How to Determine Lewis Acid Deficiency in Catalyst?
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has emerged as a cornerstone in modern synthetic chemistry since the pioneering work of Gilbert N. Lewis in 1923, who defined Lewis acids as electron pair acceptors. This fundamental concept has evolved significantly over the past century, transforming from theoretical chemistry into practical applications across numerous industrial processes, particularly in petrochemical refining, pharmaceutical synthesis, and fine chemical production.
The evolution of Lewis acid catalysis has been marked by several significant milestones. Initially limited to simple metal halides like AlCl3 and BF3, the field expanded dramatically in the 1980s with the development of chiral Lewis acids for asymmetric synthesis. The 1990s witnessed the integration of Lewis acid catalysis with transition metal chemistry, while the early 2000s saw the emergence of Lewis acid-surfactant-combined catalysts and supported Lewis acid systems.
Current technological trends in this domain include the development of water-tolerant Lewis acids, heterogeneous Lewis acid catalysts for green chemistry applications, and the exploration of frustrated Lewis pairs (FLPs) that exhibit unique reactivity patterns. The growing emphasis on sustainability has driven research toward recyclable catalysts and those derived from earth-abundant metals rather than precious metals.
The primary objective of investigating Lewis acid deficiency in catalysts is to enhance catalytic efficiency and selectivity while minimizing catalyst deactivation. Lewis acid sites in catalysts are crucial active centers that facilitate numerous transformations, including Friedel-Crafts reactions, Diels-Alder cycloadditions, and various carbonyl activations. When these sites become deficient through processes such as poisoning, leaching, or structural changes, catalytic performance deteriorates significantly.
Determining Lewis acid deficiency represents a critical challenge in catalyst development and maintenance. Accurate assessment methods are essential for quality control in industrial catalysis, optimization of catalyst regeneration protocols, and the rational design of next-generation catalytic materials. Furthermore, understanding the mechanisms of Lewis acid site deactivation can provide valuable insights for developing more robust catalytic systems with extended operational lifetimes.
The technological goals of this investigation include developing reliable, preferably in-situ methods for quantifying Lewis acid site density and strength, establishing correlations between Lewis acidity and catalytic performance metrics, and creating predictive models for catalyst deactivation under various reaction conditions. Additionally, we aim to explore innovative approaches for preserving and regenerating Lewis acid functionality in catalytic materials, potentially through novel synthesis methods or protective strategies.
The evolution of Lewis acid catalysis has been marked by several significant milestones. Initially limited to simple metal halides like AlCl3 and BF3, the field expanded dramatically in the 1980s with the development of chiral Lewis acids for asymmetric synthesis. The 1990s witnessed the integration of Lewis acid catalysis with transition metal chemistry, while the early 2000s saw the emergence of Lewis acid-surfactant-combined catalysts and supported Lewis acid systems.
Current technological trends in this domain include the development of water-tolerant Lewis acids, heterogeneous Lewis acid catalysts for green chemistry applications, and the exploration of frustrated Lewis pairs (FLPs) that exhibit unique reactivity patterns. The growing emphasis on sustainability has driven research toward recyclable catalysts and those derived from earth-abundant metals rather than precious metals.
The primary objective of investigating Lewis acid deficiency in catalysts is to enhance catalytic efficiency and selectivity while minimizing catalyst deactivation. Lewis acid sites in catalysts are crucial active centers that facilitate numerous transformations, including Friedel-Crafts reactions, Diels-Alder cycloadditions, and various carbonyl activations. When these sites become deficient through processes such as poisoning, leaching, or structural changes, catalytic performance deteriorates significantly.
Determining Lewis acid deficiency represents a critical challenge in catalyst development and maintenance. Accurate assessment methods are essential for quality control in industrial catalysis, optimization of catalyst regeneration protocols, and the rational design of next-generation catalytic materials. Furthermore, understanding the mechanisms of Lewis acid site deactivation can provide valuable insights for developing more robust catalytic systems with extended operational lifetimes.
The technological goals of this investigation include developing reliable, preferably in-situ methods for quantifying Lewis acid site density and strength, establishing correlations between Lewis acidity and catalytic performance metrics, and creating predictive models for catalyst deactivation under various reaction conditions. Additionally, we aim to explore innovative approaches for preserving and regenerating Lewis acid functionality in catalytic materials, potentially through novel synthesis methods or protective strategies.
Market Analysis of Lewis Acid Catalysts
The global market for Lewis acid catalysts has experienced significant growth over the past decade, driven primarily by increasing demand in petrochemical processing, fine chemical synthesis, and pharmaceutical manufacturing. Current market valuations place the Lewis acid catalyst sector at approximately $4.2 billion, with projections indicating a compound annual growth rate of 5.7% through 2028, according to recent industry analyses.
Geographically, the market demonstrates distinct regional characteristics. North America and Europe currently dominate with a combined market share of 58%, largely due to their established chemical and pharmaceutical industries. However, the Asia-Pacific region, particularly China and India, is witnessing the fastest growth rates, exceeding 8% annually, as these economies rapidly expand their manufacturing capabilities and chemical production infrastructure.
From an application perspective, the petrochemical sector remains the largest consumer of Lewis acid catalysts, accounting for approximately 42% of total market demand. This is followed by polymer production (23%), pharmaceutical synthesis (18%), and fine chemicals (12%), with various other applications comprising the remaining 5%. The pharmaceutical segment is projected to show the highest growth potential due to increasing drug development activities and the shift toward more complex molecular structures requiring sophisticated catalytic processes.
The market for Lewis acid deficiency detection technologies represents a specialized but crucial subsector. Current estimates value this segment at approximately $320 million, with specialized analytical equipment and testing services constituting the majority of this value. The demand for more precise and efficient methods to determine Lewis acid deficiency in catalysts is growing at nearly 7% annually, outpacing the overall catalyst market.
Key market drivers include increasingly stringent environmental regulations that necessitate more efficient catalytic processes, growing demand for high-purity chemicals in pharmaceutical and electronics industries, and the push toward sustainable manufacturing practices. Additionally, the trend toward catalyst recycling and regeneration has created new market opportunities for technologies that can accurately assess catalyst activity and identify deficiencies.
Market challenges include high costs associated with advanced analytical techniques, technical complexity requiring specialized expertise, and the highly customized nature of catalyst systems across different industrial applications. These factors have created significant barriers to entry for new market participants and have contributed to market consolidation among established players with comprehensive technical capabilities and service offerings.
Geographically, the market demonstrates distinct regional characteristics. North America and Europe currently dominate with a combined market share of 58%, largely due to their established chemical and pharmaceutical industries. However, the Asia-Pacific region, particularly China and India, is witnessing the fastest growth rates, exceeding 8% annually, as these economies rapidly expand their manufacturing capabilities and chemical production infrastructure.
From an application perspective, the petrochemical sector remains the largest consumer of Lewis acid catalysts, accounting for approximately 42% of total market demand. This is followed by polymer production (23%), pharmaceutical synthesis (18%), and fine chemicals (12%), with various other applications comprising the remaining 5%. The pharmaceutical segment is projected to show the highest growth potential due to increasing drug development activities and the shift toward more complex molecular structures requiring sophisticated catalytic processes.
The market for Lewis acid deficiency detection technologies represents a specialized but crucial subsector. Current estimates value this segment at approximately $320 million, with specialized analytical equipment and testing services constituting the majority of this value. The demand for more precise and efficient methods to determine Lewis acid deficiency in catalysts is growing at nearly 7% annually, outpacing the overall catalyst market.
Key market drivers include increasingly stringent environmental regulations that necessitate more efficient catalytic processes, growing demand for high-purity chemicals in pharmaceutical and electronics industries, and the push toward sustainable manufacturing practices. Additionally, the trend toward catalyst recycling and regeneration has created new market opportunities for technologies that can accurately assess catalyst activity and identify deficiencies.
Market challenges include high costs associated with advanced analytical techniques, technical complexity requiring specialized expertise, and the highly customized nature of catalyst systems across different industrial applications. These factors have created significant barriers to entry for new market participants and have contributed to market consolidation among established players with comprehensive technical capabilities and service offerings.
Current Detection Methods and Limitations
The detection of Lewis acid deficiency in catalysts currently relies on several established methodologies, each with specific advantages and inherent limitations. Spectroscopic techniques represent the cornerstone of Lewis acid site characterization, with Fourier Transform Infrared Spectroscopy (FTIR) using probe molecules such as pyridine, CO, and NH3 being widely employed. While FTIR provides valuable information about the nature and strength of acid sites, it often struggles to differentiate between closely related Lewis acid species and requires careful sample preparation to avoid moisture contamination.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state 27Al NMR, offers insights into the coordination environment of aluminum atoms in zeolites and other catalysts. However, this technique suffers from low sensitivity for certain nuclei and cannot always detect small concentrations of Lewis acid sites that may still significantly impact catalytic performance. Additionally, the interpretation of complex NMR spectra requires considerable expertise and reference standards.
Temperature-Programmed Desorption (TPD) methods measure the strength of acid sites by analyzing the desorption temperature of basic probe molecules. While quantitative in nature, TPD often provides limited information about the specific type of acidity and can be influenced by diffusion limitations within catalyst pores, leading to potential misinterpretation of acid strength distribution.
X-ray Absorption Spectroscopy (XAS) techniques, including XANES and EXAFS, offer element-specific information about the local environment of metal centers that function as Lewis acids. These methods require synchrotron radiation facilities, limiting their accessibility for routine analysis, and the data interpretation demands specialized knowledge and computational modeling.
Catalytic probe reactions, where the catalyst's performance in model reactions sensitive to Lewis acidity is evaluated, provide practical but indirect assessment of acid properties. The challenge lies in selecting appropriate test reactions that specifically respond to Lewis acidity rather than other catalyst properties, and results can be confounded by mass transfer limitations or competing reaction pathways.
Recent advancements in computational chemistry have enabled theoretical prediction of Lewis acid properties, but these approaches require validation against experimental data and often struggle with complex, heterogeneous catalyst systems where multiple factors influence acidity.
A significant limitation across all current methods is the difficulty in performing in-situ or operando measurements under realistic reaction conditions. Most techniques characterize catalysts in idealized environments that may not reflect the dynamic changes in Lewis acidity occurring during actual catalytic processes. This disconnect between characterization conditions and operating conditions represents a major challenge in accurately determining Lewis acid deficiency in practical applications.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly solid-state 27Al NMR, offers insights into the coordination environment of aluminum atoms in zeolites and other catalysts. However, this technique suffers from low sensitivity for certain nuclei and cannot always detect small concentrations of Lewis acid sites that may still significantly impact catalytic performance. Additionally, the interpretation of complex NMR spectra requires considerable expertise and reference standards.
Temperature-Programmed Desorption (TPD) methods measure the strength of acid sites by analyzing the desorption temperature of basic probe molecules. While quantitative in nature, TPD often provides limited information about the specific type of acidity and can be influenced by diffusion limitations within catalyst pores, leading to potential misinterpretation of acid strength distribution.
X-ray Absorption Spectroscopy (XAS) techniques, including XANES and EXAFS, offer element-specific information about the local environment of metal centers that function as Lewis acids. These methods require synchrotron radiation facilities, limiting their accessibility for routine analysis, and the data interpretation demands specialized knowledge and computational modeling.
Catalytic probe reactions, where the catalyst's performance in model reactions sensitive to Lewis acidity is evaluated, provide practical but indirect assessment of acid properties. The challenge lies in selecting appropriate test reactions that specifically respond to Lewis acidity rather than other catalyst properties, and results can be confounded by mass transfer limitations or competing reaction pathways.
Recent advancements in computational chemistry have enabled theoretical prediction of Lewis acid properties, but these approaches require validation against experimental data and often struggle with complex, heterogeneous catalyst systems where multiple factors influence acidity.
A significant limitation across all current methods is the difficulty in performing in-situ or operando measurements under realistic reaction conditions. Most techniques characterize catalysts in idealized environments that may not reflect the dynamic changes in Lewis acidity occurring during actual catalytic processes. This disconnect between characterization conditions and operating conditions represents a major challenge in accurately determining Lewis acid deficiency in practical applications.
Established Techniques for Lewis Acidity Measurement
01 Lewis acid catalysts in polymerization reactions
Lewis acid catalysts are widely used in polymerization reactions to overcome acid deficiency issues. These catalysts, such as metal halides and organometallic compounds, can activate monomers and initiate polymerization processes. By carefully selecting and optimizing Lewis acid catalysts, improved polymer properties, higher conversion rates, and better control over molecular weight distribution can be achieved, addressing the limitations of conventional acid catalysts.- Lewis acid catalysts in polymerization reactions: Lewis acid catalysts are used in various polymerization processes to overcome acid deficiency issues. These catalysts facilitate the formation of polymer chains by activating monomers and controlling reaction kinetics. By carefully selecting appropriate Lewis acid catalysts, polymerization reactions can proceed efficiently even in conditions where traditional acid catalysis might be insufficient. The catalysts help in achieving desired molecular weight distributions and polymer properties.
- Modified Lewis acid catalysts with enhanced acidity: Various modifications can be made to Lewis acid catalysts to enhance their acidity and overcome deficiency issues. These modifications include the addition of promoters, support materials, or co-catalysts that can increase the acid strength. Enhanced Lewis acid catalysts show improved catalytic performance in reactions requiring strong acid sites. The modifications can involve structural changes or the incorporation of additional functional groups to boost the acid properties.
- Lewis acid catalysts in hydrocarbon processing: Lewis acid catalysts are employed in various hydrocarbon processing applications to address acid deficiency challenges. These catalysts facilitate isomerization, alkylation, and cracking reactions in petroleum refining processes. When traditional acid catalysis is insufficient, specially designed Lewis acid systems can provide the necessary catalytic activity. The catalysts help in achieving higher conversion rates and selectivity in hydrocarbon transformation reactions.
- Supported Lewis acid catalyst systems: Supporting Lewis acid catalysts on various materials can help overcome acid deficiency issues by increasing the dispersion and stability of acid sites. These supported catalyst systems provide enhanced surface area and accessibility to reactants. Common support materials include silica, alumina, and zeolites, which can also contribute additional acid functionality. The supported catalysts show improved reusability and reduced deactivation compared to their unsupported counterparts.
- Lewis acid catalysts with water tolerance: Water-tolerant Lewis acid catalysts have been developed to address the acid deficiency that occurs when conventional Lewis acids are deactivated by moisture. These specialized catalysts maintain their acidity even in the presence of water, allowing for reactions in aqueous or high-humidity environments. The water tolerance is achieved through specific structural features or by incorporating hydrophobic elements into the catalyst design. These catalysts are particularly useful in industrial processes where complete water removal is impractical.
02 Modified Lewis acid catalysts with enhanced acidity
Various modifications can be made to Lewis acid catalysts to enhance their acidity and overcome deficiency issues. These modifications include the addition of promoters, support materials, or co-catalysts that can increase the acid strength and catalytic activity. Modified Lewis acid catalysts demonstrate improved performance in reactions requiring strong acid sites, such as alkylation, isomerization, and cracking processes, while maintaining stability under reaction conditions.Expand Specific Solutions03 Lewis acid catalysts in hydrocarbon processing
Lewis acid catalysts are employed in various hydrocarbon processing applications to address acid deficiency issues. These catalysts facilitate important reactions such as alkylation, isomerization, and cracking by providing the necessary acid sites for these transformations. The selection of appropriate Lewis acid catalysts can overcome limitations in conventional acid catalysis, leading to improved product selectivity, higher conversion rates, and reduced byproduct formation in petroleum refining and petrochemical processes.Expand Specific Solutions04 Supported Lewis acid catalysts for enhanced stability
Supporting Lewis acid catalysts on various materials can address acid deficiency issues by improving catalyst stability, dispersing active sites, and preventing deactivation. Common support materials include silica, alumina, zeolites, and carbon-based materials. These supported catalysts show enhanced performance in reactions requiring strong acidity while maintaining longer catalyst lifetimes, improved recyclability, and resistance to leaching or deactivation under harsh reaction conditions.Expand Specific Solutions05 Novel Lewis acid catalyst systems for specific applications
Novel Lewis acid catalyst systems have been developed to address specific acid deficiency issues in targeted applications. These innovative catalysts incorporate unique structural features, metal combinations, or functional groups designed to enhance catalytic performance in challenging reactions. Applications include fine chemical synthesis, pharmaceutical manufacturing, and environmentally friendly processes where conventional acid catalysts may be insufficient, providing improved selectivity, yield, and environmental compatibility.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid deficiency in catalysts represents a critical challenge in the evolving field of catalytic chemistry, currently in a growth phase with increasing market demand for more efficient catalytic processes. The global catalyst market is expanding steadily, driven by petrochemical, environmental, and fine chemical applications. Technologically, this field shows moderate maturity with significant room for innovation. Leading players like W.R. Grace, Sinopec, ExxonMobil Chemical, and Haldor Topsøe have developed proprietary methods for Lewis acid characterization and optimization, while academic institutions such as Zhejiang University and University of Stuttgart contribute fundamental research. Recent advancements from UOP LLC and IFP Energies Nouvelles focus on in-situ measurement techniques, indicating a collaborative ecosystem where industry-academic partnerships are accelerating solutions for Lewis acid deficiency detection and remediation.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a systematic approach to determine Lewis acid deficiency in catalysts through a combination of advanced characterization techniques and correlative catalytic testing. Their methodology centers on using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) with carefully selected probe molecules (CO, pyridine, and CD3CN) to quantify Lewis acid site density and strength distribution. Sinopec has enhanced this approach by implementing solid-state 27Al MAS NMR spectroscopy to distinguish between framework and extra-framework aluminum species in zeolite catalysts, directly correlating with Lewis acidity. Their innovation includes developing a "Lewis Acid Index" that combines spectroscopic measurements with catalytic performance data in model reactions (isopropanol dehydration, cumene cracking) to predict catalyst behavior in industrial processes. Sinopec has also pioneered the use of in-situ UV-Vis spectroscopy to monitor the coordination environment of transition metal centers during catalyst activation and reaction, providing real-time assessment of Lewis acid site availability and strength under operating conditions.
Strengths: Comprehensive characterization combining multiple spectroscopic techniques; established correlation between measured Lewis acidity and industrial catalyst performance; ability to distinguish between different types of Lewis acid sites. Weaknesses: Requires specialized equipment and expertise; some characterization methods may not be suitable for all catalyst types; challenging to implement for routine quality control in production environments.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed a sophisticated approach to determine Lewis acid deficiency in catalysts, particularly for polymerization and chemical transformation processes. Their methodology combines traditional probe molecule techniques (NH3-TPD, pyridine-FTIR) with advanced solid-state NMR spectroscopy (27Al, 29Si, 11B MAS NMR) to characterize the nature, density, and strength of Lewis acid sites. Dow's innovation lies in their development of structure-property relationships that correlate spectroscopic measurements with catalyst performance metrics. Their proprietary "Acid Site Accessibility Index" quantifies not only the presence of Lewis acid sites but also their accessibility to reactant molecules of varying sizes, which is crucial for processes involving bulky substrates. Dow has implemented in-situ and operando characterization techniques that monitor Lewis acid site availability during catalyst activation and under reaction conditions, providing insights into deactivation mechanisms. Their approach includes correlating measured Lewis acidity with computational models to predict catalyst behavior in industrial processes, enabling rational catalyst design and optimization for specific applications in polymer production and chemical manufacturing.
Strengths: Comprehensive characterization of Lewis acid site properties including accessibility; integration of experimental measurements with computational modeling; direct correlation with industrial catalyst performance. Weaknesses: Complex methodology requiring multiple analytical techniques; some measurements may not translate directly to industrial conditions; challenging to implement for high-throughput catalyst screening.
Key Spectroscopic and Computational Approaches
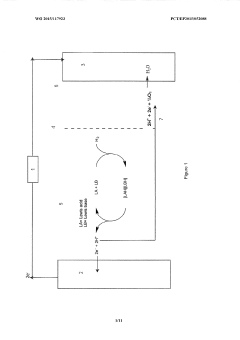
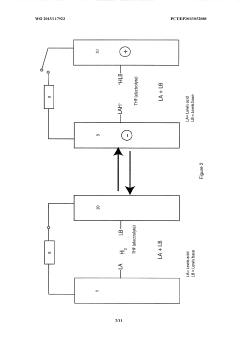
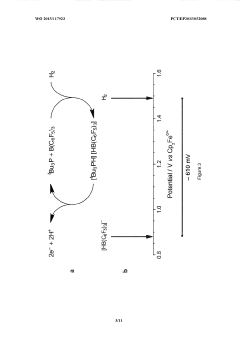
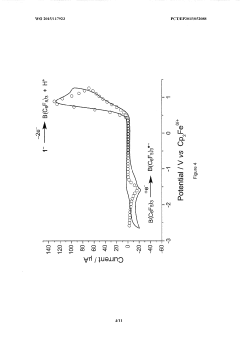
Lewis acid electrocatalysed fuel cell & battery
PatentWO2015117923A1
Innovation
- The use of an electrocatalytic frustrated Lewis pair system comprising a Lewis acid and a Lewis base that heterolytically cleaves dihydrogen, generating a hydride and a protonated base, which can then be oxidized to produce electrical energy in a fuel cell, eliminating the need for expensive metal catalysts.
Methods of preparing quinolone analogs
PatentActiveUS7834180B2
Innovation
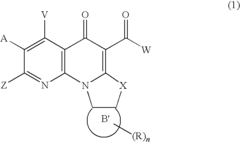
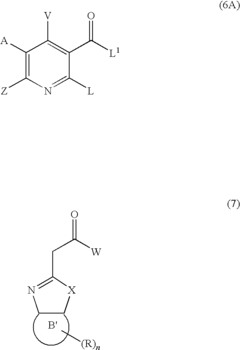
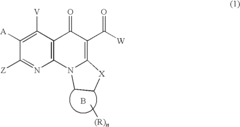
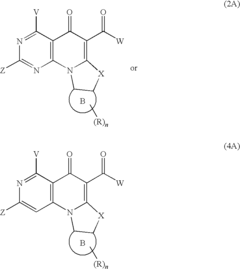
- A method involving the synthesis of compounds with specific formulas, such as (1), (2A), (4), (8), and (11), by contacting compounds with leaving groups and bases in the presence of Lewis acids, to produce pharmaceutically acceptable salts, esters, and prodrugs that can interact with DNA quadruplexes and treat cell proliferative disorders.
Regulatory Standards for Catalyst Characterization
The regulatory landscape for catalyst characterization has evolved significantly in response to the growing importance of Lewis acid sites in catalytic processes. International standards organizations such as ISO, ASTM, and IUPAC have established specific protocols for quantifying Lewis acid properties in catalytic materials. These standards typically require multiple complementary techniques to ensure accurate characterization, with pyridine-FTIR spectroscopy being recognized as a primary method in many jurisdictions.
The European Chemicals Agency (ECHA) has implemented guidelines under REACH regulation that specifically address catalyst characterization, requiring manufacturers to document Lewis acid site density and strength for catalysts used in industrial processes. Similarly, the U.S. Environmental Protection Agency (EPA) has incorporated Lewis acid characterization requirements into its Significant New Use Rules (SNURs) for novel catalytic materials, particularly those employed in petroleum refining and chemical synthesis.
In Asia, Japan's Ministry of Economy, Trade and Industry (METI) has established the JIS K 2547 standard specifically for zeolite catalyst characterization, which includes detailed procedures for Lewis acid site determination using temperature-programmed desorption of ammonia. China's recent environmental regulations have also introduced mandatory testing for Lewis acid properties in catalysts used in pollution control technologies.
For pharmaceutical applications, regulatory bodies including the FDA and EMA require comprehensive catalyst characterization data as part of the drug master file, with specific emphasis on Lewis acid properties when these catalysts are used in API synthesis. These requirements are outlined in ICH Q11 guidelines for drug substance manufacturing.
Industry-specific standards have also emerged, with the American Petroleum Institute (API) publishing recommended practices for catalyst characterization in refining processes. These standards specify minimum reporting requirements for Lewis acid site concentration, strength distribution, and accessibility metrics, which must be determined using validated analytical methods.
Compliance with these regulatory standards necessitates regular calibration of analytical instruments and participation in interlaboratory comparison studies. Many jurisdictions now require third-party verification of catalyst characterization data before commercial deployment, particularly for environmental applications. Certification bodies such as TÜV and SGS offer accredited testing services that align with these regulatory requirements.
The trend toward global harmonization of catalyst characterization standards is evident in recent initiatives by the International Conference on Harmonisation (ICH) and ISO Technical Committee 229, which aim to establish unified protocols for determining Lewis acid properties across different catalyst families and applications.
The European Chemicals Agency (ECHA) has implemented guidelines under REACH regulation that specifically address catalyst characterization, requiring manufacturers to document Lewis acid site density and strength for catalysts used in industrial processes. Similarly, the U.S. Environmental Protection Agency (EPA) has incorporated Lewis acid characterization requirements into its Significant New Use Rules (SNURs) for novel catalytic materials, particularly those employed in petroleum refining and chemical synthesis.
In Asia, Japan's Ministry of Economy, Trade and Industry (METI) has established the JIS K 2547 standard specifically for zeolite catalyst characterization, which includes detailed procedures for Lewis acid site determination using temperature-programmed desorption of ammonia. China's recent environmental regulations have also introduced mandatory testing for Lewis acid properties in catalysts used in pollution control technologies.
For pharmaceutical applications, regulatory bodies including the FDA and EMA require comprehensive catalyst characterization data as part of the drug master file, with specific emphasis on Lewis acid properties when these catalysts are used in API synthesis. These requirements are outlined in ICH Q11 guidelines for drug substance manufacturing.
Industry-specific standards have also emerged, with the American Petroleum Institute (API) publishing recommended practices for catalyst characterization in refining processes. These standards specify minimum reporting requirements for Lewis acid site concentration, strength distribution, and accessibility metrics, which must be determined using validated analytical methods.
Compliance with these regulatory standards necessitates regular calibration of analytical instruments and participation in interlaboratory comparison studies. Many jurisdictions now require third-party verification of catalyst characterization data before commercial deployment, particularly for environmental applications. Certification bodies such as TÜV and SGS offer accredited testing services that align with these regulatory requirements.
The trend toward global harmonization of catalyst characterization standards is evident in recent initiatives by the International Conference on Harmonisation (ICH) and ISO Technical Committee 229, which aim to establish unified protocols for determining Lewis acid properties across different catalyst families and applications.
Sustainability Aspects of Lewis Acid Catalysts
The sustainability of Lewis acid catalysts represents a critical dimension in modern catalytic processes, particularly as industries worldwide shift toward greener chemistry practices. Lewis acid catalysts, while highly effective in numerous chemical transformations, present several sustainability challenges that must be addressed through comprehensive deficiency detection and management strategies.
Environmental impact assessment of Lewis acid catalysts reveals significant concerns regarding metal leaching, particularly with traditional metal-based Lewis acids. When catalyst deficiency occurs due to deactivation or poisoning, these metals can contaminate product streams and waste effluents, potentially causing ecological damage. Advanced monitoring techniques such as ICP-MS and atomic absorption spectroscopy enable precise quantification of metal content in waste streams, serving as indirect indicators of catalyst deficiency.
Resource efficiency constitutes another crucial sustainability aspect, as many conventional Lewis acids incorporate rare or precious metals with limited global reserves. Catalyst deficiency detection through performance monitoring helps optimize catalyst usage and replacement cycles, thereby conserving these valuable resources. Techniques such as reaction yield tracking and selectivity monitoring provide early warning signs of diminishing catalytic activity before complete failure occurs.
Life cycle assessment (LCA) methodologies have emerged as valuable tools for evaluating the holistic environmental footprint of Lewis acid catalysts. These assessments consider raw material extraction, catalyst synthesis, operational performance, and end-of-life management. Catalyst deficiency directly impacts LCA metrics by necessitating more frequent replacement and regeneration cycles, thus increasing the overall environmental burden of catalytic processes.
Regeneration and recycling protocols represent promising approaches to extending catalyst lifespan and addressing deficiency issues. Techniques such as thermal reactivation, solvent washing, and chemical treatments can restore catalytic activity in many cases. The development of robust regeneration protocols requires precise deficiency characterization to target specific deactivation mechanisms.
Recent advances in green chemistry have led to the development of bio-based and biodegradable Lewis acid catalysts derived from renewable resources. These sustainable alternatives often exhibit different deficiency patterns compared to traditional catalysts, necessitating adapted monitoring strategies. Spectroscopic techniques combined with activity measurements provide valuable insights into the unique deactivation mechanisms of these emerging catalyst systems.
Regulatory frameworks increasingly emphasize sustainability metrics in industrial catalytic processes, with particular focus on waste reduction and hazardous substance elimination. Comprehensive catalyst deficiency monitoring programs help organizations demonstrate regulatory compliance while simultaneously optimizing process economics through extended catalyst lifetimes and reduced replacement costs.
Environmental impact assessment of Lewis acid catalysts reveals significant concerns regarding metal leaching, particularly with traditional metal-based Lewis acids. When catalyst deficiency occurs due to deactivation or poisoning, these metals can contaminate product streams and waste effluents, potentially causing ecological damage. Advanced monitoring techniques such as ICP-MS and atomic absorption spectroscopy enable precise quantification of metal content in waste streams, serving as indirect indicators of catalyst deficiency.
Resource efficiency constitutes another crucial sustainability aspect, as many conventional Lewis acids incorporate rare or precious metals with limited global reserves. Catalyst deficiency detection through performance monitoring helps optimize catalyst usage and replacement cycles, thereby conserving these valuable resources. Techniques such as reaction yield tracking and selectivity monitoring provide early warning signs of diminishing catalytic activity before complete failure occurs.
Life cycle assessment (LCA) methodologies have emerged as valuable tools for evaluating the holistic environmental footprint of Lewis acid catalysts. These assessments consider raw material extraction, catalyst synthesis, operational performance, and end-of-life management. Catalyst deficiency directly impacts LCA metrics by necessitating more frequent replacement and regeneration cycles, thus increasing the overall environmental burden of catalytic processes.
Regeneration and recycling protocols represent promising approaches to extending catalyst lifespan and addressing deficiency issues. Techniques such as thermal reactivation, solvent washing, and chemical treatments can restore catalytic activity in many cases. The development of robust regeneration protocols requires precise deficiency characterization to target specific deactivation mechanisms.
Recent advances in green chemistry have led to the development of bio-based and biodegradable Lewis acid catalysts derived from renewable resources. These sustainable alternatives often exhibit different deficiency patterns compared to traditional catalysts, necessitating adapted monitoring strategies. Spectroscopic techniques combined with activity measurements provide valuable insights into the unique deactivation mechanisms of these emerging catalyst systems.
Regulatory frameworks increasingly emphasize sustainability metrics in industrial catalytic processes, with particular focus on waste reduction and hazardous substance elimination. Comprehensive catalyst deficiency monitoring programs help organizations demonstrate regulatory compliance while simultaneously optimizing process economics through extended catalyst lifetimes and reduced replacement costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!