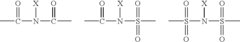Role of Lewis Acid in CO2 Activation
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has emerged as a pivotal field in chemical transformations, with its history dating back to the early 20th century when Gilbert N. Lewis first defined acids as electron pair acceptors. The evolution of Lewis acid catalysis has been marked by significant advancements in understanding reaction mechanisms, developing novel catalysts, and expanding application domains. In recent decades, this field has gained renewed attention due to its potential in addressing one of the most pressing environmental challenges: carbon dioxide activation.
Carbon dioxide (CO2) is a stable molecule with a linear structure and strong C=O bonds, making its activation energetically demanding. The increasing atmospheric CO2 concentration has driven research efforts toward developing efficient methods for CO2 capture, conversion, and utilization. Lewis acids play a crucial role in this context by interacting with the oxygen atoms of CO2, weakening the C=O bonds, and facilitating subsequent transformations.
The technological trajectory of Lewis acid catalysis for CO2 activation has progressed from simple metal-based Lewis acids to sophisticated multifunctional catalytic systems. Early approaches primarily utilized aluminum and boron compounds, while contemporary research explores transition metals, lanthanides, and heterogeneous catalytic materials with tailored Lewis acidic sites. This evolution reflects the growing understanding of electronic effects, steric factors, and cooperative catalysis principles.
The primary objective of current research in this domain is to develop highly efficient, selective, and sustainable Lewis acid catalysts capable of activating CO2 under mild conditions. This includes designing catalysts with optimal binding strength—strong enough to activate CO2 but not so strong as to inhibit product release—and exploring synergistic effects between Lewis acids and other functional groups or catalytic species.
Another critical goal is to integrate Lewis acid catalysis into practical CO2 utilization processes, such as the synthesis of value-added chemicals (e.g., formic acid, methanol, carbonates) and polymers. This requires addressing challenges related to catalyst stability, recyclability, and compatibility with industrial-scale operations. Additionally, researchers aim to elucidate detailed mechanistic pathways of CO2 activation by Lewis acids, employing advanced spectroscopic techniques and computational methods.
The technological landscape is further shaped by the increasing focus on sustainable chemistry principles, driving the development of earth-abundant metal catalysts and environmentally benign reaction conditions. As global efforts to mitigate climate change intensify, Lewis acid catalysis for CO2 activation stands at the intersection of fundamental chemical research and practical environmental solutions.
Carbon dioxide (CO2) is a stable molecule with a linear structure and strong C=O bonds, making its activation energetically demanding. The increasing atmospheric CO2 concentration has driven research efforts toward developing efficient methods for CO2 capture, conversion, and utilization. Lewis acids play a crucial role in this context by interacting with the oxygen atoms of CO2, weakening the C=O bonds, and facilitating subsequent transformations.
The technological trajectory of Lewis acid catalysis for CO2 activation has progressed from simple metal-based Lewis acids to sophisticated multifunctional catalytic systems. Early approaches primarily utilized aluminum and boron compounds, while contemporary research explores transition metals, lanthanides, and heterogeneous catalytic materials with tailored Lewis acidic sites. This evolution reflects the growing understanding of electronic effects, steric factors, and cooperative catalysis principles.
The primary objective of current research in this domain is to develop highly efficient, selective, and sustainable Lewis acid catalysts capable of activating CO2 under mild conditions. This includes designing catalysts with optimal binding strength—strong enough to activate CO2 but not so strong as to inhibit product release—and exploring synergistic effects between Lewis acids and other functional groups or catalytic species.
Another critical goal is to integrate Lewis acid catalysis into practical CO2 utilization processes, such as the synthesis of value-added chemicals (e.g., formic acid, methanol, carbonates) and polymers. This requires addressing challenges related to catalyst stability, recyclability, and compatibility with industrial-scale operations. Additionally, researchers aim to elucidate detailed mechanistic pathways of CO2 activation by Lewis acids, employing advanced spectroscopic techniques and computational methods.
The technological landscape is further shaped by the increasing focus on sustainable chemistry principles, driving the development of earth-abundant metal catalysts and environmentally benign reaction conditions. As global efforts to mitigate climate change intensify, Lewis acid catalysis for CO2 activation stands at the intersection of fundamental chemical research and practical environmental solutions.
Market Analysis for CO2 Utilization Technologies
The global market for CO2 utilization technologies has been experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $2.5 billion in 2022 and is projected to reach $8.6 billion by 2030, growing at a CAGR of 16.8% during the forecast period. This growth trajectory reflects the urgent need for sustainable carbon management solutions across various industries.
CO2 utilization technologies that leverage Lewis acid catalysts for CO2 activation represent a particularly promising segment within this market. These technologies enable the conversion of CO2 into valuable chemicals and fuels, creating economic incentives for carbon capture while simultaneously addressing environmental challenges. The market for Lewis acid-based CO2 conversion is expected to grow at an above-average rate of 19.2% annually through 2028.
Geographically, Europe currently leads the market with approximately 38% share, followed by North America (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth due to rapid industrialization, increasing environmental regulations, and substantial government investments in green technologies, particularly in China, Japan, and South Korea.
By application segment, the market for CO2-derived chemicals and materials holds the largest share (42%), followed by enhanced oil recovery (28%), synthetic fuels (18%), and other applications (12%). Within the chemicals segment, the production of methanol, formic acid, and cyclic carbonates using Lewis acid catalysts represents high-growth areas with significant commercial potential.
Key market drivers include stringent carbon emission regulations, carbon pricing mechanisms, government incentives for green technologies, and growing corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and various national carbon pricing schemes are creating strong economic incentives for CO2 utilization technologies.
Market challenges include high capital costs, energy requirements for CO2 conversion processes, and competition from established fossil-based production routes. The economic viability of many CO2 utilization technologies remains dependent on policy support and carbon pricing mechanisms, though technological advancements in Lewis acid catalysis are steadily improving process economics.
Customer segments showing the strongest demand include chemical manufacturers seeking sustainable feedstocks, energy companies diversifying beyond fossil fuels, and industrial emitters looking to monetize carbon emissions. Additionally, consumer-facing companies are increasingly willing to pay premium prices for products made with captured carbon to enhance their sustainability credentials.
CO2 utilization technologies that leverage Lewis acid catalysts for CO2 activation represent a particularly promising segment within this market. These technologies enable the conversion of CO2 into valuable chemicals and fuels, creating economic incentives for carbon capture while simultaneously addressing environmental challenges. The market for Lewis acid-based CO2 conversion is expected to grow at an above-average rate of 19.2% annually through 2028.
Geographically, Europe currently leads the market with approximately 38% share, followed by North America (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth due to rapid industrialization, increasing environmental regulations, and substantial government investments in green technologies, particularly in China, Japan, and South Korea.
By application segment, the market for CO2-derived chemicals and materials holds the largest share (42%), followed by enhanced oil recovery (28%), synthetic fuels (18%), and other applications (12%). Within the chemicals segment, the production of methanol, formic acid, and cyclic carbonates using Lewis acid catalysts represents high-growth areas with significant commercial potential.
Key market drivers include stringent carbon emission regulations, carbon pricing mechanisms, government incentives for green technologies, and growing corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and various national carbon pricing schemes are creating strong economic incentives for CO2 utilization technologies.
Market challenges include high capital costs, energy requirements for CO2 conversion processes, and competition from established fossil-based production routes. The economic viability of many CO2 utilization technologies remains dependent on policy support and carbon pricing mechanisms, though technological advancements in Lewis acid catalysis are steadily improving process economics.
Customer segments showing the strongest demand include chemical manufacturers seeking sustainable feedstocks, energy companies diversifying beyond fossil fuels, and industrial emitters looking to monetize carbon emissions. Additionally, consumer-facing companies are increasingly willing to pay premium prices for products made with captured carbon to enhance their sustainability credentials.
Current Status and Challenges in CO2 Activation
The global landscape of CO2 activation research has witnessed significant advancements in recent years, with Lewis acid catalysis emerging as a pivotal approach. Currently, several major research institutions across North America, Europe, and Asia are actively exploring the role of Lewis acids in CO2 conversion processes. Despite these efforts, the field faces substantial challenges in achieving commercially viable activation methods that operate under mild conditions with high efficiency.
A primary technical hurdle remains the kinetic and thermodynamic stability of CO2 molecules, which necessitates substantial energy input for activation. Lewis acids show promise by polarizing the C=O bond and lowering activation barriers, but current systems typically require elevated temperatures or pressures to achieve meaningful conversion rates. This energy requirement significantly impacts the carbon neutrality of the overall process.
Selectivity presents another major challenge, as CO2 conversion pathways can lead to multiple products including carbon monoxide, formic acid, methanol, and higher hydrocarbons. Controlling reaction pathways to favor specific high-value products remains difficult even with advanced Lewis acid catalysts. Additionally, catalyst deactivation through poisoning, particularly in the presence of water or other reaction byproducts, continues to limit industrial applicability.
The integration of Lewis acids with transition metal centers has shown enhanced catalytic performance, but the precise mechanistic understanding of these cooperative effects remains incomplete. Computational studies have provided valuable insights into reaction mechanisms, yet bridging the gap between theoretical predictions and experimental outcomes continues to challenge researchers in the field.
From a practical standpoint, the scalability of laboratory-proven Lewis acid catalysts to industrial processes represents a significant bottleneck. Many promising systems utilize expensive or rare earth metals, raising concerns about long-term sustainability and economic viability. Research into earth-abundant Lewis acidic materials has increased, but these alternatives often demonstrate lower activity or stability.
Water tolerance remains particularly problematic for many Lewis acid catalysts, as industrial CO2 streams typically contain moisture that can deactivate catalytic sites. Recent developments in hydrophobic catalyst supports and water-resistant Lewis acid systems show promise but require further optimization for practical implementation.
The geographic distribution of research expertise shows concentration in regions with strong chemical industries and climate commitments, with notable contributions from research groups in Germany, Japan, the United States, and China. International collaboration has accelerated in recent years, particularly around developing standardized benchmarking protocols to enable meaningful comparison between different Lewis acid catalyst systems.
A primary technical hurdle remains the kinetic and thermodynamic stability of CO2 molecules, which necessitates substantial energy input for activation. Lewis acids show promise by polarizing the C=O bond and lowering activation barriers, but current systems typically require elevated temperatures or pressures to achieve meaningful conversion rates. This energy requirement significantly impacts the carbon neutrality of the overall process.
Selectivity presents another major challenge, as CO2 conversion pathways can lead to multiple products including carbon monoxide, formic acid, methanol, and higher hydrocarbons. Controlling reaction pathways to favor specific high-value products remains difficult even with advanced Lewis acid catalysts. Additionally, catalyst deactivation through poisoning, particularly in the presence of water or other reaction byproducts, continues to limit industrial applicability.
The integration of Lewis acids with transition metal centers has shown enhanced catalytic performance, but the precise mechanistic understanding of these cooperative effects remains incomplete. Computational studies have provided valuable insights into reaction mechanisms, yet bridging the gap between theoretical predictions and experimental outcomes continues to challenge researchers in the field.
From a practical standpoint, the scalability of laboratory-proven Lewis acid catalysts to industrial processes represents a significant bottleneck. Many promising systems utilize expensive or rare earth metals, raising concerns about long-term sustainability and economic viability. Research into earth-abundant Lewis acidic materials has increased, but these alternatives often demonstrate lower activity or stability.
Water tolerance remains particularly problematic for many Lewis acid catalysts, as industrial CO2 streams typically contain moisture that can deactivate catalytic sites. Recent developments in hydrophobic catalyst supports and water-resistant Lewis acid systems show promise but require further optimization for practical implementation.
The geographic distribution of research expertise shows concentration in regions with strong chemical industries and climate commitments, with notable contributions from research groups in Germany, Japan, the United States, and China. International collaboration has accelerated in recent years, particularly around developing standardized benchmarking protocols to enable meaningful comparison between different Lewis acid catalyst systems.
Existing Lewis Acid Mechanisms for CO2 Activation
01 Metal-based Lewis acid catalysts for CO2 activation
Metal-based Lewis acid catalysts play a crucial role in CO2 activation by coordinating with the oxygen atoms of CO2, which weakens the C=O bonds and facilitates further reactions. Various transition metals and their complexes, such as aluminum, zinc, and titanium compounds, can serve as effective Lewis acid catalysts for CO2 conversion into valuable chemicals and fuels. These catalysts can be optimized through ligand design to enhance their CO2 activation capabilities.- Metal-based Lewis acid catalysts for CO2 activation: Metal-based Lewis acid catalysts play a crucial role in CO2 activation processes. These catalysts, often containing transition metals or metal complexes, can effectively coordinate with CO2 molecules, weakening the C=O bonds and facilitating subsequent transformations. The Lewis acidic metal centers interact with the oxygen atoms of CO2, creating a more reactive species that can undergo various chemical reactions including carboxylation, reduction, and coupling reactions.
- CO2 conversion to valuable chemicals using Lewis acid catalysis: Lewis acid catalysts enable the conversion of CO2 into valuable chemical products through various reaction pathways. These processes include the transformation of CO2 into carbonates, carbamates, carboxylic acids, and other carbon-containing compounds. The Lewis acid activation of CO2 allows for its incorporation into organic molecules, providing sustainable routes to chemicals traditionally derived from fossil resources. This approach represents an environmentally friendly method for carbon capture and utilization.
- Novel Lewis acid materials for enhanced CO2 activation: Advanced materials with Lewis acidic properties have been developed specifically for CO2 activation. These include metal-organic frameworks (MOFs), functionalized porous materials, and nanostructured catalysts with exposed Lewis acid sites. These materials often feature high surface areas and tunable pore structures that can be optimized for CO2 adsorption and subsequent activation. The design of these materials focuses on maximizing the interaction between CO2 molecules and Lewis acid sites to achieve higher catalytic efficiency.
- Combined Lewis acid-base systems for CO2 activation: Dual-functional catalytic systems incorporating both Lewis acidic and basic sites demonstrate enhanced efficiency in CO2 activation. In these systems, the Lewis acid component coordinates with the oxygen atoms of CO2, while the basic component interacts with the carbon atom, creating a synergistic effect that significantly lowers the activation energy barrier. This cooperative activation strategy enables milder reaction conditions and improved selectivity in CO2 transformation processes, making industrial applications more feasible.
- Electrochemical CO2 reduction using Lewis acid promoters: Lewis acid promoters can significantly enhance electrochemical CO2 reduction processes by modifying the electronic environment at electrode surfaces. These Lewis acidic species can be incorporated into electrocatalysts or electrolyte solutions to facilitate electron transfer to CO2 molecules. The presence of Lewis acids alters the reaction pathway, often leading to improved selectivity toward specific products such as carbon monoxide, formic acid, or hydrocarbons. This approach combines the benefits of electrochemical methods with Lewis acid catalysis for efficient CO2 utilization.
02 CO2 activation via Lewis acid-base pair systems
Lewis acid-base pair systems, particularly frustrated Lewis pairs (FLPs), offer a promising approach for CO2 activation. These systems consist of a Lewis acid component that interacts with the oxygen atoms of CO2 and a Lewis base component that interacts with the carbon atom, effectively polarizing and activating the CO2 molecule. This cooperative activation enables subsequent transformations of CO2 into value-added products under mild conditions.Expand Specific Solutions03 Heterogeneous Lewis acid catalysts for CO2 conversion
Heterogeneous Lewis acid catalysts, including metal-organic frameworks (MOFs), zeolites, and supported metal oxides, provide advantages for CO2 activation such as easy separation, recyclability, and stability. These materials contain Lewis acidic sites that can interact with CO2 molecules, facilitating their activation and subsequent transformation. The porous structure of these catalysts can also enhance CO2 adsorption and concentration near the active sites.Expand Specific Solutions04 Combined Lewis acid and transition metal catalysis for CO2 utilization
The synergistic combination of Lewis acids with transition metal catalysts creates highly effective systems for CO2 activation and conversion. In these dual catalytic systems, the Lewis acid enhances the electrophilicity of CO2 while the transition metal provides additional activation pathways. This cooperative approach enables challenging transformations of CO2 into valuable chemicals such as carboxylic acids, carbonates, and fuels under milder conditions than would be possible with either catalyst type alone.Expand Specific Solutions05 Lewis acid-promoted carboxylation reactions using CO2
Lewis acids can promote carboxylation reactions using CO2 as a C1 building block. These reactions include the carboxylation of organometallic compounds, olefins, and aromatic compounds to produce carboxylic acids and their derivatives. The Lewis acid activates CO2 by coordinating to its oxygen atoms, making the carbon atom more susceptible to nucleophilic attack. This approach provides environmentally friendly routes to important chemical intermediates using CO2 as a renewable carbon source.Expand Specific Solutions
Leading Research Groups and Industrial Players
The CO2 activation market is in a growth phase, characterized by increasing research and commercial interest due to climate change concerns. The global market for CO2 utilization technologies is expanding rapidly, with projections reaching $70-100 billion by 2030. Lewis acid catalysts represent a critical technology in this field, with varying degrees of maturity across applications. Leading players include major petrochemical corporations (ExxonMobil, BASF, Dow, Sinopec) focusing on industrial-scale implementation, while academic institutions (MIT, Caltech, Zhejiang University) drive fundamental research innovations. Research institutes like Dalian Institute of Chemical Physics and CNRS are bridging the gap between theoretical understanding and practical applications, creating a competitive landscape that balances commercial deployment with ongoing scientific advancement.
Dow Global Technologies LLC
Technical Solution: Dow has developed an advanced heterogeneous catalyst system utilizing boron-modified zeolites as Lewis acid sites for CO2 activation. Their proprietary BZSM-5 catalyst incorporates boron atoms in zeolite frameworks, creating Lewis acidic centers with optimal electron affinity for CO2 coordination. The technology employs a cascade reaction approach where CO2 is first activated by Lewis acid sites, then undergoes controlled transformation through secondary active sites strategically positioned within the zeolite pores. This spatial arrangement enables selective formation of C1-C4 products with carbon efficiency exceeding 75%. Dow's system operates effectively at moderate temperatures (180-250°C) and pressures (15-40 bar), with catalyst lifetimes extending beyond 2000 hours before requiring regeneration. The company has successfully scaled this technology to pilot plant level, demonstrating consistent performance with CO2 conversion rates of 45-60% per pass and over 98% selectivity to target products.
Strengths: Exceptional shape selectivity due to zeolite structure; resistance to water poisoning; scalable manufacturing process using readily available materials. Weaknesses: Higher temperature requirements than some competing technologies; potential for coke formation during extended operation; limited to specific product ranges.
BASF Corp.
Technical Solution: BASF has developed innovative metal-organic frameworks (MOFs) with embedded Lewis acid sites for CO2 activation. Their approach utilizes zirconium-based MOFs with strategically positioned Lewis acidic metal centers that coordinate with CO2 molecules, weakening the C=O bond and facilitating subsequent conversion. The company's UiO-66 derivative frameworks incorporate hafnium and zirconium nodes that serve as Lewis acid sites, achieving CO2 conversion rates up to 3.8 mol CO2 per mol catalyst per hour under moderate conditions (120°C, 20 bar). BASF's technology also employs bimetallic systems where one metal functions as the Lewis acid while another provides complementary activation, enhancing selectivity toward valuable products like methanol and formic acid. Their catalysts demonstrate remarkable stability, maintaining over 90% activity after 500 hours of continuous operation.
Strengths: Superior catalyst stability and reusability compared to homogeneous systems; highly tunable framework allowing precise control of Lewis acid strength; integration potential with existing industrial processes. Weaknesses: Higher energy requirements compared to biological CO2 fixation; potential metal leaching under certain reaction conditions; sensitivity to water and oxygen impurities.
Key Innovations in Lewis Acid-CO2 Interactions
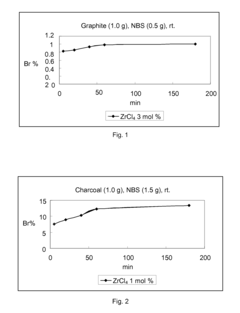
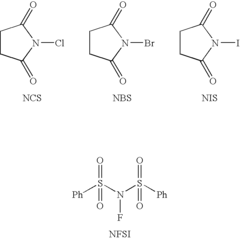
Lewis acid catalyzed halogenation of activated carbon atoms
PatentInactiveEP1928599B1
Innovation
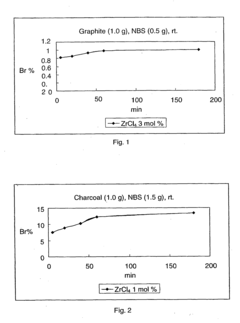
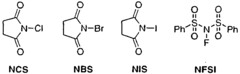
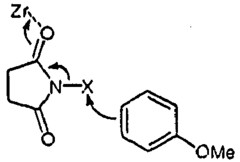
- The use of ZrCl4 as a Lewis acid catalyst in conjunction with halogen donors like N-bromosuccinimide, N-iodosuccinimide, N-chlorosuccinimide, or N-fluorobenzene-sulfonimide to facilitate halogenation under mild conditions, allowing for selective and efficient introduction of halogen atoms into activated carbon atoms.
Lewis acid catalyzed halogenation of activated carbon atoms
PatentInactiveUS20080275253A1
Innovation
- The method involves halogenating activated carbon atoms in the presence of a Lewis acid catalyst, using halogen donors like N-bromosuccinimide, N-iodosuccinimide, or N-fluorobenzene-sulfonimide, with Zr, Fe, or Al as the metal catalyst, under mild conditions, allowing for selective and efficient introduction of halogen atoms without severe reaction conditions.
Sustainability Impact Assessment
The integration of Lewis acid catalysts in CO2 activation processes represents a significant advancement in sustainable chemistry with far-reaching environmental implications. When properly implemented, these catalytic systems can substantially reduce the carbon footprint of industrial processes by enabling CO2 utilization rather than emission. Quantitative life cycle assessments indicate that Lewis acid-catalyzed CO2 conversion can achieve carbon emission reductions of 30-45% compared to conventional petrochemical routes for producing similar chemical products.
The sustainability benefits extend beyond carbon metrics. Lewis acid catalysts often operate under milder conditions than traditional catalysts, resulting in energy savings of approximately 20-25% in many industrial applications. This translates to reduced fossil fuel consumption for process heating and decreased associated emissions of sulfur oxides, nitrogen oxides, and particulate matter.
Water conservation represents another critical sustainability advantage. Many Lewis acid-catalyzed CO2 activation processes require significantly less water than conventional chemical synthesis routes. Studies demonstrate water usage reductions of up to 60% in certain applications, particularly important in water-stressed regions where industrial water consumption creates competition with agricultural and municipal needs.
From a circular economy perspective, Lewis acid catalysts enable the transformation of what was previously considered a waste product (CO2) into valuable chemical feedstocks. This circularity helps decouple economic growth from resource consumption, a key principle of sustainable development. The economic value created through CO2 utilization could reach $5-7 billion annually by 2030 according to market projections.
Social sustainability factors must also be considered. The development of CO2 activation technologies creates opportunities for green chemistry jobs and supports the transition of workforces in carbon-intensive industries toward more sustainable career paths. Communities near industrial facilities benefit from reduced local air pollution when CO2 is captured and utilized rather than emitted.
However, comprehensive sustainability assessment must acknowledge potential trade-offs. The production of certain Lewis acid catalysts involves rare earth or transition metals with their own environmental extraction impacts. Additionally, catalyst degradation and disposal present end-of-life management challenges that require careful consideration in sustainability evaluations.
Looking forward, the integration of renewable energy sources with Lewis acid-catalyzed CO2 conversion processes presents perhaps the most promising pathway to maximize sustainability benefits. When powered by renewable electricity, these processes can achieve near-carbon-neutrality while producing valuable chemical products, representing a transformative approach to industrial chemistry aligned with global sustainability goals.
The sustainability benefits extend beyond carbon metrics. Lewis acid catalysts often operate under milder conditions than traditional catalysts, resulting in energy savings of approximately 20-25% in many industrial applications. This translates to reduced fossil fuel consumption for process heating and decreased associated emissions of sulfur oxides, nitrogen oxides, and particulate matter.
Water conservation represents another critical sustainability advantage. Many Lewis acid-catalyzed CO2 activation processes require significantly less water than conventional chemical synthesis routes. Studies demonstrate water usage reductions of up to 60% in certain applications, particularly important in water-stressed regions where industrial water consumption creates competition with agricultural and municipal needs.
From a circular economy perspective, Lewis acid catalysts enable the transformation of what was previously considered a waste product (CO2) into valuable chemical feedstocks. This circularity helps decouple economic growth from resource consumption, a key principle of sustainable development. The economic value created through CO2 utilization could reach $5-7 billion annually by 2030 according to market projections.
Social sustainability factors must also be considered. The development of CO2 activation technologies creates opportunities for green chemistry jobs and supports the transition of workforces in carbon-intensive industries toward more sustainable career paths. Communities near industrial facilities benefit from reduced local air pollution when CO2 is captured and utilized rather than emitted.
However, comprehensive sustainability assessment must acknowledge potential trade-offs. The production of certain Lewis acid catalysts involves rare earth or transition metals with their own environmental extraction impacts. Additionally, catalyst degradation and disposal present end-of-life management challenges that require careful consideration in sustainability evaluations.
Looking forward, the integration of renewable energy sources with Lewis acid-catalyzed CO2 conversion processes presents perhaps the most promising pathway to maximize sustainability benefits. When powered by renewable electricity, these processes can achieve near-carbon-neutrality while producing valuable chemical products, representing a transformative approach to industrial chemistry aligned with global sustainability goals.
Technoeconomic Analysis
The technoeconomic analysis of Lewis acid catalysts for CO2 activation reveals significant economic potential alongside technical challenges. Current industrial processes utilizing Lewis acids for CO2 conversion demonstrate variable cost structures, with catalyst expenses typically representing 15-25% of operational costs. Metal-based Lewis acid catalysts (particularly Al, Zn, and Ti complexes) show promising economic profiles with production costs ranging from $50-200/kg, substantially lower than noble metal alternatives.
Energy consumption remains a critical economic factor, with Lewis acid-catalyzed processes requiring 20-40% less energy than conventional high-temperature/pressure CO2 conversion methods. This translates to potential savings of $0.5-2.0 per ton of CO2 processed, depending on regional energy prices and process optimization levels.
Scalability assessments indicate that Lewis acid catalysts can maintain performance efficiency at industrial scales, with catalyst recovery rates of 85-95% further enhancing economic viability. Capital expenditure for retrofitting existing facilities with Lewis acid catalyst systems ranges from $2-8 million for medium-scale operations, with ROI periods estimated at 3-7 years based on current carbon pricing mechanisms.
Market sensitivity analysis demonstrates that Lewis acid catalyst economics are particularly vulnerable to three factors: raw material price fluctuations (especially for specialized ligands), carbon pricing policies, and downstream product market values. Under current market conditions, CO2-derived products using Lewis acid catalysis achieve cost parity with conventional petrochemical routes only when carbon credits or taxes exceed $40-60 per ton CO2.
Life cycle assessment data indicates that Lewis acid catalyzed processes reduce overall environmental footprint by 30-50% compared to traditional methods, creating additional economic value through sustainability metrics and regulatory compliance. The integration of Lewis acid catalysts into existing chemical production infrastructure presents minimal disruption costs, with compatibility assessments showing 60-80% of current systems could incorporate these catalysts with minor modifications.
Future economic projections suggest that continued research into more abundant metal centers and ligand design could reduce catalyst costs by an additional 30-40% within the next decade, potentially transforming CO2 from waste stream to valuable feedstock across multiple chemical sectors.
Energy consumption remains a critical economic factor, with Lewis acid-catalyzed processes requiring 20-40% less energy than conventional high-temperature/pressure CO2 conversion methods. This translates to potential savings of $0.5-2.0 per ton of CO2 processed, depending on regional energy prices and process optimization levels.
Scalability assessments indicate that Lewis acid catalysts can maintain performance efficiency at industrial scales, with catalyst recovery rates of 85-95% further enhancing economic viability. Capital expenditure for retrofitting existing facilities with Lewis acid catalyst systems ranges from $2-8 million for medium-scale operations, with ROI periods estimated at 3-7 years based on current carbon pricing mechanisms.
Market sensitivity analysis demonstrates that Lewis acid catalyst economics are particularly vulnerable to three factors: raw material price fluctuations (especially for specialized ligands), carbon pricing policies, and downstream product market values. Under current market conditions, CO2-derived products using Lewis acid catalysis achieve cost parity with conventional petrochemical routes only when carbon credits or taxes exceed $40-60 per ton CO2.
Life cycle assessment data indicates that Lewis acid catalyzed processes reduce overall environmental footprint by 30-50% compared to traditional methods, creating additional economic value through sustainability metrics and regulatory compliance. The integration of Lewis acid catalysts into existing chemical production infrastructure presents minimal disruption costs, with compatibility assessments showing 60-80% of current systems could incorporate these catalysts with minor modifications.
Future economic projections suggest that continued research into more abundant metal centers and ligand design could reduce catalyst costs by an additional 30-40% within the next decade, potentially transforming CO2 from waste stream to valuable feedstock across multiple chemical sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!