complicate the understanding of acid-mediated pathways. Lewis acid coordination strength often shows non-linear temperature dependence, while proton acid activity can be dramatically affected by pressure changes that influence hydrogen bonding networks. These multidimensional parameter spaces make comprehensive mapping of reaction conditions challenging.
Finally, the field faces challenges in developing unified theoretical frameworks that can accurately predict and compare the effects of structurally diverse Lewis and proton acids across different reaction types. Current structure-activity relationship models often fail when applied outside their training reaction classes, limiting their predictive power for novel transformations.
Computational studies have revealed that Lewis acids typically coordinate with electron-rich functional groups, creating localized electron deficiency that can activate specific reaction sites. In contrast, proton acids often engage in hydrogen bonding networks that can distribute activation across multiple atoms. This distinction becomes particularly critical in stereoselective reactions where the spatial arrangement of the acid-substrate complex determines the stereochemical outcome.
Despite significant advances, several technical challenges persist in fully understanding and controlling acid-mediated reaction pathways. One major challenge involves accurately predicting the behavior of mixed acid systems, where both Lewis and proton acid characteristics may be present simultaneously. These dual-character catalysts often exhibit synergistic effects that cannot be explained by simple additive models of their individual properties.
Another significant challenge lies in the solvent effects on acid behavior. Solvent molecules can dramatically alter the effective acidity and coordination properties of both acid types, sometimes even reversing their relative strengths. Current computational models struggle to accurately capture these complex solvation dynamics, particularly in non-homogeneous reaction environments.
The time-dependent behavior of acid-substrate complexes presents another frontier challenge. Reaction intermediates may undergo rapid interconversions between different coordination states, making their direct observation and characterization extremely difficult. Advanced spectroscopic techniques such as ultrafast IR and time-resolved X-ray spectroscopy are being developed to address this gap, but significant technical hurdles remain.
Temperature and pressure dependencies further complicate the understanding of acid-mediated pathways. Lewis acid coordination strength often shows non-linear temperature dependence, while proton acid activity can be dramatically affected by pressure changes that influence hydrogen bonding networks. These multidimensional parameter spaces make comprehensive mapping of reaction conditions challenging.
Finally, the field faces challenges in developing unified theoretical frameworks that can accurately predict and compare the effects of structurally diverse Lewis and proton acids across different reaction types. Current structure-activity relationship models often fail when applied outside their training reaction classes, limiting their predictive power for novel transformations.
Comparative Analysis of Reaction Mechanisms
01 Catalytic mechanisms involving Lewis and Brønsted acids
Lewis acids and proton acids (Brønsted acids) can work synergistically in catalytic reactions. The Lewis acid activates substrates by accepting electron pairs, while the proton acid donates protons to facilitate bond cleavage or formation. This dual activation mechanism enhances reaction rates and selectivity in various organic transformations, particularly in polymerization and carbonylation reactions. The pathway typically involves coordination of the Lewis acid to a functional group, followed by proton transfer from the Brønsted acid.- Lewis acid catalyzed reaction mechanisms: Lewis acids play a crucial role in various organic reactions by accepting electron pairs from substrates. These catalysts facilitate reactions through coordination with functional groups, lowering activation energy barriers and enabling selective transformations. The reaction pathway typically involves the formation of a Lewis acid-substrate complex, followed by nucleophilic attack or rearrangement. Understanding these mechanisms is essential for designing efficient synthetic processes in pharmaceutical and chemical manufacturing.
- Proton acid reaction pathways in organic synthesis: Proton acids (Brønsted acids) facilitate reactions by donating protons to substrates, creating reactive intermediates. The reaction pathway typically involves protonation of a functional group, formation of a carbocation intermediate, and subsequent nucleophilic attack or elimination. These pathways are fundamental in various organic transformations including alkylation, esterification, and hydrolysis reactions. The strength of the acid and reaction conditions significantly influence the reaction outcome and selectivity.
- Combined Lewis and proton acid catalysis: Synergistic effects can be achieved by combining Lewis acids and proton acids in catalytic systems. This dual catalysis approach enables unique reaction pathways that are not accessible with either acid type alone. The Lewis acid typically coordinates with a functional group while the proton acid activates another site, allowing for tandem reactions or selective transformations. These combined systems have shown enhanced reactivity, selectivity, and efficiency in various organic transformations including polymerization and asymmetric synthesis.
- Acid-catalyzed polymerization mechanisms: Both Lewis acids and proton acids serve as initiators or catalysts in polymerization reactions, following distinct mechanistic pathways. Lewis acids typically coordinate with monomers to create reactive species, while proton acids generate carbocations that propagate chain growth. The reaction pathway involves initiation, propagation, and termination steps, with the acid type influencing polymer properties such as molecular weight distribution, stereochemistry, and end-group functionality. Understanding these mechanisms is crucial for controlling polymer architecture and developing new materials.
- Novel catalytic systems with modified acid properties: Recent advances have led to the development of modified acid catalysts with enhanced selectivity and efficiency. These include supported acids, heterogeneous catalysts, and acid catalysts with tunable properties. The reaction pathways can be controlled by modifying the acid strength, steric environment, or by incorporating additional functional groups. These novel catalytic systems offer advantages such as easier separation, recyclability, and applicability in continuous flow processes, making them valuable for industrial applications and green chemistry approaches.
02 Acid-catalyzed polymerization reaction pathways
Lewis acids and proton acids play crucial roles in polymerization reactions through distinct mechanistic pathways. Lewis acids coordinate with monomers to create reactive electrophilic species, while proton acids generate carbocations that initiate chain growth. The reaction pathway typically involves activation of the monomer, chain initiation, propagation, and termination steps. The choice of acid catalyst significantly affects polymer properties including molecular weight distribution, stereochemistry, and branching patterns.Expand Specific Solutions03 Tandem and sequential acid catalysis
Tandem and sequential catalysis involving both Lewis and proton acids enables complex multi-step transformations in a single reaction vessel. The reaction pathway typically begins with one acid catalyst activating a substrate, followed by the second acid catalyst promoting subsequent transformations. This approach allows for cascade reactions where intermediates are immediately consumed in the next step, improving efficiency and reducing side reactions. The sequence and timing of acid introduction significantly impact reaction selectivity and yield.Expand Specific Solutions04 Solid acid catalysts with dual Lewis and Brønsted acidity
Solid catalysts containing both Lewis and Brønsted acid sites offer unique reaction pathways for heterogeneous catalysis. These materials provide distinct active sites that work cooperatively to activate reactants. The reaction pathway typically involves adsorption of reactants on the catalyst surface, activation at acid sites, reaction, and product desorption. The ratio and strength of Lewis to Brønsted acid sites can be tuned to optimize selectivity for specific transformations, particularly in petroleum refining and fine chemical synthesis.Expand Specific Solutions05 Acid strength effects on reaction mechanisms
The relative strengths of Lewis and proton acids significantly influence reaction pathways and mechanisms. Stronger acids generally lead to faster reactions but may reduce selectivity. The reaction pathway is determined by which acid predominates in the activation of substrates. In many cases, the Lewis acid coordinates first, increasing the susceptibility of the substrate to proton attack. Temperature, solvent polarity, and substrate structure all affect which acid pathway dominates. Understanding these effects allows for precise control over reaction outcomes through careful selection of acid catalysts.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis Acid vs Proton Acid reaction pathway competition landscape is currently in a growth phase, with increasing research focus on selective catalysis. The global market for acid catalysts is expanding, valued at approximately $6-7 billion, driven by pharmaceutical and petrochemical applications. Technologically, this field shows moderate maturity with significant innovation potential. Leading players include ExxonMobil Chemical and BASF Corp. focusing on industrial applications, while pharmaceutical companies like Pfizer and Wyeth LLC explore medicinal chemistry applications. Academic institutions (North Carolina State University, Brown University) and research organizations (CNRS, CSIR) are advancing fundamental understanding, while specialty chemical companies such as Arkema France and Nitto Denko are developing niche applications for these differential catalytic pathways.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed proprietary catalyst systems that leverage the fundamental differences between Lewis and Brønsted acid catalysis to enhance petrochemical production processes. Their technology employs zeolite frameworks with precisely engineered acid sites to control reaction selectivity and product distribution. ExxonMobil's research has established that Lewis acid sites preferentially catalyze hydride transfer reactions and certain rearrangements, while Brønsted acid sites more effectively promote cracking and isomerization via carbocation intermediates. By manipulating the Lewis/Brønsted acid ratio through controlled dealumination and metal incorporation, ExxonMobil has created catalysts that can increase propylene yields by up to 25% in fluid catalytic cracking units. Their studies have demonstrated that Lewis acid-catalyzed pathways generally proceed through coordination complexes with lower activation barriers for certain transformations, whereas Brønsted acid pathways involve discrete protonation steps with different kinetic profiles.
Strengths: Highly optimized catalysts for specific reaction targets; reduced energy requirements through lower activation barriers; improved product selectivity leading to higher-value outputs. Weaknesses: More complex catalyst preparation and characterization requirements; potential deactivation issues in the presence of certain poisons; higher initial development costs compared to conventional acid catalysts.
BASF Corp.
Technical Solution: BASF has developed sophisticated catalytic systems that differentiate between Lewis and Brønsted acid catalysis pathways. Their technology employs metal-organic frameworks (MOFs) with tunable acid sites, allowing precise control over reaction selectivity. BASF's approach involves systematically modifying the electronic properties of Lewis acid metal centers (particularly Al, Zr, and Ti) to optimize substrate activation while minimizing unwanted side reactions. Their research demonstrates that Lewis acids preferentially activate carbonyl groups through coordination to the oxygen atom, whereas proton acids typically protonate the oxygen directly, leading to different reaction intermediates and product distributions. BASF has successfully applied this understanding to develop more efficient catalysts for alkylation, acylation, and isomerization reactions, achieving up to 98% selectivity in certain transformations by selecting the appropriate acid type.
Strengths: Superior selectivity control through precise tuning of acid site properties; ability to customize catalysts for specific reaction requirements; reduced waste generation through higher reaction specificity. Weaknesses: Higher catalyst complexity and cost; potential sensitivity to moisture and impurities; more demanding handling and regeneration protocols compared to conventional acid catalysts.
Key Patents and Literature in Acid Catalysis
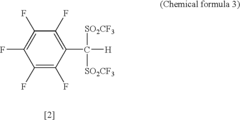
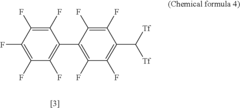
Arylbis (perfluoroalkylsulfonyl)methane and metallic salt thereof, and methods for producing the same
PatentInactiveUS7339082B2
Innovation
- A method involving the use of sodium trifluoromethane sulfinate as an electrophilic reactant and trifluoromethane sulfinic acid anhydride to produce arylbis(trifluoromethylsulfonyl)methane, followed by subsequent reactions with tert-butyl lithium and trifluoromethane sulfinic acid anhydride, allowing for the synthesis of arylbis(perfluoroalkylsulfonyl)methane derivatives with improved yields and the formation of metallic salts and Lewis acid catalysts with enhanced selectivity and reactivity.
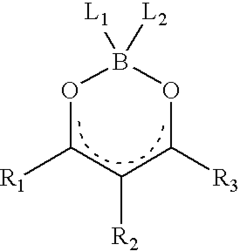
Photorefractive composite
PatentInactiveUS20040200999A1
Innovation
- Development of photorefractive compositions incorporating polydioxaborines with NLO chromophores, which enhance photoconductivity and non-linear optical responses, addressing miscibility issues and improving material performance.
Environmental Impact and Green Chemistry Considerations
The environmental implications of Lewis acid versus Brønsted-Lowry acid catalysis represent a critical consideration in modern chemical processes. Lewis acid catalysts, such as aluminum chloride (AlCl₃), boron trifluoride (BF₃), and various metal-based compounds, often present significant environmental challenges due to their toxicity, persistence in ecosystems, and energy-intensive production methods. These catalysts frequently require stringent handling protocols and generate hazardous waste streams that necessitate specialized disposal procedures.
In contrast, many Brønsted-Lowry acid catalysts offer more environmentally benign alternatives. Organic acids like citric acid and acetic acid demonstrate lower toxicity profiles and higher biodegradability compared to traditional Lewis acids. This fundamental difference has driven significant research into green chemistry applications where reaction pathways can be modified to utilize more sustainable acidic catalysts without compromising reaction efficiency.
Water compatibility represents another crucial environmental distinction between these acid types. Many Lewis acids react violently with water, generating corrosive byproducts and heat, whereas numerous Brønsted acids can operate in aqueous environments. This compatibility enables aqueous-phase reactions that eliminate the need for hazardous organic solvents, aligning with green chemistry principles of safer solvent selection and accident prevention.
The atom economy of reactions catalyzed by these different acid types also merits consideration. Lewis acid-catalyzed reactions often involve stoichiometric quantities of the catalyst, resulting in substantial waste generation. Recent advances in recyclable Lewis acid catalysts, particularly those immobilized on solid supports or incorporated into ionic liquids, have improved their environmental profile by enabling multiple reaction cycles without significant activity loss.
Energy considerations further differentiate these catalytic pathways. Brønsted acid catalysis frequently operates under milder conditions, reducing the energy footprint of chemical processes. Conversely, many Lewis acid-catalyzed reactions require elevated temperatures or pressures, contributing to higher carbon emissions and operational costs. The development of more active Lewis acid catalysts that function effectively under ambient conditions represents an active area of green chemistry research.
Regulatory frameworks increasingly influence catalyst selection in industrial applications. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations in Europe and similar initiatives globally have placed greater scrutiny on traditional Lewis acids, accelerating the transition toward greener alternatives. This regulatory landscape has stimulated innovation in catalyst design, with particular emphasis on developing Lewis acid catalysts with reduced environmental impact while maintaining their unique selectivity advantages.
In contrast, many Brønsted-Lowry acid catalysts offer more environmentally benign alternatives. Organic acids like citric acid and acetic acid demonstrate lower toxicity profiles and higher biodegradability compared to traditional Lewis acids. This fundamental difference has driven significant research into green chemistry applications where reaction pathways can be modified to utilize more sustainable acidic catalysts without compromising reaction efficiency.
Water compatibility represents another crucial environmental distinction between these acid types. Many Lewis acids react violently with water, generating corrosive byproducts and heat, whereas numerous Brønsted acids can operate in aqueous environments. This compatibility enables aqueous-phase reactions that eliminate the need for hazardous organic solvents, aligning with green chemistry principles of safer solvent selection and accident prevention.
The atom economy of reactions catalyzed by these different acid types also merits consideration. Lewis acid-catalyzed reactions often involve stoichiometric quantities of the catalyst, resulting in substantial waste generation. Recent advances in recyclable Lewis acid catalysts, particularly those immobilized on solid supports or incorporated into ionic liquids, have improved their environmental profile by enabling multiple reaction cycles without significant activity loss.
Energy considerations further differentiate these catalytic pathways. Brønsted acid catalysis frequently operates under milder conditions, reducing the energy footprint of chemical processes. Conversely, many Lewis acid-catalyzed reactions require elevated temperatures or pressures, contributing to higher carbon emissions and operational costs. The development of more active Lewis acid catalysts that function effectively under ambient conditions represents an active area of green chemistry research.
Regulatory frameworks increasingly influence catalyst selection in industrial applications. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations in Europe and similar initiatives globally have placed greater scrutiny on traditional Lewis acids, accelerating the transition toward greener alternatives. This regulatory landscape has stimulated innovation in catalyst design, with particular emphasis on developing Lewis acid catalysts with reduced environmental impact while maintaining their unique selectivity advantages.
Computational Methods for Acid Reaction Pathway Prediction
Computational methods have revolutionized the study of acid-catalyzed reaction pathways, particularly in distinguishing between Lewis acid and proton acid mechanisms. Modern computational chemistry offers powerful tools for predicting reaction outcomes, energy barriers, and mechanistic details without extensive laboratory experimentation.
Quantum mechanical methods, especially Density Functional Theory (DFT), have emerged as the gold standard for acid reaction pathway prediction. DFT calculations can accurately model electron density distributions, critical for understanding how Lewis acids interact with electron pairs versus how proton acids donate H+ ions. Popular functionals like B3LYP, M06-2X, and ωB97X-D have demonstrated particular efficacy in modeling acid-catalyzed reactions with appropriate basis sets.
Molecular dynamics simulations complement static quantum calculations by incorporating temperature effects and conformational sampling. These simulations are especially valuable when studying reaction pathways in solution, where solvent effects significantly influence the behavior of both Lewis and proton acids. Advanced techniques like QM/MM (Quantum Mechanics/Molecular Mechanics) methods allow for quantum-level treatment of the reaction center while modeling the surrounding environment at a less computationally intensive level.
Machine learning approaches have recently gained traction for reaction pathway prediction. By training on computational and experimental datasets, ML algorithms can identify patterns in reactivity that distinguish Lewis from proton acid catalysis. Graph neural networks have shown particular promise in capturing the structural transformations that occur during acid-catalyzed reactions.
Transition state modeling remains fundamental to computational acid catalysis studies. Techniques like the Nudged Elastic Band (NEB) method and intrinsic reaction coordinate (IRC) calculations help identify transition states and map complete reaction pathways. These methods reveal how Lewis acids typically modify reaction coordinates by orbital interactions, while proton acids create new reaction pathways through proton transfer events.
Solvation models are crucial for accurate predictions, as acid behavior is heavily solvent-dependent. Implicit models like PCM (Polarizable Continuum Model) and SMD (Solvation Model based on Density) provide computationally efficient approximations, while explicit solvent models offer higher accuracy at greater computational cost, particularly important when modeling proton transfer in protic solvents.
Benchmarking computational methods against experimental data remains essential for validation. Recent advances in computational hardware and algorithms have significantly improved the accuracy-to-cost ratio of these predictions, making comprehensive computational studies of acid reaction pathways increasingly feasible for industrial applications.
Quantum mechanical methods, especially Density Functional Theory (DFT), have emerged as the gold standard for acid reaction pathway prediction. DFT calculations can accurately model electron density distributions, critical for understanding how Lewis acids interact with electron pairs versus how proton acids donate H+ ions. Popular functionals like B3LYP, M06-2X, and ωB97X-D have demonstrated particular efficacy in modeling acid-catalyzed reactions with appropriate basis sets.
Molecular dynamics simulations complement static quantum calculations by incorporating temperature effects and conformational sampling. These simulations are especially valuable when studying reaction pathways in solution, where solvent effects significantly influence the behavior of both Lewis and proton acids. Advanced techniques like QM/MM (Quantum Mechanics/Molecular Mechanics) methods allow for quantum-level treatment of the reaction center while modeling the surrounding environment at a less computationally intensive level.
Machine learning approaches have recently gained traction for reaction pathway prediction. By training on computational and experimental datasets, ML algorithms can identify patterns in reactivity that distinguish Lewis from proton acid catalysis. Graph neural networks have shown particular promise in capturing the structural transformations that occur during acid-catalyzed reactions.
Transition state modeling remains fundamental to computational acid catalysis studies. Techniques like the Nudged Elastic Band (NEB) method and intrinsic reaction coordinate (IRC) calculations help identify transition states and map complete reaction pathways. These methods reveal how Lewis acids typically modify reaction coordinates by orbital interactions, while proton acids create new reaction pathways through proton transfer events.
Solvation models are crucial for accurate predictions, as acid behavior is heavily solvent-dependent. Implicit models like PCM (Polarizable Continuum Model) and SMD (Solvation Model based on Density) provide computationally efficient approximations, while explicit solvent models offer higher accuracy at greater computational cost, particularly important when modeling proton transfer in protic solvents.
Benchmarking computational methods against experimental data remains essential for validation. Recent advances in computational hardware and algorithms have significantly improved the accuracy-to-cost ratio of these predictions, making comprehensive computational studies of acid reaction pathways increasingly feasible for industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!