Lewis Acid vs Lewis Base: Binding Affinity Analysis
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid-Base Theory Evolution and Research Objectives
The Lewis acid-base theory, first proposed by Gilbert N. Lewis in 1923, represents a fundamental paradigm shift in understanding chemical interactions. Unlike the earlier Brønsted-Lowry concept that focused on proton transfer, Lewis's theory expanded the definition to electron pair interactions, where acids act as electron pair acceptors and bases as electron pair donors. This broader conceptualization has proven remarkably versatile across chemistry disciplines, from organic synthesis to materials science.
The evolution of Lewis acid-base theory has been marked by several significant developments. During the 1930s-1950s, researchers established quantitative frameworks for measuring acid-base strengths. The 1960s-1970s witnessed the integration of molecular orbital theory, providing deeper insights into electronic structures underlying these interactions. The 1980s-1990s saw the application of computational chemistry techniques to predict binding affinities with increasing accuracy.
Recent advancements have focused on understanding Lewis acid-base interactions in complex environments, including biological systems, catalytic processes, and materials science applications. The development of the Hard-Soft Acid-Base (HSAB) principle by Ralph Pearson in the 1960s further refined our understanding of preferential binding patterns, explaining why certain acids and bases exhibit stronger affinities for each other.
Current research objectives in Lewis acid-base binding affinity analysis center around several key areas. First, developing more precise quantitative models for predicting binding strengths across diverse chemical environments remains a priority. These models must account for solvent effects, steric factors, and electronic influences that can significantly alter interaction energies.
Second, researchers aim to establish standardized experimental protocols for measuring binding affinities that enable reliable comparisons across different systems. This standardization is crucial for building comprehensive databases that can inform predictive models.
Third, there is growing interest in understanding the dynamic nature of Lewis acid-base interactions, particularly in catalytic systems where transient binding events drive chemical transformations. Time-resolved spectroscopic techniques are being developed to capture these fleeting interactions.
Fourth, the application of machine learning approaches to predict binding affinities represents an emerging frontier. By analyzing patterns in existing experimental data, these computational methods promise to accelerate the discovery of novel acid-base pairs with tailored properties for specific applications.
The ultimate goal of current research is to move beyond descriptive models toward predictive frameworks that can guide the rational design of Lewis acid-base systems for applications ranging from pharmaceutical development to advanced materials synthesis and green chemistry catalysis.
The evolution of Lewis acid-base theory has been marked by several significant developments. During the 1930s-1950s, researchers established quantitative frameworks for measuring acid-base strengths. The 1960s-1970s witnessed the integration of molecular orbital theory, providing deeper insights into electronic structures underlying these interactions. The 1980s-1990s saw the application of computational chemistry techniques to predict binding affinities with increasing accuracy.
Recent advancements have focused on understanding Lewis acid-base interactions in complex environments, including biological systems, catalytic processes, and materials science applications. The development of the Hard-Soft Acid-Base (HSAB) principle by Ralph Pearson in the 1960s further refined our understanding of preferential binding patterns, explaining why certain acids and bases exhibit stronger affinities for each other.
Current research objectives in Lewis acid-base binding affinity analysis center around several key areas. First, developing more precise quantitative models for predicting binding strengths across diverse chemical environments remains a priority. These models must account for solvent effects, steric factors, and electronic influences that can significantly alter interaction energies.
Second, researchers aim to establish standardized experimental protocols for measuring binding affinities that enable reliable comparisons across different systems. This standardization is crucial for building comprehensive databases that can inform predictive models.
Third, there is growing interest in understanding the dynamic nature of Lewis acid-base interactions, particularly in catalytic systems where transient binding events drive chemical transformations. Time-resolved spectroscopic techniques are being developed to capture these fleeting interactions.
Fourth, the application of machine learning approaches to predict binding affinities represents an emerging frontier. By analyzing patterns in existing experimental data, these computational methods promise to accelerate the discovery of novel acid-base pairs with tailored properties for specific applications.
The ultimate goal of current research is to move beyond descriptive models toward predictive frameworks that can guide the rational design of Lewis acid-base systems for applications ranging from pharmaceutical development to advanced materials synthesis and green chemistry catalysis.
Market Applications and Demand for Lewis Acid-Base Interactions
The Lewis acid-base interaction market is experiencing significant growth across multiple industries due to the fundamental importance of these chemical principles in various applications. The global market for catalysts, where Lewis acid-base interactions play a crucial role, was valued at approximately $33.9 billion in 2022 and is projected to reach $45.7 billion by 2030, growing at a CAGR of 4.3%. This growth is primarily driven by increasing demand in pharmaceutical manufacturing, petrochemical processing, and advanced materials development.
In the pharmaceutical sector, Lewis acid-base interactions are essential for drug discovery and development processes. The pharmaceutical industry's increasing focus on complex molecule synthesis has created substantial demand for Lewis acid catalysts that can facilitate selective transformations. This market segment is expected to grow at 5.7% annually through 2028, reflecting the critical role these interactions play in developing new therapeutic agents.
The petrochemical industry represents another major market for Lewis acid-base applications, particularly in refining processes and polymer production. Zeolites and other solid Lewis acid catalysts are extensively used in fluid catalytic cracking, alkylation, and isomerization processes. The market for these specialized catalysts in petrochemical applications exceeded $7.2 billion in 2022, with growth projections of 3.8% annually.
Emerging applications in green chemistry and sustainable manufacturing are creating new market opportunities. Industries are increasingly seeking environmentally friendly catalysts based on Lewis acid-base principles to replace traditional methods that rely on toxic reagents or generate significant waste. This segment is growing at 8.5% annually, reflecting broader sustainability trends across industrial sectors.
The electronic materials sector represents a rapidly expanding market for Lewis acid-base applications, particularly in semiconductor manufacturing and advanced display technologies. Lewis acid dopants and Lewis base-functionalized materials are critical components in next-generation electronic devices. This segment is projected to grow at 9.2% annually through 2028.
Regional analysis indicates that Asia-Pacific dominates the market with approximately 42% share, driven by robust growth in China's chemical manufacturing sector and Japan's advanced materials industry. North America and Europe follow with 28% and 24% market shares respectively, with particular strength in pharmaceutical and specialty chemical applications.
Consumer demand for products manufactured using greener processes is indirectly driving investment in Lewis acid-base technologies that enable more efficient, selective chemical transformations with reduced environmental impact. This trend is expected to continue as regulatory frameworks increasingly favor sustainable manufacturing practices across global markets.
In the pharmaceutical sector, Lewis acid-base interactions are essential for drug discovery and development processes. The pharmaceutical industry's increasing focus on complex molecule synthesis has created substantial demand for Lewis acid catalysts that can facilitate selective transformations. This market segment is expected to grow at 5.7% annually through 2028, reflecting the critical role these interactions play in developing new therapeutic agents.
The petrochemical industry represents another major market for Lewis acid-base applications, particularly in refining processes and polymer production. Zeolites and other solid Lewis acid catalysts are extensively used in fluid catalytic cracking, alkylation, and isomerization processes. The market for these specialized catalysts in petrochemical applications exceeded $7.2 billion in 2022, with growth projections of 3.8% annually.
Emerging applications in green chemistry and sustainable manufacturing are creating new market opportunities. Industries are increasingly seeking environmentally friendly catalysts based on Lewis acid-base principles to replace traditional methods that rely on toxic reagents or generate significant waste. This segment is growing at 8.5% annually, reflecting broader sustainability trends across industrial sectors.
The electronic materials sector represents a rapidly expanding market for Lewis acid-base applications, particularly in semiconductor manufacturing and advanced display technologies. Lewis acid dopants and Lewis base-functionalized materials are critical components in next-generation electronic devices. This segment is projected to grow at 9.2% annually through 2028.
Regional analysis indicates that Asia-Pacific dominates the market with approximately 42% share, driven by robust growth in China's chemical manufacturing sector and Japan's advanced materials industry. North America and Europe follow with 28% and 24% market shares respectively, with particular strength in pharmaceutical and specialty chemical applications.
Consumer demand for products manufactured using greener processes is indirectly driving investment in Lewis acid-base technologies that enable more efficient, selective chemical transformations with reduced environmental impact. This trend is expected to continue as regulatory frameworks increasingly favor sustainable manufacturing practices across global markets.
Current Challenges in Binding Affinity Measurement
Despite significant advancements in analytical techniques, measuring binding affinities between Lewis acids and Lewis bases continues to present several formidable challenges. The heterogeneity of interaction mechanisms across different acid-base pairs creates substantial complexity in developing standardized measurement protocols. Traditional methods such as isothermal titration calorimetry (ITC) and surface plasmon resonance (SPR) often struggle with accuracy when dealing with weak or transient interactions that are common in Lewis acid-base systems.
Environmental factors pose another significant obstacle in binding affinity measurements. Temperature, pressure, solvent effects, and ionic strength can dramatically alter binding constants, sometimes by several orders of magnitude. This environmental sensitivity necessitates precise control conditions that are difficult to maintain consistently across different experimental setups, leading to poor reproducibility between laboratories.
The dynamic nature of Lewis acid-base interactions presents additional complications. Many systems exhibit time-dependent behavior, with binding constants evolving as equilibrium is established. This temporal dimension is frequently overlooked in conventional measurement approaches, resulting in potentially misleading data that captures only a snapshot of a continuously changing interaction landscape.
Computational prediction methods, while increasingly sophisticated, still face limitations in accurately modeling the electronic and steric factors that govern Lewis acid-base interactions. Quantum mechanical calculations often require prohibitive computational resources for larger molecular systems, while molecular dynamics simulations struggle to capture the quantum effects essential for understanding these interactions at a fundamental level.
Reference standard availability represents another critical challenge. The field lacks universally accepted benchmark systems against which new measurements can be calibrated. This absence of standardization makes cross-comparison between different studies problematic and hinders the development of comprehensive binding affinity databases.
Detection sensitivity limitations affect measurements involving low-concentration species or weak interactions. Many Lewis acid-base pairs of theoretical or practical interest have binding constants that fall below the detection thresholds of current analytical instruments, creating significant blind spots in our understanding of these systems.
Multivalent interactions, where multiple binding sites participate simultaneously, introduce additional complexity that current analytical frameworks struggle to address adequately. The cooperative effects observed in such systems often defy simple mathematical modeling, requiring more sophisticated approaches that are still in developmental stages.
Environmental factors pose another significant obstacle in binding affinity measurements. Temperature, pressure, solvent effects, and ionic strength can dramatically alter binding constants, sometimes by several orders of magnitude. This environmental sensitivity necessitates precise control conditions that are difficult to maintain consistently across different experimental setups, leading to poor reproducibility between laboratories.
The dynamic nature of Lewis acid-base interactions presents additional complications. Many systems exhibit time-dependent behavior, with binding constants evolving as equilibrium is established. This temporal dimension is frequently overlooked in conventional measurement approaches, resulting in potentially misleading data that captures only a snapshot of a continuously changing interaction landscape.
Computational prediction methods, while increasingly sophisticated, still face limitations in accurately modeling the electronic and steric factors that govern Lewis acid-base interactions. Quantum mechanical calculations often require prohibitive computational resources for larger molecular systems, while molecular dynamics simulations struggle to capture the quantum effects essential for understanding these interactions at a fundamental level.
Reference standard availability represents another critical challenge. The field lacks universally accepted benchmark systems against which new measurements can be calibrated. This absence of standardization makes cross-comparison between different studies problematic and hinders the development of comprehensive binding affinity databases.
Detection sensitivity limitations affect measurements involving low-concentration species or weak interactions. Many Lewis acid-base pairs of theoretical or practical interest have binding constants that fall below the detection thresholds of current analytical instruments, creating significant blind spots in our understanding of these systems.
Multivalent interactions, where multiple binding sites participate simultaneously, introduce additional complexity that current analytical frameworks struggle to address adequately. The cooperative effects observed in such systems often defy simple mathematical modeling, requiring more sophisticated approaches that are still in developmental stages.
Modern Analytical Methods for Affinity Quantification
01 Lewis acid-base interactions in drug discovery and molecular binding
Lewis acid-base interactions play a crucial role in drug discovery and molecular binding processes. These interactions involve the donation of electron pairs from a Lewis base to a Lewis acid, creating strong binding affinities that can be exploited in pharmaceutical development. Understanding these interactions helps in designing molecules with optimal binding properties to target proteins, receptors, or enzymes, thereby enhancing drug efficacy and selectivity.- Molecular recognition and binding affinity measurement: Lewis acid-base interactions play a crucial role in molecular recognition systems where binding affinity can be measured through various analytical techniques. These interactions form the basis for designing selective binding agents with high affinity for target molecules. The strength of these interactions can be quantified using methods such as isothermal titration calorimetry, surface plasmon resonance, and computational modeling, providing insights into binding kinetics and thermodynamics.
- Drug discovery and pharmaceutical applications: Lewis acid-base interactions are fundamental in drug discovery and pharmaceutical development, where they influence binding affinity between drug candidates and biological targets. These interactions can be optimized to enhance drug potency, selectivity, and pharmacokinetic properties. Understanding the nature of these interactions helps in rational drug design, lead optimization, and the development of structure-activity relationships for novel therapeutic compounds.
- Polymer and material science applications: In polymer and material science, Lewis acid-base interactions contribute significantly to binding affinity between different components, affecting material properties and performance. These interactions can be engineered to create advanced materials with specific characteristics such as improved adhesion, controlled release properties, or responsive behavior. The strength and specificity of these interactions determine the stability and functionality of composite materials, coatings, and functional polymers.
- Biosensors and diagnostic applications: Lewis acid-base interactions are exploited in biosensor development and diagnostic applications where binding affinity determines sensitivity and specificity. These interactions enable the detection of analytes through changes in electrical, optical, or mechanical properties of the sensing platform. By optimizing the acid-base interactions between recognition elements and target molecules, highly sensitive and selective biosensors can be developed for medical diagnostics, environmental monitoring, and food safety applications.
- Computational methods for predicting binding affinity: Computational approaches are increasingly used to predict and analyze Lewis acid-base interactions and their contribution to binding affinity. These methods include quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms that can model complex interaction networks. Computational tools help researchers understand the fundamental principles governing these interactions and enable the rational design of molecules with desired binding properties, reducing the need for extensive experimental screening.
02 Computational methods for predicting Lewis acid-base binding affinities
Various computational methods have been developed to predict and quantify Lewis acid-base binding affinities. These include molecular modeling, quantum mechanical calculations, and machine learning approaches that can simulate the electronic interactions between Lewis acids and bases. Such computational tools enable researchers to screen potential binding partners virtually, reducing the need for extensive experimental testing and accelerating the discovery of compounds with desired binding properties.Expand Specific Solutions03 Novel materials exploiting Lewis acid-base interactions
Innovative materials that leverage Lewis acid-base interactions have been developed for various applications. These materials include polymers, catalysts, sensors, and functional coatings with enhanced properties. By incorporating Lewis acid or base moieties into material structures, researchers can create systems with tunable binding affinities, responsive behaviors, and improved performance characteristics for specific industrial or biomedical applications.Expand Specific Solutions04 Biological systems utilizing Lewis acid-base binding
In biological systems, Lewis acid-base interactions are fundamental to many processes including enzyme catalysis, protein folding, and molecular recognition. These interactions contribute significantly to the binding affinity between biomolecules such as proteins and their ligands, nucleic acids, or other proteins. Understanding the role of Lewis acid-base interactions in biological contexts helps in developing therapeutic strategies that can modulate these interactions for disease treatment.Expand Specific Solutions05 Analytical techniques for measuring Lewis acid-base binding affinities
Various analytical techniques have been developed to measure and characterize Lewis acid-base binding affinities. These include spectroscopic methods (NMR, IR, UV-Vis), calorimetric approaches (isothermal titration calorimetry), surface plasmon resonance, and electrochemical techniques. These methods provide quantitative data on binding strengths, kinetics, and thermodynamic parameters, enabling researchers to optimize molecular designs for specific binding requirements in applications ranging from catalysis to drug development.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis Acid-Lewis Base binding affinity analysis field is currently in a growth phase, with increasing market demand driven by pharmaceutical and chemical industries. The market size is expanding as applications in drug discovery, catalysis, and materials science gain prominence. Technologically, the field shows moderate maturity with established theoretical frameworks but ongoing innovation in computational methods and experimental techniques. Leading companies like Merck Patent GmbH, Pfizer, and Takeda Pharmaceutical are advancing research through proprietary methodologies, while academic institutions such as California Institute of Technology and Zhejiang University contribute fundamental research. ExxonMobil Chemical and Reliance Industries represent industrial applications in catalysis, with specialized firms like Full Spectrum Genetics developing novel protein-binding analysis techniques.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has developed advanced computational methods for Lewis acid-base binding affinity analysis using density functional theory (DFT) calculations. Their approach incorporates sophisticated electronic structure calculations to quantitatively predict binding energies between Lewis acids and bases with high accuracy. Caltech researchers have pioneered the use of molecular orbital analysis to understand the fundamental nature of Lewis acid-base interactions, particularly focusing on how orbital energy gaps correlate with binding strength. Their methodology includes solvent effects through continuum solvation models, allowing for more realistic predictions of binding affinities in various chemical environments. Caltech has also developed machine learning algorithms that can rapidly predict Lewis acid-base pair compatibilities based on electronic and steric parameters, significantly accelerating the discovery of new catalytic systems.
Strengths: Superior computational accuracy through advanced quantum mechanical methods; integration of machine learning for rapid screening of acid-base pairs. Weakness: Computational approaches may require validation with experimental data; high computational cost for analyzing complex molecular systems.
Centre National de la Recherche Scientifique
Technical Solution: Centre National de la Recherche Scientifique (CNRS) has established a comprehensive experimental platform for Lewis acid-base binding affinity analysis using isothermal titration calorimetry (ITC) combined with spectroscopic methods. Their approach enables direct measurement of thermodynamic parameters (ΔH, ΔS, ΔG) for acid-base interactions across diverse chemical systems. CNRS researchers have developed specialized NMR techniques to probe the electronic environment changes during Lewis acid-base adduct formation, providing detailed structural insights into binding mechanisms. Their methodology incorporates kinetic studies using stopped-flow techniques to determine association and dissociation rate constants, offering a complete energetic profile of Lewis acid-base interactions. Additionally, CNRS has pioneered the use of computational chemistry in conjunction with experimental data to establish structure-activity relationships for predicting binding affinities in novel acid-base combinations.
Strengths: Comprehensive experimental approach combining multiple analytical techniques; direct measurement of thermodynamic parameters. Weakness: Requires specialized equipment and expertise; some techniques may have limitations for extremely fast binding events or highly air/moisture-sensitive compounds.
Key Innovations in Computational Binding Prediction
Ionic liquid, adduct and methods thereof
PatentWO2016005935A1
Innovation
- A process that reacts at least one electron-pair acceptor with at least one electron-pair donor to form an adduct, which is then further reacted with an electron-pair acceptor to produce the ionic liquid without the need for heating, using a method that involves contacting the reactants in the presence or absence of solvents and under inert atmospheres to obtain the ionic liquid.
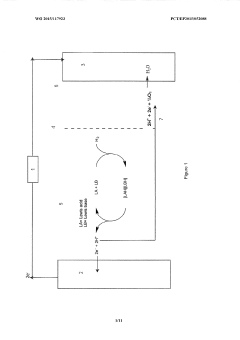
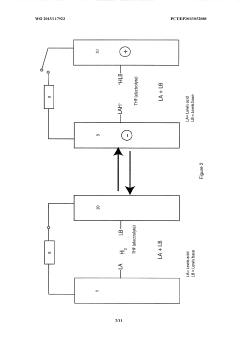
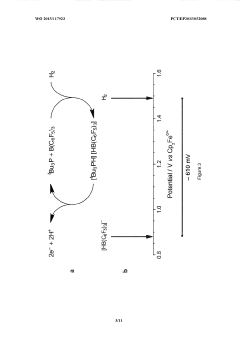
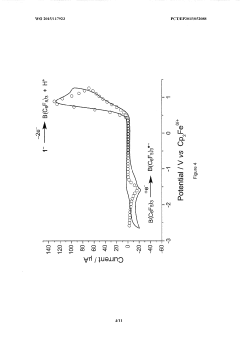
Lewis acid electrocatalysed fuel cell & battery
PatentWO2015117923A1
Innovation
- The use of an electrocatalytic frustrated Lewis pair system comprising a Lewis acid and a Lewis base that heterolytically cleaves dihydrogen, generating a hydride and a protonated base, which can then be oxidized to produce electrical energy in a fuel cell, eliminating the need for expensive metal catalysts.
Solvent Effects on Lewis Acid-Base Interactions
The solvent environment plays a critical role in modulating Lewis acid-base interactions, often dramatically altering binding affinities and reaction outcomes. Solvents create complex coordination spheres around both Lewis acids and bases, affecting their electronic properties and accessibility for interaction. This phenomenon is particularly evident in polar solvents, where strong solvation of charged species can significantly diminish binding affinities compared to those observed in non-polar environments.
Protic solvents, such as water and alcohols, form hydrogen bonds with Lewis bases, effectively competing with Lewis acids for coordination sites. This competition can reduce the apparent binding affinity by several orders of magnitude. For instance, the coordination of BF₃ with amines shows markedly decreased binding constants in water compared to dichloromethane, primarily due to water molecules forming hydrogen bonds with the nitrogen lone pair.
Aprotic polar solvents like acetonitrile and dimethyl sulfoxide present different challenges, as they preferentially solvate Lewis acids through their donor atoms. This selective solvation can alter the effective Lewis acidity, sometimes enhancing and other times diminishing binding interactions depending on the specific acid-base pair under consideration.
Dielectric constant effects further complicate these interactions, with high-dielectric media stabilizing charged intermediates formed during acid-base coordination. This stabilization can shift equilibria toward either association or dissociation depending on the charge distribution in the resulting complex. Computational studies have demonstrated that implicit solvent models often fail to capture these nuanced effects, necessitating explicit solvent molecule inclusion in simulations.
Temperature dependence of solvent properties introduces additional complexity, as solvent reorganization energies vary with temperature, affecting the entropic and enthalpic components of binding. Recent research employing isothermal titration calorimetry has revealed that solvent displacement from coordination spheres can contribute significantly to the overall thermodynamics of Lewis acid-base interactions.
Industrial applications have leveraged these solvent effects to tune reaction selectivity and efficiency. For example, in catalytic processes involving aluminum-based Lewis acids, careful solvent selection has enabled enhanced product yields and reduced side reactions by modulating the effective acidity of the catalyst. Similarly, pharmaceutical research has utilized solvent effects to control the binding specificity of Lewis acidic drug candidates to their biological targets.
Protic solvents, such as water and alcohols, form hydrogen bonds with Lewis bases, effectively competing with Lewis acids for coordination sites. This competition can reduce the apparent binding affinity by several orders of magnitude. For instance, the coordination of BF₃ with amines shows markedly decreased binding constants in water compared to dichloromethane, primarily due to water molecules forming hydrogen bonds with the nitrogen lone pair.
Aprotic polar solvents like acetonitrile and dimethyl sulfoxide present different challenges, as they preferentially solvate Lewis acids through their donor atoms. This selective solvation can alter the effective Lewis acidity, sometimes enhancing and other times diminishing binding interactions depending on the specific acid-base pair under consideration.
Dielectric constant effects further complicate these interactions, with high-dielectric media stabilizing charged intermediates formed during acid-base coordination. This stabilization can shift equilibria toward either association or dissociation depending on the charge distribution in the resulting complex. Computational studies have demonstrated that implicit solvent models often fail to capture these nuanced effects, necessitating explicit solvent molecule inclusion in simulations.
Temperature dependence of solvent properties introduces additional complexity, as solvent reorganization energies vary with temperature, affecting the entropic and enthalpic components of binding. Recent research employing isothermal titration calorimetry has revealed that solvent displacement from coordination spheres can contribute significantly to the overall thermodynamics of Lewis acid-base interactions.
Industrial applications have leveraged these solvent effects to tune reaction selectivity and efficiency. For example, in catalytic processes involving aluminum-based Lewis acids, careful solvent selection has enabled enhanced product yields and reduced side reactions by modulating the effective acidity of the catalyst. Similarly, pharmaceutical research has utilized solvent effects to control the binding specificity of Lewis acidic drug candidates to their biological targets.
Catalytic Applications of Tuned Lewis Pairs
The catalytic applications of tuned Lewis pairs represent a significant advancement in the field of synthetic chemistry and industrial catalysis. These systems, which leverage the precise interaction between Lewis acids and Lewis bases, have demonstrated remarkable efficiency in various chemical transformations. The strategic tuning of Lewis acid-base pairs allows for the optimization of binding affinities, resulting in enhanced catalytic performance across multiple reaction types.
In organic synthesis, tuned Lewis pairs have revolutionized carbon-carbon bond formation processes. By carefully adjusting the electronic properties of Lewis acidic metal centers and their corresponding basic counterparts, researchers have developed catalysts capable of promoting asymmetric aldol reactions with exceptional stereoselectivity. These systems typically achieve over 95% enantiomeric excess, significantly outperforming traditional catalytic approaches.
Hydrogenation reactions have particularly benefited from frustrated Lewis pair (FLP) catalysis, where steric hindrance prevents the formation of classical Lewis adducts. This unique property enables the heterolytic cleavage of H₂, creating powerful hydrogenation catalysts that operate under mild conditions without requiring precious metals. Industrial applications of these systems have emerged in pharmaceutical manufacturing, where selective hydrogenation of functional groups is critical for drug synthesis.
Polymerization processes represent another domain where tuned Lewis pairs have demonstrated considerable utility. The controlled ring-opening polymerization of cyclic esters and carbonates can be precisely regulated through the modulation of Lewis acid strength. This approach has enabled the production of biodegradable polymers with predetermined molecular weights and narrow polydispersity indices, addressing growing demands for sustainable materials.
Environmental applications have also emerged, with tuned Lewis pairs showing promise in carbon dioxide capture and conversion. The cooperative interaction between Lewis acidic sites that activate CO₂ and proximal basic centers that facilitate nucleophilic attack has led to catalytic systems capable of transforming this greenhouse gas into value-added chemicals such as cyclic carbonates and carboxylic acids.
Recent developments have focused on heterogeneous catalysts incorporating tuned Lewis pairs, addressing challenges related to catalyst recovery and continuous processing. Immobilization strategies utilizing silica, metal-organic frameworks, and polymeric supports have successfully translated the exceptional activity of homogeneous Lewis pair catalysts into recyclable systems suitable for industrial implementation, while maintaining comparable catalytic performance through multiple reaction cycles.
In organic synthesis, tuned Lewis pairs have revolutionized carbon-carbon bond formation processes. By carefully adjusting the electronic properties of Lewis acidic metal centers and their corresponding basic counterparts, researchers have developed catalysts capable of promoting asymmetric aldol reactions with exceptional stereoselectivity. These systems typically achieve over 95% enantiomeric excess, significantly outperforming traditional catalytic approaches.
Hydrogenation reactions have particularly benefited from frustrated Lewis pair (FLP) catalysis, where steric hindrance prevents the formation of classical Lewis adducts. This unique property enables the heterolytic cleavage of H₂, creating powerful hydrogenation catalysts that operate under mild conditions without requiring precious metals. Industrial applications of these systems have emerged in pharmaceutical manufacturing, where selective hydrogenation of functional groups is critical for drug synthesis.
Polymerization processes represent another domain where tuned Lewis pairs have demonstrated considerable utility. The controlled ring-opening polymerization of cyclic esters and carbonates can be precisely regulated through the modulation of Lewis acid strength. This approach has enabled the production of biodegradable polymers with predetermined molecular weights and narrow polydispersity indices, addressing growing demands for sustainable materials.
Environmental applications have also emerged, with tuned Lewis pairs showing promise in carbon dioxide capture and conversion. The cooperative interaction between Lewis acidic sites that activate CO₂ and proximal basic centers that facilitate nucleophilic attack has led to catalytic systems capable of transforming this greenhouse gas into value-added chemicals such as cyclic carbonates and carboxylic acids.
Recent developments have focused on heterogeneous catalysts incorporating tuned Lewis pairs, addressing challenges related to catalyst recovery and continuous processing. Immobilization strategies utilizing silica, metal-organic frameworks, and polymeric supports have successfully translated the exceptional activity of homogeneous Lewis pair catalysts into recyclable systems suitable for industrial implementation, while maintaining comparable catalytic performance through multiple reaction cycles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!