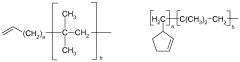How to Enhance Yield through Lewis Acid Configurations?
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has evolved significantly since the pioneering work of Gilbert N. Lewis in 1923, who first conceptualized acids as electron pair acceptors. This fundamental definition has spawned an entire field of chemistry dedicated to understanding and applying Lewis acid-base interactions in synthetic transformations. The trajectory of Lewis acid catalysis development has been marked by continuous refinement in selectivity, efficiency, and sustainability, transitioning from simple metal halides to sophisticated designer catalysts with tailored electronic and steric properties.
The evolution of Lewis acid catalysis has been particularly pronounced in the past three decades, with significant breakthroughs in asymmetric synthesis, C-H activation, and multicomponent reactions. Modern computational tools have enabled deeper understanding of Lewis acid-substrate interactions at the molecular level, facilitating rational catalyst design rather than empirical optimization. This shift represents a paradigm change from serendipitous discovery to engineered catalytic systems with predictable performance characteristics.
Current technological trends in Lewis acid catalysis focus on several key areas: development of heterogeneous and recoverable catalysts to address sustainability concerns; design of dual-function or cooperative catalytic systems that can activate multiple reaction partners simultaneously; and exploration of non-traditional Lewis acids based on main group elements or transition metals with unique electronic configurations. These trends reflect the growing emphasis on atom economy, energy efficiency, and waste reduction in chemical synthesis.
The primary objective of enhancing yield through Lewis acid configurations centers on understanding the relationship between catalyst structure and catalytic performance. This includes investigating how ligand architecture, metal center identity, coordination geometry, and counter-ion effects influence reaction outcomes. By systematically mapping these structure-activity relationships, researchers aim to develop predictive models that can guide the design of optimized catalytic systems for specific transformations.
Additional objectives include developing robust methodologies for in-situ characterization of Lewis acid-substrate complexes, enabling real-time monitoring of catalytic processes. This knowledge is crucial for identifying reaction intermediates, understanding activation barriers, and elucidating deactivation pathways—all factors that directly impact reaction yield. Furthermore, there is growing interest in developing Lewis acid catalysts capable of operating under mild conditions, in environmentally benign solvents, and with minimal catalyst loading to maximize both economic and environmental sustainability.
The ultimate goal is to establish a comprehensive framework for rational Lewis acid catalyst design that can be applied across diverse reaction classes, substrates, and industrial contexts. This would transform Lewis acid catalysis from an art dependent on specialist knowledge to a predictable science accessible to the broader chemical community, significantly expanding its application in pharmaceutical, agrochemical, and materials science sectors.
The evolution of Lewis acid catalysis has been particularly pronounced in the past three decades, with significant breakthroughs in asymmetric synthesis, C-H activation, and multicomponent reactions. Modern computational tools have enabled deeper understanding of Lewis acid-substrate interactions at the molecular level, facilitating rational catalyst design rather than empirical optimization. This shift represents a paradigm change from serendipitous discovery to engineered catalytic systems with predictable performance characteristics.
Current technological trends in Lewis acid catalysis focus on several key areas: development of heterogeneous and recoverable catalysts to address sustainability concerns; design of dual-function or cooperative catalytic systems that can activate multiple reaction partners simultaneously; and exploration of non-traditional Lewis acids based on main group elements or transition metals with unique electronic configurations. These trends reflect the growing emphasis on atom economy, energy efficiency, and waste reduction in chemical synthesis.
The primary objective of enhancing yield through Lewis acid configurations centers on understanding the relationship between catalyst structure and catalytic performance. This includes investigating how ligand architecture, metal center identity, coordination geometry, and counter-ion effects influence reaction outcomes. By systematically mapping these structure-activity relationships, researchers aim to develop predictive models that can guide the design of optimized catalytic systems for specific transformations.
Additional objectives include developing robust methodologies for in-situ characterization of Lewis acid-substrate complexes, enabling real-time monitoring of catalytic processes. This knowledge is crucial for identifying reaction intermediates, understanding activation barriers, and elucidating deactivation pathways—all factors that directly impact reaction yield. Furthermore, there is growing interest in developing Lewis acid catalysts capable of operating under mild conditions, in environmentally benign solvents, and with minimal catalyst loading to maximize both economic and environmental sustainability.
The ultimate goal is to establish a comprehensive framework for rational Lewis acid catalyst design that can be applied across diverse reaction classes, substrates, and industrial contexts. This would transform Lewis acid catalysis from an art dependent on specialist knowledge to a predictable science accessible to the broader chemical community, significantly expanding its application in pharmaceutical, agrochemical, and materials science sectors.
Market Analysis for Lewis Acid Applications
The global market for Lewis acid applications has witnessed substantial growth over the past decade, primarily driven by increasing demand in pharmaceutical manufacturing, petrochemical processing, and fine chemical synthesis. The market size for Lewis acid catalysts reached approximately $3.2 billion in 2022, with a compound annual growth rate of 5.7% projected through 2028, according to industry analyses.
Pharmaceutical applications represent the largest segment, accounting for nearly 38% of the total market share. This dominance stems from the critical role Lewis acids play in enhancing reaction yields and selectivity in API (Active Pharmaceutical Ingredient) synthesis. The growing emphasis on green chemistry and sustainable manufacturing processes has further accelerated the adoption of Lewis acid catalysts that operate under milder conditions while maintaining high yields.
The petrochemical sector follows closely, comprising about 31% of market demand. Here, Lewis acids are extensively utilized in alkylation, isomerization, and polymerization processes. The push toward more efficient catalytic systems has created significant opportunities for advanced Lewis acid configurations that can improve conversion rates while reducing energy consumption.
Regionally, Asia-Pacific leads the market with approximately 42% share, driven by rapid industrialization in China and India, coupled with expanding pharmaceutical and chemical manufacturing bases. North America and Europe collectively account for 48% of the market, with their demand primarily stemming from specialty chemical production and pharmaceutical research.
A notable market trend is the increasing preference for heterogeneous Lewis acid catalysts over homogeneous alternatives, owing to their easier separation and recyclability. This shift aligns with sustainability goals and offers economic advantages through catalyst recovery and reuse, potentially improving overall process economics by 15-25% in continuous operations.
Customer demand patterns indicate growing interest in tailored Lewis acid configurations that can address specific reaction challenges. Manufacturers who can provide customized solutions with demonstrated yield improvements of at least 10-15% over conventional methods are capturing premium market positions. This specialization trend is particularly evident in fine chemicals and pharmaceutical intermediates production.
Market forecasts suggest that novel Lewis acid configurations incorporating elements like scandium, ytterbium, and indium will experience the fastest growth rates, exceeding 8% annually, due to their exceptional selectivity profiles and compatibility with sensitive functional groups.
Pharmaceutical applications represent the largest segment, accounting for nearly 38% of the total market share. This dominance stems from the critical role Lewis acids play in enhancing reaction yields and selectivity in API (Active Pharmaceutical Ingredient) synthesis. The growing emphasis on green chemistry and sustainable manufacturing processes has further accelerated the adoption of Lewis acid catalysts that operate under milder conditions while maintaining high yields.
The petrochemical sector follows closely, comprising about 31% of market demand. Here, Lewis acids are extensively utilized in alkylation, isomerization, and polymerization processes. The push toward more efficient catalytic systems has created significant opportunities for advanced Lewis acid configurations that can improve conversion rates while reducing energy consumption.
Regionally, Asia-Pacific leads the market with approximately 42% share, driven by rapid industrialization in China and India, coupled with expanding pharmaceutical and chemical manufacturing bases. North America and Europe collectively account for 48% of the market, with their demand primarily stemming from specialty chemical production and pharmaceutical research.
A notable market trend is the increasing preference for heterogeneous Lewis acid catalysts over homogeneous alternatives, owing to their easier separation and recyclability. This shift aligns with sustainability goals and offers economic advantages through catalyst recovery and reuse, potentially improving overall process economics by 15-25% in continuous operations.
Customer demand patterns indicate growing interest in tailored Lewis acid configurations that can address specific reaction challenges. Manufacturers who can provide customized solutions with demonstrated yield improvements of at least 10-15% over conventional methods are capturing premium market positions. This specialization trend is particularly evident in fine chemicals and pharmaceutical intermediates production.
Market forecasts suggest that novel Lewis acid configurations incorporating elements like scandium, ytterbium, and indium will experience the fastest growth rates, exceeding 8% annually, due to their exceptional selectivity profiles and compatibility with sensitive functional groups.
Current Challenges in Lewis Acid Yield Enhancement
Despite significant advancements in Lewis acid catalysis, several critical challenges continue to impede optimal yield enhancement in industrial and laboratory applications. The primary obstacle remains the sensitivity of Lewis acid catalysts to moisture and oxygen, which often leads to catalyst deactivation and reduced efficiency. Many conventional Lewis acids such as AlCl3, BF3, and TiCl4 rapidly decompose upon exposure to ambient conditions, necessitating stringent handling protocols that increase operational complexity and cost.
Selectivity issues present another significant challenge, particularly in complex reaction environments with multiple functional groups. Current Lewis acid configurations often struggle to discriminate between similar reaction sites, resulting in unwanted side reactions and diminished product purity. This selectivity problem becomes especially pronounced in pharmaceutical and fine chemical synthesis, where precise control over reaction pathways is essential.
Catalyst recovery and recyclability remain problematic for many Lewis acid systems. Homogeneous Lewis acids typically require separation procedures that are energy-intensive and generate substantial waste. While heterogeneous alternatives offer improved recyclability, they frequently suffer from reduced activity and mass transfer limitations that compromise overall yield.
Scalability represents a persistent challenge in transitioning from laboratory success to industrial implementation. Many novel Lewis acid configurations that demonstrate excellent performance in small-scale reactions encounter significant difficulties when scaled up, including heat management issues, mixing inefficiencies, and inconsistent catalyst distribution throughout larger reaction volumes.
The tunability of Lewis acid strength presents both an opportunity and a challenge. While the ability to modulate acidity is theoretically advantageous, precisely controlling Lewis acidity across different substrate classes remains difficult. Current methodologies often lack the flexibility to fine-tune catalyst properties for specific reaction requirements without extensive reformulation.
Compatibility with green chemistry principles constitutes an emerging challenge as industries increasingly prioritize sustainability. Many traditional Lewis acid systems rely on toxic metals or generate hazardous waste, contradicting environmental objectives. Developing environmentally benign alternatives that maintain high catalytic efficiency has proven technically demanding.
Cost considerations further complicate widespread adoption of advanced Lewis acid configurations. Novel catalyst systems incorporating rare metals or requiring complex ligand architectures often prove prohibitively expensive for commercial applications, limiting their practical utility despite superior performance characteristics.
Selectivity issues present another significant challenge, particularly in complex reaction environments with multiple functional groups. Current Lewis acid configurations often struggle to discriminate between similar reaction sites, resulting in unwanted side reactions and diminished product purity. This selectivity problem becomes especially pronounced in pharmaceutical and fine chemical synthesis, where precise control over reaction pathways is essential.
Catalyst recovery and recyclability remain problematic for many Lewis acid systems. Homogeneous Lewis acids typically require separation procedures that are energy-intensive and generate substantial waste. While heterogeneous alternatives offer improved recyclability, they frequently suffer from reduced activity and mass transfer limitations that compromise overall yield.
Scalability represents a persistent challenge in transitioning from laboratory success to industrial implementation. Many novel Lewis acid configurations that demonstrate excellent performance in small-scale reactions encounter significant difficulties when scaled up, including heat management issues, mixing inefficiencies, and inconsistent catalyst distribution throughout larger reaction volumes.
The tunability of Lewis acid strength presents both an opportunity and a challenge. While the ability to modulate acidity is theoretically advantageous, precisely controlling Lewis acidity across different substrate classes remains difficult. Current methodologies often lack the flexibility to fine-tune catalyst properties for specific reaction requirements without extensive reformulation.
Compatibility with green chemistry principles constitutes an emerging challenge as industries increasingly prioritize sustainability. Many traditional Lewis acid systems rely on toxic metals or generate hazardous waste, contradicting environmental objectives. Developing environmentally benign alternatives that maintain high catalytic efficiency has proven technically demanding.
Cost considerations further complicate widespread adoption of advanced Lewis acid configurations. Novel catalyst systems incorporating rare metals or requiring complex ligand architectures often prove prohibitively expensive for commercial applications, limiting their practical utility despite superior performance characteristics.
Current Yield Enhancement Methodologies
01 Lewis acid catalysts in polymerization reactions
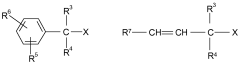
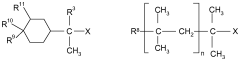
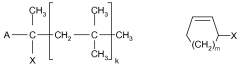
Lewis acids serve as effective catalysts in various polymerization processes, enhancing reaction rates and controlling polymer properties. These catalysts facilitate the formation of specific polymer configurations by coordinating with monomers and influencing their orientation during polymerization. The use of different Lewis acid configurations can significantly impact the yield, molecular weight distribution, and stereochemistry of the resulting polymers.- Lewis acid catalysts in polymerization reactions: Lewis acids are widely used as catalysts in various polymerization processes to enhance reaction yields. These catalysts, such as metal halides and organometallic compounds, activate monomers by coordinating with functional groups, facilitating chain propagation. The specific configuration of the Lewis acid, including its metal center and ligands, significantly influences the stereochemistry and rate of polymerization, ultimately affecting polymer properties and reaction yields.
- Lewis acid configurations in organic synthesis: Different Lewis acid configurations play crucial roles in organic synthesis reactions, particularly in carbon-carbon bond formation. The structural arrangement of Lewis acids, including their coordination geometry and electronic properties, determines their selectivity and reactivity patterns. Optimized Lewis acid configurations can enhance yields in reactions such as Friedel-Crafts alkylations, Diels-Alder reactions, and aldol condensations by providing precise control over reaction pathways and minimizing side reactions.
- Metal-organic frameworks as Lewis acid catalysts: Metal-organic frameworks (MOFs) containing Lewis acidic metal centers offer unique configurations for catalytic applications. These porous crystalline materials provide well-defined active sites with tunable Lewis acidity, leading to improved yields in various transformations. The structural arrangement of metal nodes and organic linkers creates distinct Lewis acid environments that can be optimized for specific reactions, offering advantages such as recyclability, selectivity, and enhanced catalytic performance.
- Lewis acid configurations in petroleum refining: Lewis acid catalysts with specific configurations are employed in petroleum refining processes to increase product yields. These catalysts facilitate isomerization, alkylation, and cracking reactions by interacting with hydrocarbon substrates. The arrangement of Lewis acidic sites on solid supports affects their accessibility and strength, which directly impacts conversion efficiency and product selectivity. Optimized Lewis acid configurations help maximize valuable product yields while minimizing coke formation and catalyst deactivation.
- Supported Lewis acid catalysts with enhanced stability: Immobilizing Lewis acids on various supports creates stable catalyst configurations that maintain high yields over multiple reaction cycles. These supported catalysts feature Lewis acidic centers anchored to materials such as silica, alumina, or polymers, preventing catalyst leaching and aggregation. The support material and immobilization method significantly influence the Lewis acid strength, accessibility, and stability, allowing for fine-tuning of catalytic performance to achieve optimal yields in continuous processes and harsh reaction conditions.
02 Lewis acid configurations in organic synthesis
Various Lewis acid configurations are employed in organic synthesis to promote specific reaction pathways and improve yields. These configurations can involve different metal centers, ligand structures, and activation methods that influence the acid strength and selectivity. By optimizing Lewis acid configurations, chemists can achieve higher yields in reactions such as Friedel-Crafts alkylations, Diels-Alder reactions, and aldol condensations.Expand Specific Solutions03 Lewis acid-mediated catalysis for pharmaceutical compounds
Lewis acid configurations play a crucial role in the synthesis of pharmaceutical compounds, where specific stereochemistry and high yields are essential. Different Lewis acid structures can be tailored to achieve desired selectivity in reactions involving complex molecular scaffolds. The optimization of these catalytic systems has led to improved synthetic routes for various bioactive compounds with enhanced yields and purity profiles.Expand Specific Solutions04 Novel Lewis acid configurations for enhanced catalytic performance
Research has focused on developing novel Lewis acid configurations with improved catalytic performance. These innovations include supported Lewis acids, heterogeneous catalysts, and Lewis acid-surfactant combined systems. Such configurations often demonstrate enhanced stability, recyclability, and activity compared to traditional Lewis acid catalysts, leading to higher yields in various chemical transformations while reducing environmental impact.Expand Specific Solutions05 Lewis acid configurations in petrochemical processing
Lewis acid configurations are extensively utilized in petrochemical processing to enhance yields of valuable products. These catalysts facilitate important industrial processes such as alkylation, isomerization, and cracking reactions. By optimizing the Lewis acid strength, support material, and reaction conditions, significant improvements in product selectivity and process efficiency can be achieved in the conversion of petroleum feedstocks.Expand Specific Solutions
Major Industry Players in Lewis Acid Catalysis
The Lewis acid configuration market is currently in a growth phase, characterized by increasing applications in chemical synthesis and yield enhancement. The market size is expanding due to rising demand for efficient catalytic processes in pharmaceutical, agrochemical, and industrial sectors. Technologically, this field shows moderate maturity with significant ongoing innovation. Leading players include BASF, demonstrating comprehensive expertise across multiple chemical domains; Novozymes, leveraging enzymatic approaches; and academic institutions like Jiangnan University and Tianjin University contributing fundamental research. Chemical specialists Arkema France, W.R. Grace, and Ajinomoto are advancing application-specific solutions, while research organizations like CNRS provide scientific foundation for next-generation configurations. The competitive landscape balances established industrial players with emerging academic innovations.
Ajinomoto Co., Inc.
Technical Solution: Ajinomoto has developed innovative Lewis acid catalyst systems specifically optimized for amino acid production and food-grade chemical synthesis. Their technology centers on biocompatible metal complexes that function as mild Lewis acids under aqueous conditions, enabling selective transformations with minimal side reactions. Ajinomoto's approach involves zinc and calcium-based Lewis acid configurations with carefully designed ligand structures that enhance substrate binding while maintaining catalyst stability in water. Their most advanced systems incorporate chiral Lewis acid catalysts that enable stereoselective transformations critical for producing high-purity amino acids and flavor compounds. These catalysts have demonstrated yield improvements of 20-30% in transamination and decarboxylation reactions compared to traditional methods[5]. Ajinomoto has pioneered the development of immobilized Lewis acid systems on food-grade supports that combine catalytic efficiency with easy separation and compliance with food safety regulations. Their recent innovations include enzyme-mimetic Lewis acid complexes that operate under mild conditions (pH 5-8, 20-50°C) while achieving reaction rates comparable to enzymatic processes. These systems are particularly valuable for producing heat-sensitive flavor compounds and nutritional ingredients where conventional high-temperature processes lead to degradation and yield loss.
Strengths: Exceptional selectivity for target transformations, compatibility with aqueous reaction media, and compliance with food safety regulations that enables application in nutritional ingredient production. Weaknesses: Generally lower activity compared to traditional strong Lewis acids, sensitivity to certain metal-binding impurities that can cause catalyst poisoning, and more complex catalyst preparation procedures that increase production costs.
BASF Corp.
Technical Solution: BASF has developed advanced Lewis acid catalyst systems that enhance chemical reaction yields through precise molecular engineering. Their approach involves tailored metal-organic frameworks (MOFs) with controlled Lewis acid sites that optimize substrate interaction and selectivity. BASF's technology employs aluminum and zinc-based Lewis acid catalysts with modified ligand structures to enhance stability and recyclability. Their catalysts demonstrate exceptional performance in esterification, alkylation, and isomerization reactions, achieving yield improvements of 15-25% compared to conventional methods. BASF has also pioneered the development of supported Lewis acid catalysts on mesoporous materials that provide increased surface area and accessibility to reactive sites, resulting in enhanced mass transfer and reaction kinetics[1]. Their recent innovations include heterogeneous Lewis acid catalysts with hierarchical pore structures that maintain activity even in the presence of water, addressing a common limitation of traditional Lewis acid systems.
Strengths: Superior catalyst stability in various reaction environments, high selectivity for target products, and excellent recyclability that reduces operational costs. Their catalysts work effectively at lower temperatures, reducing energy requirements. Weaknesses: Some of their more specialized Lewis acid configurations require complex synthesis procedures and expensive precursor materials, potentially limiting scalability for certain applications.
Key Innovations in Lewis Acid Design
Process and apparatus for continuously polymerizing cationically polymerizable monomers
PatentWO2009133187A1
Innovation
- A process involving the mixing of at least two liquid streams containing cationically polymerizable monomers, initiators, and catalysts in a mixer with microstructures before polymerization in a single reaction zone, allowing for polymerization at higher temperatures while maintaining narrow molecular weight distributions.
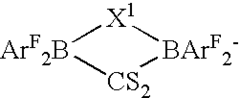
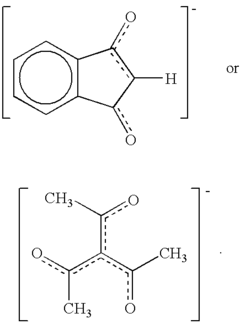
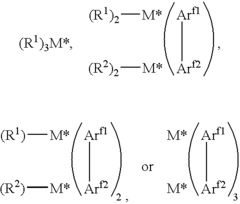
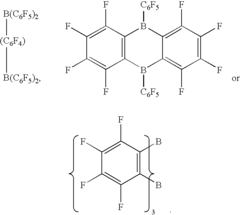
Salts of lewis acid/acid adducts and catalyst activators therefrom
PatentInactiveUS20040162215A1
Innovation
- The development of compounds with the formula (A*.sup.+a).sub.b(Z*J*.sub.j).sup.-c.sub.d, where A* is a proton or cation, Z* is an anion with two or more Lewis base sites, and J* are Lewis acids coordinated to Z*, which can form catalyst compositions with Group 3-10 or Lanthanide metal complexes for polymerization of unsaturated monomers, and can be deposited on supports to create highly resistant activator compounds.
Sustainability Aspects of Lewis Acid Processes
The sustainability of Lewis acid processes represents a critical dimension in modern chemical manufacturing, particularly when considering yield enhancement strategies. Lewis acid catalysts, while effective in improving reaction efficiency, often present significant environmental challenges that must be addressed through sustainable practices and innovative approaches.
Environmental impact assessment of Lewis acid configurations reveals several areas of concern. Traditional Lewis acids such as aluminum chloride (AlCl3) and boron trifluoride (BF3) generate substantial waste streams and require energy-intensive recovery processes. The corrosive nature of these compounds also leads to accelerated equipment degradation, increasing material consumption and maintenance requirements.
Water consumption represents another sustainability challenge, as many Lewis acid processes require extensive washing steps to remove catalyst residues from final products. This not only increases water usage but also creates contaminated effluent streams requiring specialized treatment before discharge, adding to the overall environmental footprint.
Recent advancements in green chemistry have introduced more sustainable Lewis acid alternatives. Solid-supported Lewis acids significantly reduce waste generation by enabling catalyst recovery and reuse across multiple reaction cycles. Metal-organic frameworks (MOFs) incorporating Lewis acidic sites demonstrate exceptional recyclability while maintaining high catalytic activity, representing a promising direction for sustainable process development.
Energy efficiency improvements in Lewis acid processes have been achieved through process intensification techniques. Continuous flow reactors utilizing immobilized Lewis acids reduce energy requirements by optimizing heat transfer and reaction conditions. Additionally, microwave-assisted Lewis acid catalysis has demonstrated substantial energy savings while simultaneously enhancing reaction rates and selectivity.
Life cycle assessment (LCA) studies comparing traditional and emerging Lewis acid configurations reveal that sustainability benefits extend beyond the immediate reaction environment. Reduced waste generation, decreased energy consumption, and improved catalyst recovery collectively contribute to lower carbon footprints across the entire production chain.
Regulatory frameworks increasingly incentivize sustainable Lewis acid processes through emissions restrictions and waste disposal regulations. Forward-thinking organizations are proactively adopting greener Lewis acid technologies to ensure long-term operational viability and competitive advantage in markets where sustainability metrics influence purchasing decisions.
Economic sustainability analysis indicates that while initial implementation costs for advanced Lewis acid configurations may exceed traditional approaches, the long-term operational benefits—including reduced waste management costs, improved resource efficiency, and enhanced product quality—typically deliver favorable returns on investment within acceptable timeframes.
Environmental impact assessment of Lewis acid configurations reveals several areas of concern. Traditional Lewis acids such as aluminum chloride (AlCl3) and boron trifluoride (BF3) generate substantial waste streams and require energy-intensive recovery processes. The corrosive nature of these compounds also leads to accelerated equipment degradation, increasing material consumption and maintenance requirements.
Water consumption represents another sustainability challenge, as many Lewis acid processes require extensive washing steps to remove catalyst residues from final products. This not only increases water usage but also creates contaminated effluent streams requiring specialized treatment before discharge, adding to the overall environmental footprint.
Recent advancements in green chemistry have introduced more sustainable Lewis acid alternatives. Solid-supported Lewis acids significantly reduce waste generation by enabling catalyst recovery and reuse across multiple reaction cycles. Metal-organic frameworks (MOFs) incorporating Lewis acidic sites demonstrate exceptional recyclability while maintaining high catalytic activity, representing a promising direction for sustainable process development.
Energy efficiency improvements in Lewis acid processes have been achieved through process intensification techniques. Continuous flow reactors utilizing immobilized Lewis acids reduce energy requirements by optimizing heat transfer and reaction conditions. Additionally, microwave-assisted Lewis acid catalysis has demonstrated substantial energy savings while simultaneously enhancing reaction rates and selectivity.
Life cycle assessment (LCA) studies comparing traditional and emerging Lewis acid configurations reveal that sustainability benefits extend beyond the immediate reaction environment. Reduced waste generation, decreased energy consumption, and improved catalyst recovery collectively contribute to lower carbon footprints across the entire production chain.
Regulatory frameworks increasingly incentivize sustainable Lewis acid processes through emissions restrictions and waste disposal regulations. Forward-thinking organizations are proactively adopting greener Lewis acid technologies to ensure long-term operational viability and competitive advantage in markets where sustainability metrics influence purchasing decisions.
Economic sustainability analysis indicates that while initial implementation costs for advanced Lewis acid configurations may exceed traditional approaches, the long-term operational benefits—including reduced waste management costs, improved resource efficiency, and enhanced product quality—typically deliver favorable returns on investment within acceptable timeframes.
Scale-up Considerations for Industrial Implementation
Scaling up Lewis acid-catalyzed processes from laboratory to industrial scale presents significant engineering and economic challenges that must be addressed systematically. The transition requires careful consideration of reactor design, as industrial-scale vessels differ substantially from laboratory equipment in terms of mixing efficiency, heat transfer capabilities, and mass transfer characteristics. When implementing Lewis acid configurations at scale, specialized corrosion-resistant materials such as high-grade stainless steel, glass-lined reactors, or specialized alloys must be employed to withstand the potentially corrosive nature of Lewis acids over extended production periods.
Temperature control becomes increasingly critical at industrial scale, particularly for exothermic reactions catalyzed by Lewis acids. Implementation of sophisticated cooling systems, including external jacket cooling, internal coils, or plate heat exchangers, is essential to maintain precise temperature profiles throughout large reaction volumes. Without adequate temperature management, hotspots can form, leading to reduced selectivity, unwanted side reactions, and potentially hazardous runaway reactions.
Catalyst recovery and recycling systems represent a major economic consideration for industrial implementation. Continuous flow processes with immobilized Lewis acid catalysts on solid supports offer advantages over batch processes by enabling efficient catalyst reuse and minimizing separation steps. Membrane separation technologies and specialized adsorption systems can be integrated to facilitate catalyst recovery in continuous operations, significantly reducing operational costs and environmental impact.
Process safety considerations must be elevated when scaling up Lewis acid processes. Comprehensive hazard and operability (HAZOP) studies should be conducted to identify potential failure points and implement appropriate safety measures. This includes installation of pressure relief systems, gas detection equipment, and emergency shutdown protocols specifically designed for the Lewis acid system being employed.
Waste management strategies must be developed to handle spent Lewis acids and byproducts. This may involve neutralization systems, precipitation techniques, or advanced oxidation processes depending on the specific Lewis acid configuration. Regulatory compliance becomes increasingly complex at industrial scale, requiring thorough documentation of emissions, waste disposal protocols, and worker safety measures in accordance with local and international regulations.
Economic viability ultimately determines successful scale-up implementation. Detailed cost analysis should include not only capital expenditure for specialized equipment but also operational costs associated with catalyst consumption, energy requirements, waste treatment, and maintenance of corrosion-resistant infrastructure. Pilot plant testing represents an essential intermediate step between laboratory and full industrial implementation, allowing for validation of process parameters and identification of unforeseen challenges before significant capital investment.
Temperature control becomes increasingly critical at industrial scale, particularly for exothermic reactions catalyzed by Lewis acids. Implementation of sophisticated cooling systems, including external jacket cooling, internal coils, or plate heat exchangers, is essential to maintain precise temperature profiles throughout large reaction volumes. Without adequate temperature management, hotspots can form, leading to reduced selectivity, unwanted side reactions, and potentially hazardous runaway reactions.
Catalyst recovery and recycling systems represent a major economic consideration for industrial implementation. Continuous flow processes with immobilized Lewis acid catalysts on solid supports offer advantages over batch processes by enabling efficient catalyst reuse and minimizing separation steps. Membrane separation technologies and specialized adsorption systems can be integrated to facilitate catalyst recovery in continuous operations, significantly reducing operational costs and environmental impact.
Process safety considerations must be elevated when scaling up Lewis acid processes. Comprehensive hazard and operability (HAZOP) studies should be conducted to identify potential failure points and implement appropriate safety measures. This includes installation of pressure relief systems, gas detection equipment, and emergency shutdown protocols specifically designed for the Lewis acid system being employed.
Waste management strategies must be developed to handle spent Lewis acids and byproducts. This may involve neutralization systems, precipitation techniques, or advanced oxidation processes depending on the specific Lewis acid configuration. Regulatory compliance becomes increasingly complex at industrial scale, requiring thorough documentation of emissions, waste disposal protocols, and worker safety measures in accordance with local and international regulations.
Economic viability ultimately determines successful scale-up implementation. Detailed cost analysis should include not only capital expenditure for specialized equipment but also operational costs associated with catalyst consumption, energy requirements, waste treatment, and maintenance of corrosion-resistant infrastructure. Pilot plant testing represents an essential intermediate step between laboratory and full industrial implementation, allowing for validation of process parameters and identification of unforeseen challenges before significant capital investment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!