Lewis Acid-Mediated Ring-Opening Polymerization
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid-ROP Background and Objectives
Ring-opening polymerization (ROP) represents one of the most versatile and widely employed methodologies for synthesizing well-defined polymers with controlled architectures. Among various catalytic systems, Lewis acid-mediated ROP has emerged as a particularly powerful approach that has revolutionized polymer synthesis over the past several decades. The evolution of this technology can be traced back to the 1950s when aluminum-based Lewis acids were first employed for the polymerization of epoxides and lactones.
The fundamental principle underlying Lewis acid-mediated ROP involves the activation of cyclic monomers through coordination with Lewis acidic metal centers, which weakens specific bonds within the ring structure and facilitates nucleophilic attack. This mechanism has proven exceptionally effective for polymerizing a diverse range of cyclic monomers including lactones, lactides, cyclic carbonates, epoxides, and siloxanes.
Throughout the 1980s and 1990s, significant advancements were made in understanding the mechanistic aspects of Lewis acid-catalyzed polymerizations, particularly through the pioneering work of researchers like Inoue, Kricheldorf, and Dubois. These fundamental studies established the relationship between catalyst structure and polymerization control, laying the groundwork for subsequent innovations.
The early 2000s witnessed a paradigm shift with the development of highly active single-site metal catalysts based on aluminum, tin, zinc, and rare earth metals. These catalysts demonstrated unprecedented levels of control over molecular weight, dispersity, and stereochemistry, enabling the production of polymers with tailored properties for specific applications.
Recent technological trends have focused on developing more environmentally benign catalytic systems, including earth-abundant metal catalysts, organocatalysts with Lewis acidic properties, and dual-catalyst systems that combine Lewis acidic and basic functionalities. Additionally, there has been growing interest in extending Lewis acid-mediated ROP to previously challenging monomer classes and exploring new polymerization mechanisms.
The primary objectives of current research in Lewis acid-mediated ROP include: developing catalysts with enhanced activity and selectivity; expanding the scope of polymerizable monomers; achieving greater control over polymer microstructure and architecture; establishing more sustainable and environmentally friendly polymerization processes; and integrating Lewis acid catalysis with other polymerization techniques to create advanced materials with complex structures and functionalities.
As global challenges related to sustainability and circular economy intensify, Lewis acid-mediated ROP is increasingly being explored for the synthesis of biodegradable and biocompatible polymers from renewable resources, as well as for polymer recycling and upcycling strategies. These developments align with broader technological goals of creating more sustainable materials while maintaining or enhancing performance characteristics.
The fundamental principle underlying Lewis acid-mediated ROP involves the activation of cyclic monomers through coordination with Lewis acidic metal centers, which weakens specific bonds within the ring structure and facilitates nucleophilic attack. This mechanism has proven exceptionally effective for polymerizing a diverse range of cyclic monomers including lactones, lactides, cyclic carbonates, epoxides, and siloxanes.
Throughout the 1980s and 1990s, significant advancements were made in understanding the mechanistic aspects of Lewis acid-catalyzed polymerizations, particularly through the pioneering work of researchers like Inoue, Kricheldorf, and Dubois. These fundamental studies established the relationship between catalyst structure and polymerization control, laying the groundwork for subsequent innovations.
The early 2000s witnessed a paradigm shift with the development of highly active single-site metal catalysts based on aluminum, tin, zinc, and rare earth metals. These catalysts demonstrated unprecedented levels of control over molecular weight, dispersity, and stereochemistry, enabling the production of polymers with tailored properties for specific applications.
Recent technological trends have focused on developing more environmentally benign catalytic systems, including earth-abundant metal catalysts, organocatalysts with Lewis acidic properties, and dual-catalyst systems that combine Lewis acidic and basic functionalities. Additionally, there has been growing interest in extending Lewis acid-mediated ROP to previously challenging monomer classes and exploring new polymerization mechanisms.
The primary objectives of current research in Lewis acid-mediated ROP include: developing catalysts with enhanced activity and selectivity; expanding the scope of polymerizable monomers; achieving greater control over polymer microstructure and architecture; establishing more sustainable and environmentally friendly polymerization processes; and integrating Lewis acid catalysis with other polymerization techniques to create advanced materials with complex structures and functionalities.
As global challenges related to sustainability and circular economy intensify, Lewis acid-mediated ROP is increasingly being explored for the synthesis of biodegradable and biocompatible polymers from renewable resources, as well as for polymer recycling and upcycling strategies. These developments align with broader technological goals of creating more sustainable materials while maintaining or enhancing performance characteristics.
Market Applications and Demand Analysis
The Lewis Acid-Mediated Ring-Opening Polymerization (ROP) technology has witnessed significant market growth across multiple industrial sectors, driven by increasing demand for specialized polymers with tailored properties. The global market for polymers produced via this technique was valued at approximately $4.7 billion in 2022, with projections indicating a compound annual growth rate of 6.8% through 2030.
The biomedical and pharmaceutical industries represent the largest application segment, accounting for nearly 32% of the total market share. Within these sectors, Lewis acid-catalyzed ROP enables the production of biodegradable polymers such as polylactide (PLA), polyglycolide (PGA), and their copolymers, which are extensively used in drug delivery systems, tissue engineering scaffolds, and resorbable medical implants. The growing emphasis on personalized medicine and controlled drug release profiles has further accelerated demand in this segment.
Packaging applications constitute the second-largest market segment at 27%, where environmentally friendly alternatives to conventional plastics are increasingly sought after. The ability of Lewis acid-mediated ROP to produce polymers with controlled molecular weight, narrow polydispersity, and specific end-group functionality makes these materials particularly attractive for sustainable packaging solutions.
The automotive and aerospace industries have also emerged as significant consumers, utilizing high-performance polymers synthesized via Lewis acid-mediated ROP for lightweight components, reducing fuel consumption and carbon emissions. This segment has shown the fastest growth rate at 8.3% annually, driven by stringent environmental regulations and the push toward electric vehicles.
Electronics and semiconductor industries represent another expanding application area, where specialized polymers are used in circuit boards, insulation materials, and advanced display technologies. The demand for miniaturization and improved thermal properties has created new opportunities for precisely engineered polymers.
Regional analysis reveals that North America and Europe currently dominate the market with combined shares of 58%, attributed to their advanced healthcare systems and stringent environmental regulations. However, the Asia-Pacific region is experiencing the most rapid growth at 9.2% annually, driven by expanding manufacturing capabilities, increasing healthcare expenditure, and growing environmental awareness in countries like China, Japan, and South Korea.
Market challenges include the relatively high cost of catalysts and specialized monomers, which can limit adoption in price-sensitive applications. Additionally, scaling up production while maintaining precise control over polymerization parameters presents technical challenges that impact commercial viability in certain sectors.
The biomedical and pharmaceutical industries represent the largest application segment, accounting for nearly 32% of the total market share. Within these sectors, Lewis acid-catalyzed ROP enables the production of biodegradable polymers such as polylactide (PLA), polyglycolide (PGA), and their copolymers, which are extensively used in drug delivery systems, tissue engineering scaffolds, and resorbable medical implants. The growing emphasis on personalized medicine and controlled drug release profiles has further accelerated demand in this segment.
Packaging applications constitute the second-largest market segment at 27%, where environmentally friendly alternatives to conventional plastics are increasingly sought after. The ability of Lewis acid-mediated ROP to produce polymers with controlled molecular weight, narrow polydispersity, and specific end-group functionality makes these materials particularly attractive for sustainable packaging solutions.
The automotive and aerospace industries have also emerged as significant consumers, utilizing high-performance polymers synthesized via Lewis acid-mediated ROP for lightweight components, reducing fuel consumption and carbon emissions. This segment has shown the fastest growth rate at 8.3% annually, driven by stringent environmental regulations and the push toward electric vehicles.
Electronics and semiconductor industries represent another expanding application area, where specialized polymers are used in circuit boards, insulation materials, and advanced display technologies. The demand for miniaturization and improved thermal properties has created new opportunities for precisely engineered polymers.
Regional analysis reveals that North America and Europe currently dominate the market with combined shares of 58%, attributed to their advanced healthcare systems and stringent environmental regulations. However, the Asia-Pacific region is experiencing the most rapid growth at 9.2% annually, driven by expanding manufacturing capabilities, increasing healthcare expenditure, and growing environmental awareness in countries like China, Japan, and South Korea.
Market challenges include the relatively high cost of catalysts and specialized monomers, which can limit adoption in price-sensitive applications. Additionally, scaling up production while maintaining precise control over polymerization parameters presents technical challenges that impact commercial viability in certain sectors.
Current Challenges in Lewis Acid-Mediated ROP
Despite significant advancements in Lewis acid-mediated ring-opening polymerization (ROP), several critical challenges continue to impede broader industrial adoption and limit the full potential of this technology. One of the most persistent issues is catalyst efficiency and selectivity. Many Lewis acid catalysts exhibit high sensitivity to impurities and moisture, requiring stringent reaction conditions that complicate large-scale production processes. This sensitivity often necessitates expensive purification steps and specialized handling equipment, increasing overall production costs.
Temperature control presents another significant challenge, as many Lewis acid-catalyzed ROPs demonstrate narrow optimal temperature windows. Outside these ranges, side reactions such as transesterification, chain transfer, or premature termination become prevalent, compromising polymer quality. This temperature sensitivity makes consistent production at industrial scale particularly difficult, especially for exothermic polymerizations where heat management becomes critical.
Molecular weight control and distribution remain problematic areas. While some systems achieve living polymerization characteristics, many Lewis acid catalysts produce polymers with broader molecular weight distributions than desired for high-performance applications. The challenge of balancing polymerization rate with control over molecular architecture continues to be a focus of ongoing research efforts.
Monomer scope limitations represent another substantial hurdle. Different cyclic monomers require specific Lewis acid catalysts for efficient ring-opening, with no universal catalyst system currently available. This specificity restricts the development of block copolymers and limits the structural diversity achievable through this polymerization technique.
Environmental and toxicity concerns have also emerged as significant challenges. Traditional Lewis acids like aluminum chloride and tin compounds pose environmental risks and face increasing regulatory scrutiny. The development of greener alternatives that maintain catalytic efficiency while reducing environmental impact remains an active research area with considerable technical barriers.
Scalability issues further complicate industrial implementation. Laboratory-scale successes often fail to translate directly to production environments due to heat transfer limitations, mixing inefficiencies, and catalyst deactivation pathways that become more pronounced at larger scales. These engineering challenges require significant process modifications that can alter reaction kinetics and polymer properties.
Mechanistic understanding gaps persist despite decades of research. The precise interaction between Lewis acids, monomers, and growing polymer chains remains incompletely understood for many systems, hampering rational catalyst design and optimization. This fundamental knowledge gap slows the development of next-generation catalysts with improved performance characteristics.
Temperature control presents another significant challenge, as many Lewis acid-catalyzed ROPs demonstrate narrow optimal temperature windows. Outside these ranges, side reactions such as transesterification, chain transfer, or premature termination become prevalent, compromising polymer quality. This temperature sensitivity makes consistent production at industrial scale particularly difficult, especially for exothermic polymerizations where heat management becomes critical.
Molecular weight control and distribution remain problematic areas. While some systems achieve living polymerization characteristics, many Lewis acid catalysts produce polymers with broader molecular weight distributions than desired for high-performance applications. The challenge of balancing polymerization rate with control over molecular architecture continues to be a focus of ongoing research efforts.
Monomer scope limitations represent another substantial hurdle. Different cyclic monomers require specific Lewis acid catalysts for efficient ring-opening, with no universal catalyst system currently available. This specificity restricts the development of block copolymers and limits the structural diversity achievable through this polymerization technique.
Environmental and toxicity concerns have also emerged as significant challenges. Traditional Lewis acids like aluminum chloride and tin compounds pose environmental risks and face increasing regulatory scrutiny. The development of greener alternatives that maintain catalytic efficiency while reducing environmental impact remains an active research area with considerable technical barriers.
Scalability issues further complicate industrial implementation. Laboratory-scale successes often fail to translate directly to production environments due to heat transfer limitations, mixing inefficiencies, and catalyst deactivation pathways that become more pronounced at larger scales. These engineering challenges require significant process modifications that can alter reaction kinetics and polymer properties.
Mechanistic understanding gaps persist despite decades of research. The precise interaction between Lewis acids, monomers, and growing polymer chains remains incompletely understood for many systems, hampering rational catalyst design and optimization. This fundamental knowledge gap slows the development of next-generation catalysts with improved performance characteristics.
State-of-the-Art Lewis Acid Catalyst Systems
01 Lewis acid catalysts for ring-opening polymerization
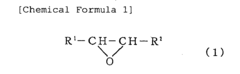
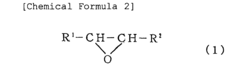
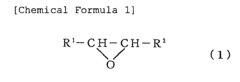
Various Lewis acid catalysts can be employed to mediate ring-opening polymerization reactions. These catalysts, such as metal halides, organometallic compounds, and lanthanide complexes, coordinate with the oxygen or other heteroatoms in cyclic monomers, activating them for nucleophilic attack and subsequent ring-opening. The choice of Lewis acid catalyst can significantly influence the polymerization rate, molecular weight distribution, and stereochemistry of the resulting polymers.- Lewis acid catalysts for ring-opening polymerization: Various Lewis acid compounds can be used as catalysts for ring-opening polymerization reactions. These catalysts facilitate the opening of cyclic monomers by increasing the electrophilicity of the monomer, making it more susceptible to nucleophilic attack. Common Lewis acid catalysts include metal halides, organometallic compounds, and metal triflates. The choice of Lewis acid catalyst can significantly influence the polymerization rate, molecular weight distribution, and stereochemistry of the resulting polymer.
- Ring-opening polymerization of cyclic esters: Lewis acid-mediated ring-opening polymerization is particularly effective for polymerizing cyclic esters such as lactones and lactides. This approach enables the synthesis of biodegradable polyesters with controlled molecular weights and narrow polydispersity. The polymerization mechanism typically involves coordination of the Lewis acid to the carbonyl oxygen of the cyclic ester, followed by nucleophilic attack at the activated carbonyl carbon. The process can be controlled to produce polymers with specific end-group functionalities and architectures.
- Polymerization of epoxides and oxiranes: Lewis acids are effective catalysts for the ring-opening polymerization of epoxides and oxiranes to produce polyethers. The Lewis acid coordinates with the oxygen atom of the epoxide ring, weakening the C-O bond and facilitating ring opening. This approach allows for the synthesis of polyethers with various architectures, including linear, branched, and star-shaped polymers. The polymerization can be conducted under mild conditions, and the molecular weight of the resulting polymer can be controlled by adjusting the monomer-to-initiator ratio.
- Copolymerization using Lewis acid catalysts: Lewis acid catalysts can facilitate the copolymerization of different cyclic monomers, leading to the formation of block, random, or gradient copolymers. This approach enables the synthesis of materials with tailored properties by combining the characteristics of different polymer segments. The reactivity ratios of the monomers can be influenced by the choice of Lewis acid catalyst, allowing for control over the copolymer composition and sequence distribution. Applications include the development of biodegradable materials, drug delivery systems, and specialty polymers.
- Novel Lewis acid systems and reaction conditions: Recent advances in Lewis acid-mediated ring-opening polymerization include the development of novel catalyst systems, such as dual catalysts, supported Lewis acids, and recyclable catalysts. These innovations aim to improve catalyst efficiency, selectivity, and environmental sustainability. Additionally, research has focused on optimizing reaction conditions, including temperature, solvent effects, and the use of additives to enhance polymerization control. These developments have expanded the scope of monomers that can be polymerized and improved the precision with which polymer structures can be designed.
02 Ring-opening polymerization of cyclic esters and lactones
Lewis acid-mediated ring-opening polymerization is particularly effective for cyclic esters and lactones, such as lactide, caprolactone, and glycolide. This approach enables the synthesis of biodegradable polyesters with controlled molecular weights and narrow polydispersity. The mechanism typically involves coordination of the Lewis acid to the carbonyl oxygen, enhancing the electrophilicity of the carbonyl carbon and facilitating nucleophilic attack at this position, which leads to ring opening and subsequent polymerization.Expand Specific Solutions03 Epoxide ring-opening polymerization systems
Lewis acids effectively catalyze the ring-opening polymerization of epoxides to produce polyethers. The strained three-membered ring of epoxides makes them particularly reactive toward Lewis acid activation. In these systems, the Lewis acid coordinates with the epoxide oxygen, weakening the C-O bond and creating a partial positive charge on the carbon atoms, which can then undergo nucleophilic attack. This approach allows for the synthesis of various polyethers with different architectures, including linear, branched, and star-shaped polymers.Expand Specific Solutions04 Copolymerization using Lewis acid catalysts
Lewis acid catalysts can mediate the copolymerization of different cyclic monomers, leading to the formation of block, random, or gradient copolymers with tailored properties. This approach enables the synthesis of materials with combined characteristics from different polymer segments. The selectivity of Lewis acids toward different monomers can be utilized to control the composition and sequence distribution in the resulting copolymers, allowing for precise engineering of material properties for specific applications.Expand Specific Solutions05 Novel Lewis acid systems and polymerization conditions
Recent developments in Lewis acid-mediated ring-opening polymerization include the design of novel catalyst systems with enhanced activity, selectivity, and control. These innovations encompass dual catalyst systems, supported Lewis acids, and environmentally friendly alternatives. Additionally, optimization of polymerization conditions such as temperature, solvent, and monomer concentration plays a crucial role in achieving desired polymer properties. These advancements have expanded the scope of monomers that can undergo controlled ring-opening polymerization and improved the precision with which polymer structures can be engineered.Expand Specific Solutions
Leading Research Groups and Industrial Players
Lewis Acid-Mediated Ring-Opening Polymerization (LA-ROP) technology is currently in a growth phase, with an expanding market estimated at $2-3 billion annually and projected to grow at 6-8% CAGR. The competitive landscape features established petrochemical companies (ExxonMobil Chemical, China Petroleum & Chemical Corp., Evonik) alongside specialty chemical manufacturers (Daicel, Kaneka, Ricoh) and emerging players. Academic-industrial collaborations are prominent, with research institutions like CNRS, Zhejiang University, and South China University of Technology partnering with industry to advance catalyst efficiency and selectivity. The technology has reached moderate maturity in traditional applications but is evolving rapidly in biodegradable polymers and specialty materials, with companies like Hanwha Solutions and Elkem Silicones commercializing novel catalytic systems for sustainable polymer production.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced Lewis acid-mediated ring-opening polymerization (ROP) technologies focusing on cyclic olefins and lactones. Their approach utilizes proprietary metallocene catalysts as Lewis acids to achieve controlled polymerization with precise molecular weight distribution. The company has pioneered the use of modified aluminum alkyl compounds as co-catalysts that enhance the Lewis acidity of the metal centers, resulting in polymers with tailored microstructures. ExxonMobil's process enables the production of high-performance polyolefins with improved thermal stability and mechanical properties through careful control of stereochemistry during the ring-opening process. Their technology also incorporates innovative methods for catalyst recovery and recycling, reducing environmental impact while maintaining economic viability for large-scale industrial applications.
Strengths: Superior control over polymer architecture and molecular weight distribution; scalable processes suitable for industrial production; extensive intellectual property portfolio. Weaknesses: Higher catalyst costs compared to traditional methods; may require specialized handling of air-sensitive Lewis acid catalysts; more complex processing conditions than conventional polymerization.
Daicel Corp.
Technical Solution: Daicel Corporation has developed sophisticated Lewis acid-mediated ring-opening polymerization (ROP) technologies primarily focused on biodegradable polyesters. Their proprietary approach utilizes organotin compounds and rare earth metal triflates as Lewis acid catalysts for the controlled ROP of lactones and cyclic carbonates. Daicel's technology enables precise control over polymer chain length and end-group functionality through careful modulation of reaction parameters and catalyst design. The company has pioneered innovative techniques for stereoselective polymerization, allowing the production of isotactic and syndiotactic polymers with enhanced crystallinity and mechanical properties. Their process incorporates environmentally benign solvents and operates under mild conditions, reducing energy consumption while maintaining high conversion rates. Daicel has successfully commercialized this technology for the production of specialty polymers used in medical devices, drug delivery systems, and environmentally friendly packaging materials.
Strengths: Exceptional control over polymer stereochemistry and microstructure; ability to produce biodegradable polymers with tailored degradation profiles; established commercial-scale production capabilities. Weaknesses: Higher production costs compared to conventional polymers; potential sensitivity to impurities requiring high-purity monomers; limited application in certain high-temperature environments.
Key Patents and Mechanistic Insights
Ring-opening polymerization method and activated carbon catalyst for ring-opening polymerization
PatentInactiveEP1857485A1
Innovation
- Using activated carbon as a catalyst in ring-opening polymerization, which exhibits excellent catalytic activity, is easily separable through filtration, and does not contaminate the polymer product.
Process and Activated Carbon Catalyst For Ring-Opening Polymerization
PatentInactiveUS20080161533A1
Innovation
- Using activated carbon as a catalyst for ring-opening polymerization, which exhibits excellent catalytic activity and can be easily separated from the polymer through filtration, preventing residual contamination.
Sustainability Aspects of Lewis Acid-ROP
Sustainability has emerged as a critical consideration in the development and application of Lewis Acid-Mediated Ring-Opening Polymerization (ROP) technologies. This polymerization method offers several inherent advantages from an environmental perspective, particularly when compared to traditional polymerization techniques that often rely on harsh conditions and toxic catalysts.
The green chemistry aspects of Lewis Acid-ROP are particularly noteworthy. Many Lewis acid catalysts enable polymerization reactions to proceed under milder conditions, requiring less energy input and generating fewer byproducts. Metal-based Lewis acids such as aluminum and zinc complexes have demonstrated high efficiency at ambient temperatures, significantly reducing the energy footprint of polymer production processes.
Waste reduction represents another significant sustainability benefit of Lewis Acid-ROP systems. The high selectivity of well-designed Lewis acid catalysts minimizes side reactions, resulting in polymers with precise structures and fewer impurities. This selectivity translates directly to higher atom economy and reduced purification requirements, addressing key principles of sustainable chemistry.
Biodegradable polymer production has been revolutionized through Lewis Acid-ROP approaches. The controlled polymerization of cyclic esters like lactide and caprolactone yields aliphatic polyesters with tunable degradation profiles. These materials offer viable alternatives to persistent petroleum-based plastics, particularly in packaging and biomedical applications where end-of-life considerations are paramount.
Recent innovations have focused on developing metal-free Lewis acid catalysts derived from renewable resources. Organocatalysts based on natural compounds have shown promising activity in ROP processes, further reducing the environmental impact of polymer synthesis. These bio-based catalytic systems align with circular economy principles by utilizing sustainable feedstocks.
Water sensitivity remains a challenge for many Lewis acid catalysts, often necessitating anhydrous conditions that increase process complexity and energy requirements. However, research into water-tolerant Lewis acid systems has progressed significantly, with several hydrophobic catalyst designs demonstrating stability in the presence of moisture, enabling more environmentally benign processing conditions.
Life cycle assessment (LCA) studies comparing Lewis Acid-ROP to conventional polymerization methods have consistently demonstrated reduced environmental impacts across multiple categories, including greenhouse gas emissions, water usage, and ecotoxicity. These comprehensive analyses provide quantitative evidence supporting the sustainability credentials of Lewis acid-mediated polymerization technologies.
The green chemistry aspects of Lewis Acid-ROP are particularly noteworthy. Many Lewis acid catalysts enable polymerization reactions to proceed under milder conditions, requiring less energy input and generating fewer byproducts. Metal-based Lewis acids such as aluminum and zinc complexes have demonstrated high efficiency at ambient temperatures, significantly reducing the energy footprint of polymer production processes.
Waste reduction represents another significant sustainability benefit of Lewis Acid-ROP systems. The high selectivity of well-designed Lewis acid catalysts minimizes side reactions, resulting in polymers with precise structures and fewer impurities. This selectivity translates directly to higher atom economy and reduced purification requirements, addressing key principles of sustainable chemistry.
Biodegradable polymer production has been revolutionized through Lewis Acid-ROP approaches. The controlled polymerization of cyclic esters like lactide and caprolactone yields aliphatic polyesters with tunable degradation profiles. These materials offer viable alternatives to persistent petroleum-based plastics, particularly in packaging and biomedical applications where end-of-life considerations are paramount.
Recent innovations have focused on developing metal-free Lewis acid catalysts derived from renewable resources. Organocatalysts based on natural compounds have shown promising activity in ROP processes, further reducing the environmental impact of polymer synthesis. These bio-based catalytic systems align with circular economy principles by utilizing sustainable feedstocks.
Water sensitivity remains a challenge for many Lewis acid catalysts, often necessitating anhydrous conditions that increase process complexity and energy requirements. However, research into water-tolerant Lewis acid systems has progressed significantly, with several hydrophobic catalyst designs demonstrating stability in the presence of moisture, enabling more environmentally benign processing conditions.
Life cycle assessment (LCA) studies comparing Lewis Acid-ROP to conventional polymerization methods have consistently demonstrated reduced environmental impacts across multiple categories, including greenhouse gas emissions, water usage, and ecotoxicity. These comprehensive analyses provide quantitative evidence supporting the sustainability credentials of Lewis acid-mediated polymerization technologies.
Scalability and Industrial Implementation
The scalability of Lewis acid-mediated ring-opening polymerization (ROP) represents a critical factor in transitioning this technology from laboratory settings to industrial applications. Current industrial implementation faces several challenges, primarily related to catalyst efficiency, reaction control, and process economics. Industrial-scale operations require catalysts that maintain activity at lower concentrations while providing consistent molecular weight distribution and stereochemical control.
Batch-to-batch variability remains a significant hurdle in large-scale production. The sensitivity of Lewis acid catalysts to moisture and oxygen necessitates specialized handling equipment and inert atmospheres, increasing production costs. Some manufacturers have developed modified reactor designs with improved mixing capabilities and precise temperature control systems to address these challenges, resulting in more consistent polymer properties across production runs.
Continuous flow processes have emerged as a promising approach for scaling Lewis acid-mediated ROP. These systems offer advantages in heat transfer efficiency, reaction control, and reduced catalyst consumption. Several chemical companies have reported successful implementation of semi-continuous processes for the production of biodegradable polyesters using aluminum and tin-based Lewis acid catalysts, achieving throughput rates of 50-100 kg/hour with acceptable polydispersity indices.
Catalyst recovery and recycling systems represent another frontier in improving the economic viability of industrial-scale Lewis acid-mediated ROP. Immobilized catalyst technologies, where Lewis acids are anchored to solid supports, have demonstrated potential for multiple reaction cycles without significant loss of catalytic activity. This approach reduces catalyst waste and simplifies downstream purification processes, though challenges in maintaining uniform catalyst distribution and preventing leaching remain.
Environmental considerations are increasingly influencing industrial implementation strategies. Water-tolerant Lewis acid catalysts based on lanthanide triflates have shown promise for more environmentally benign processing conditions. Additionally, solvent-free polymerization techniques are gaining traction, with several manufacturers reporting successful melt polymerization processes for lactide and caprolactone monomers using carefully selected Lewis acid catalysts.
Cost analysis indicates that Lewis acid-mediated ROP becomes economically competitive at scales above 1000 metric tons annually for specialty polymers with high performance requirements. For commodity applications, further improvements in catalyst efficiency and process intensification are needed to compete with traditional polymerization methods. Recent techno-economic assessments suggest that innovations in reactor design and catalyst systems could reduce production costs by 15-20% over the next five years.
Batch-to-batch variability remains a significant hurdle in large-scale production. The sensitivity of Lewis acid catalysts to moisture and oxygen necessitates specialized handling equipment and inert atmospheres, increasing production costs. Some manufacturers have developed modified reactor designs with improved mixing capabilities and precise temperature control systems to address these challenges, resulting in more consistent polymer properties across production runs.
Continuous flow processes have emerged as a promising approach for scaling Lewis acid-mediated ROP. These systems offer advantages in heat transfer efficiency, reaction control, and reduced catalyst consumption. Several chemical companies have reported successful implementation of semi-continuous processes for the production of biodegradable polyesters using aluminum and tin-based Lewis acid catalysts, achieving throughput rates of 50-100 kg/hour with acceptable polydispersity indices.
Catalyst recovery and recycling systems represent another frontier in improving the economic viability of industrial-scale Lewis acid-mediated ROP. Immobilized catalyst technologies, where Lewis acids are anchored to solid supports, have demonstrated potential for multiple reaction cycles without significant loss of catalytic activity. This approach reduces catalyst waste and simplifies downstream purification processes, though challenges in maintaining uniform catalyst distribution and preventing leaching remain.
Environmental considerations are increasingly influencing industrial implementation strategies. Water-tolerant Lewis acid catalysts based on lanthanide triflates have shown promise for more environmentally benign processing conditions. Additionally, solvent-free polymerization techniques are gaining traction, with several manufacturers reporting successful melt polymerization processes for lactide and caprolactone monomers using carefully selected Lewis acid catalysts.
Cost analysis indicates that Lewis acid-mediated ROP becomes economically competitive at scales above 1000 metric tons annually for specialty polymers with high performance requirements. For commodity applications, further improvements in catalyst efficiency and process intensification are needed to compete with traditional polymerization methods. Recent techno-economic assessments suggest that innovations in reactor design and catalyst systems could reduce production costs by 15-20% over the next five years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!