Lewis Acid Use in Fluorination Processes
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Fluorination Background and Objectives
Fluorination processes represent a critical area in chemical synthesis, with Lewis acids playing a pivotal role as catalysts and activating agents. The evolution of Lewis acid-mediated fluorination has progressed significantly since the early 20th century, transitioning from rudimentary methods using hydrogen fluoride to sophisticated catalytic systems employing tailored Lewis acids for selective fluorination.
The historical trajectory of Lewis acid use in fluorination began with simple metal halides such as aluminum chloride and boron trifluoride, which were employed primarily in industrial settings for bulk chemical production. The mid-20th century witnessed the development of more refined Lewis acid systems, including antimony pentafluoride and tantalum pentafluoride, which enabled more controlled fluorination reactions under milder conditions.
Recent decades have seen exponential growth in fluorination technology, driven by the pharmaceutical and agrochemical industries' demand for fluorinated compounds. This growth has catalyzed research into novel Lewis acid systems that offer enhanced selectivity, reduced environmental impact, and broader substrate compatibility. The emergence of asymmetric fluorination methodologies using chiral Lewis acids represents a particularly significant advancement in this field.
The technical objectives for Lewis acid fluorination research encompass several dimensions. Primary goals include developing catalytic systems that operate under ambient conditions, reducing the reliance on hazardous fluorinating reagents, and enhancing regioselectivity and stereoselectivity in complex molecular frameworks. Additionally, there is a pressing need for Lewis acid systems that can facilitate late-stage fluorination of complex bioactive molecules without requiring extensive protecting group strategies.
Another critical objective involves addressing the environmental and safety concerns associated with traditional fluorination processes. Many conventional methods utilize toxic or corrosive reagents such as hydrogen fluoride or sulfur tetrafluoride, presenting significant handling challenges. The development of Lewis acid systems that enable the use of benign fluoride sources represents a key sustainability goal in this field.
The integration of computational modeling with experimental design has emerged as a powerful approach for advancing Lewis acid fluorination technology. Quantum mechanical calculations can predict Lewis acid-substrate interactions, guiding the rational design of new catalytic systems with optimized performance characteristics. This computational-experimental synergy is expected to accelerate innovation in fluorination methodology.
Looking forward, the technical trajectory for Lewis acid fluorination is oriented toward achieving greater precision in C-F bond formation, particularly in complex molecular environments. The ultimate objective is to develop catalytic systems capable of site-selective fluorination with enzyme-like specificity, potentially revolutionizing the synthesis of fluorinated pharmaceuticals and materials.
The historical trajectory of Lewis acid use in fluorination began with simple metal halides such as aluminum chloride and boron trifluoride, which were employed primarily in industrial settings for bulk chemical production. The mid-20th century witnessed the development of more refined Lewis acid systems, including antimony pentafluoride and tantalum pentafluoride, which enabled more controlled fluorination reactions under milder conditions.
Recent decades have seen exponential growth in fluorination technology, driven by the pharmaceutical and agrochemical industries' demand for fluorinated compounds. This growth has catalyzed research into novel Lewis acid systems that offer enhanced selectivity, reduced environmental impact, and broader substrate compatibility. The emergence of asymmetric fluorination methodologies using chiral Lewis acids represents a particularly significant advancement in this field.
The technical objectives for Lewis acid fluorination research encompass several dimensions. Primary goals include developing catalytic systems that operate under ambient conditions, reducing the reliance on hazardous fluorinating reagents, and enhancing regioselectivity and stereoselectivity in complex molecular frameworks. Additionally, there is a pressing need for Lewis acid systems that can facilitate late-stage fluorination of complex bioactive molecules without requiring extensive protecting group strategies.
Another critical objective involves addressing the environmental and safety concerns associated with traditional fluorination processes. Many conventional methods utilize toxic or corrosive reagents such as hydrogen fluoride or sulfur tetrafluoride, presenting significant handling challenges. The development of Lewis acid systems that enable the use of benign fluoride sources represents a key sustainability goal in this field.
The integration of computational modeling with experimental design has emerged as a powerful approach for advancing Lewis acid fluorination technology. Quantum mechanical calculations can predict Lewis acid-substrate interactions, guiding the rational design of new catalytic systems with optimized performance characteristics. This computational-experimental synergy is expected to accelerate innovation in fluorination methodology.
Looking forward, the technical trajectory for Lewis acid fluorination is oriented toward achieving greater precision in C-F bond formation, particularly in complex molecular environments. The ultimate objective is to develop catalytic systems capable of site-selective fluorination with enzyme-like specificity, potentially revolutionizing the synthesis of fluorinated pharmaceuticals and materials.
Market Analysis for Lewis Acid-Catalyzed Fluorination
The global market for Lewis acid-catalyzed fluorination processes has experienced significant growth over the past decade, driven primarily by increasing demand in pharmaceutical, agrochemical, and specialty chemical sectors. Current market valuations indicate that fluorinated compounds represent approximately 20-25% of pharmaceuticals and 30-40% of agrochemicals, with the global fluorochemicals market reaching $22.3 billion in 2022 and projected to grow at a CAGR of 5.3% through 2030.
Lewis acid catalysts have become essential components in this market due to their ability to facilitate selective fluorination under milder conditions compared to traditional methods. The pharmaceutical segment dominates the demand landscape, accounting for roughly 38% of Lewis acid-catalyzed fluorination applications, followed by agrochemicals at 27% and specialty materials at 18%.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). However, the fastest growth is occurring in Asia-Pacific markets, particularly China and India, where expanding pharmaceutical manufacturing capabilities and increasing agricultural productivity demands are driving adoption rates above 7% annually.
Key market drivers include the growing preference for fluorinated pharmaceuticals due to their enhanced metabolic stability and bioavailability, stringent environmental regulations favoring catalytic processes over stoichiometric reagents, and continuous innovation in catalyst design enabling more selective transformations. The shift toward green chemistry principles has particularly accelerated market growth, as Lewis acid catalysts typically offer reduced waste generation compared to traditional fluorination methods.
Market challenges primarily revolve around high catalyst costs, especially for rare earth metal-based Lewis acids, technical complexities in catalyst recovery and recycling, and competition from emerging metal-free methodologies. Additionally, regulatory scrutiny regarding certain fluorinated compounds (particularly PFAS-related substances) has created market uncertainties in specific application segments.
Customer segmentation analysis indicates that large pharmaceutical companies represent the highest-value customer segment, willing to pay premium prices for highly selective catalytic systems that enable late-stage fluorination of complex molecules. Mid-sized specialty chemical manufacturers form the second largest customer segment, focusing primarily on cost-effective solutions for producing fluorinated building blocks and intermediates.
The market exhibits moderate fragmentation with approximately 15-20 significant players controlling about 65% of the global market share. Recent strategic movements include increased vertical integration among major chemical companies and growing investment in proprietary catalyst technologies that offer improved selectivity profiles.
Lewis acid catalysts have become essential components in this market due to their ability to facilitate selective fluorination under milder conditions compared to traditional methods. The pharmaceutical segment dominates the demand landscape, accounting for roughly 38% of Lewis acid-catalyzed fluorination applications, followed by agrochemicals at 27% and specialty materials at 18%.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). However, the fastest growth is occurring in Asia-Pacific markets, particularly China and India, where expanding pharmaceutical manufacturing capabilities and increasing agricultural productivity demands are driving adoption rates above 7% annually.
Key market drivers include the growing preference for fluorinated pharmaceuticals due to their enhanced metabolic stability and bioavailability, stringent environmental regulations favoring catalytic processes over stoichiometric reagents, and continuous innovation in catalyst design enabling more selective transformations. The shift toward green chemistry principles has particularly accelerated market growth, as Lewis acid catalysts typically offer reduced waste generation compared to traditional fluorination methods.
Market challenges primarily revolve around high catalyst costs, especially for rare earth metal-based Lewis acids, technical complexities in catalyst recovery and recycling, and competition from emerging metal-free methodologies. Additionally, regulatory scrutiny regarding certain fluorinated compounds (particularly PFAS-related substances) has created market uncertainties in specific application segments.
Customer segmentation analysis indicates that large pharmaceutical companies represent the highest-value customer segment, willing to pay premium prices for highly selective catalytic systems that enable late-stage fluorination of complex molecules. Mid-sized specialty chemical manufacturers form the second largest customer segment, focusing primarily on cost-effective solutions for producing fluorinated building blocks and intermediates.
The market exhibits moderate fragmentation with approximately 15-20 significant players controlling about 65% of the global market share. Recent strategic movements include increased vertical integration among major chemical companies and growing investment in proprietary catalyst technologies that offer improved selectivity profiles.
Current Challenges in Lewis Acid Fluorination Technology
Despite significant advancements in fluorination technology, Lewis acid-mediated processes continue to face substantial technical challenges that limit their industrial application and efficiency. One of the primary obstacles is catalyst stability under fluorination conditions. Many Lewis acids, particularly those based on transition metals, undergo rapid deactivation through fluoride binding or decomposition in the presence of fluorinating agents, necessitating higher catalyst loadings and increasing process costs.
Selectivity issues represent another major challenge, as controlling regioselectivity and stereoselectivity in Lewis acid-catalyzed fluorination remains difficult. This is particularly problematic for complex pharmaceutical intermediates where precise fluorine placement is critical for biological activity. Current systems often produce mixtures requiring costly separation processes, reducing overall yield and economic viability.
The harsh reaction conditions typically required present significant engineering challenges. Many Lewis acid fluorination processes demand strictly anhydrous environments, low temperatures, or specialized equipment resistant to corrosive fluorinating agents. These requirements substantially increase operational complexity and capital investment while limiting scalability for industrial applications.
Environmental and safety concerns constitute growing challenges for Lewis acid fluorination technology. Traditional fluorinating reagents like hydrogen fluoride and sulfur tetrafluoride pose serious health hazards and environmental risks. Although newer reagents like DAST and Selectfluor offer improved safety profiles, they remain expensive and generate substantial waste streams, conflicting with green chemistry principles and sustainability goals.
Substrate scope limitations further restrict the applicability of current Lewis acid fluorination methods. Many protocols work efficiently only for activated substrates or specific functional group environments, leaving significant gaps in synthetic capabilities for unactivated C-H bonds and electron-poor systems. This narrow applicability hampers the development of universal fluorination methodologies.
Mechanistic understanding gaps impede rational catalyst design and process optimization. The complex interplay between Lewis acids and fluorinating agents remains incompletely characterized, particularly regarding transition states and intermediate species. This knowledge deficit hinders predictive modeling and systematic improvement of catalytic systems.
Cost factors present persistent challenges, with many high-performance Lewis acid catalysts requiring expensive rare earth elements or precious metals. Combined with specialized fluorinating reagents and equipment requirements, the economic barriers to widespread adoption remain substantial, particularly for bulk chemical applications where cost sensitivity is paramount.
Selectivity issues represent another major challenge, as controlling regioselectivity and stereoselectivity in Lewis acid-catalyzed fluorination remains difficult. This is particularly problematic for complex pharmaceutical intermediates where precise fluorine placement is critical for biological activity. Current systems often produce mixtures requiring costly separation processes, reducing overall yield and economic viability.
The harsh reaction conditions typically required present significant engineering challenges. Many Lewis acid fluorination processes demand strictly anhydrous environments, low temperatures, or specialized equipment resistant to corrosive fluorinating agents. These requirements substantially increase operational complexity and capital investment while limiting scalability for industrial applications.
Environmental and safety concerns constitute growing challenges for Lewis acid fluorination technology. Traditional fluorinating reagents like hydrogen fluoride and sulfur tetrafluoride pose serious health hazards and environmental risks. Although newer reagents like DAST and Selectfluor offer improved safety profiles, they remain expensive and generate substantial waste streams, conflicting with green chemistry principles and sustainability goals.
Substrate scope limitations further restrict the applicability of current Lewis acid fluorination methods. Many protocols work efficiently only for activated substrates or specific functional group environments, leaving significant gaps in synthetic capabilities for unactivated C-H bonds and electron-poor systems. This narrow applicability hampers the development of universal fluorination methodologies.
Mechanistic understanding gaps impede rational catalyst design and process optimization. The complex interplay between Lewis acids and fluorinating agents remains incompletely characterized, particularly regarding transition states and intermediate species. This knowledge deficit hinders predictive modeling and systematic improvement of catalytic systems.
Cost factors present persistent challenges, with many high-performance Lewis acid catalysts requiring expensive rare earth elements or precious metals. Combined with specialized fluorinating reagents and equipment requirements, the economic barriers to widespread adoption remain substantial, particularly for bulk chemical applications where cost sensitivity is paramount.
State-of-the-Art Lewis Acid Fluorination Techniques
01 Lewis Acids in Catalytic Reactions
Lewis acids are widely used as catalysts in various chemical reactions, particularly in organic synthesis. They facilitate reactions by accepting electron pairs from substrates, thereby activating them for further transformations. Common Lewis acid catalysts include metal halides such as aluminum chloride, boron trifluoride, and titanium tetrachloride. These catalysts are particularly effective in reactions like Friedel-Crafts alkylation, acylation, and various carbon-carbon bond forming reactions.- Lewis Acids in Catalytic Processes: Lewis acids are widely used as catalysts in various chemical reactions, particularly in organic synthesis. They facilitate reactions by accepting electron pairs from substrates, thereby activating them for further transformations. Common Lewis acid catalysts include metal halides such as aluminum chloride, boron trifluoride, and titanium tetrachloride. These catalysts are particularly effective in reactions like Friedel-Crafts alkylation, acylation, and various carbon-carbon bond forming processes.
- Lewis Acids in Polymerization Reactions: Lewis acids play a crucial role in polymerization processes, particularly in cationic polymerization and coordination polymerization. They initiate polymerization by generating active species through interaction with monomers or co-catalysts. Metal-based Lewis acids such as titanium and zirconium compounds are commonly used in olefin polymerization, while others like boron compounds are employed in ring-opening polymerization. These catalysts control polymer properties including molecular weight, tacticity, and branching.
- Lewis Acids in Pharmaceutical Synthesis: In pharmaceutical manufacturing, Lewis acids are essential for synthesizing complex drug molecules. They enable selective transformations, stereochemical control, and activation of functional groups that would otherwise be unreactive. Lewis acids facilitate reactions such as Diels-Alder cycloadditions, aldol condensations, and rearrangements that are crucial for building the core structures of many pharmaceutical compounds. Their ability to coordinate with heteroatoms makes them valuable tools for directing regioselectivity and stereoselectivity in drug synthesis.
- Lewis Acids in Material Science Applications: Lewis acids are utilized in the development of advanced materials, including catalysts, adsorbents, and electronic materials. They serve as dopants in semiconductor materials, modifying electronic properties for specific applications. In material synthesis, Lewis acids can direct the formation of specific crystal structures, control porosity in framework materials, and modify surface properties. They are also employed in the preparation of metal-organic frameworks (MOFs) and other porous materials with applications in gas storage, separation, and catalysis.
- Novel Lewis Acid Structures and Formulations: Research continues to develop novel Lewis acid structures with enhanced properties such as increased stability, selectivity, and recyclability. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acids incorporated into polymeric frameworks. Innovations include water-tolerant Lewis acids, chiral Lewis acids for asymmetric catalysis, and Lewis acids with tunable acidity. These novel structures expand the application scope of Lewis acids in environmentally friendly processes and enable reactions under milder conditions with improved efficiency.
02 Lewis Acids in Polymerization Processes
Lewis acids play a crucial role in polymerization reactions, particularly in cationic polymerization and coordination polymerization. They can activate monomers and control the stereochemistry of the resulting polymers. Metal-based Lewis acids such as titanium and zirconium compounds are commonly used in olefin polymerization. These catalysts can influence polymer properties including molecular weight, distribution, and tacticity, making them valuable tools in polymer science and engineering.Expand Specific Solutions03 Lewis Acids in Material Science Applications
Lewis acids are utilized in various material science applications, including the preparation of advanced materials, surface treatments, and coatings. They can modify surface properties, enhance adhesion, and improve material performance. In particular, Lewis acids are employed in the synthesis of nanoparticles, thin films, and composite materials. They can also be used to create materials with specific optical, electrical, or mechanical properties for specialized applications.Expand Specific Solutions04 Lewis Acids in Pharmaceutical Synthesis
Lewis acids are essential tools in pharmaceutical synthesis, enabling the creation of complex drug molecules through selective transformations. They facilitate stereoselective reactions, allowing for the synthesis of specific isomers with desired biological activities. Lewis acid catalysts can promote reactions under milder conditions, reducing side reactions and improving yields. They are particularly valuable in the synthesis of heterocyclic compounds, which are common structural motifs in many pharmaceuticals.Expand Specific Solutions05 Novel Lewis Acid Systems and Modifications
Research continues to develop novel Lewis acid systems with enhanced properties such as increased selectivity, recyclability, and environmental compatibility. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acids with tunable acidity. Recent innovations focus on immobilized Lewis acids that can be easily recovered and reused, as well as Lewis acids designed for specific reaction environments including aqueous media and green solvents. These advancements aim to make Lewis acid catalysis more sustainable and efficient.Expand Specific Solutions
Leading Companies and Research Institutions in Fluorination
The fluorination processes market is currently in a growth phase, with increasing applications in pharmaceuticals, agrochemicals, and materials science. The global market size for Lewis acid-catalyzed fluorination is expanding steadily, driven by demand for fluorinated compounds in high-performance materials and specialty chemicals. Technologically, the field shows moderate maturity with established processes but significant room for innovation. Leading players include Solvay SA and its subsidiaries, which demonstrate advanced expertise in fluorochemicals and polymers, alongside BASF Corp. and LG Chem with strong patent portfolios. Mitsubishi Gas Chemical and Arkema have made notable advancements in selective fluorination techniques, while academic-industrial collaborations with institutions like Hokkaido University and CNRS are accelerating technological development. The competitive landscape features both specialized chemical companies and diversified conglomerates investing in proprietary fluorination technologies.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has pioneered the use of aluminum-based Lewis acid catalysts for hydrofluorination processes, particularly in the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). Their technology employs modified aluminum chloride (AlCl3) and aluminum fluoride (AlF3) catalysts with enhanced stability and activity. Honeywell's approach includes proprietary catalyst preparation methods that control surface area and acidity, optimizing fluorination efficiency. Their process technology integrates reaction and separation systems to handle the highly exothermic nature of fluorination reactions while maintaining safety and product purity. Honeywell has also developed antimony-free Lewis acid systems to address environmental concerns associated with traditional fluorination catalysts, aligning with their commitment to sustainable chemistry.
Strengths: Honeywell's catalysts demonstrate excellent activity at lower temperatures, reducing energy requirements and improving process economics. Their integrated systems offer enhanced safety features for handling hazardous fluorinating agents. Weaknesses: Some of their Lewis acid systems may experience deactivation in the presence of certain impurities, requiring additional purification steps in industrial applications.
Solvay SA
Technical Solution: Solvay has developed advanced Lewis acid catalysts for fluorination processes, particularly focusing on antimony pentafluoride (SbF5) and tantalum pentafluoride (TaF5) systems. Their proprietary technology employs these strong Lewis acids to facilitate selective fluorination of organic compounds under mild conditions. Solvay's approach involves the use of supported Lewis acid catalysts that can be easily recovered and reused, improving process economics and sustainability. Their fluorination technology enables the production of specialty fluorochemicals, including fluoropolymers and pharmaceutical intermediates, with high regioselectivity and stereoselectivity. Solvay has also developed continuous flow processes for Lewis acid-catalyzed fluorinations, allowing for safer handling of hazardous fluorinating agents and improved process control.
Strengths: Solvay's Lewis acid catalysts demonstrate exceptional activity and selectivity for fluorination reactions, reducing side reactions and improving yield. Their supported catalyst systems enable easier handling and recovery. Weaknesses: Some of their Lewis acid systems require specialized equipment due to their corrosive nature and sensitivity to moisture, potentially increasing implementation costs.
Key Patents and Literature on Lewis Acid Fluorination
Fluorination catalysts comprising antimony V
PatentInactiveEP1074300A3
Innovation
- A supported Lewis acid catalyst system utilizing fluorine-treated, moisture-free activated carbon as a support for metals such as antimony, titanium, tin, niobium, or tantalum, activated with hydrogen fluoride, allowing fluorination at higher temperatures without chlorine cofeeds.
Methods of preparing quinolone analogs
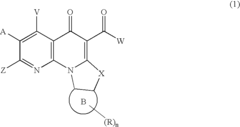
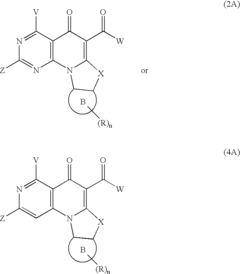
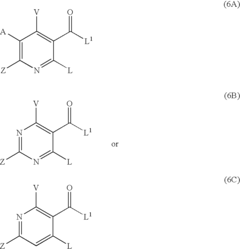
PatentActiveUS20070032652A1
Innovation
- The development of quinolone analog compounds, synthesized through specific chemical reactions involving various reactants and catalysts, which interact with DNA quadruplexes to inhibit cell proliferation and induce apoptosis in cancer cells.
Environmental Impact and Green Chemistry Considerations
The environmental impact of Lewis acid catalysts in fluorination processes represents a significant concern in modern chemical manufacturing. Traditional fluorination methods often employ harsh reagents such as hydrogen fluoride (HF) and anhydrous hydrofluoric acid, which pose serious environmental hazards including air pollution, water contamination, and potential for accidental releases. Lewis acid catalysts, while improving reaction efficiency, introduce their own environmental considerations that must be carefully evaluated.
Metal-based Lewis acids commonly used in fluorination processes, such as aluminum chloride (AlCl₃), boron trifluoride (BF₃), and antimony pentafluoride (SbF₅), present toxicity concerns and waste management challenges. These catalysts often cannot be easily recovered after reaction, resulting in substantial waste streams containing heavy metals that require specialized disposal procedures to prevent environmental contamination.
Green chemistry principles offer promising pathways to mitigate these environmental impacts. The development of recyclable and recoverable Lewis acid catalysts represents a significant advancement in sustainable fluorination technology. Heterogeneous catalysts supported on solid matrices allow for easier separation and potential reuse, substantially reducing waste generation and resource consumption compared to traditional homogeneous systems.
Water-tolerant Lewis acids have emerged as environmentally preferable alternatives to conventional moisture-sensitive catalysts. These innovative catalysts can operate in aqueous or mixed solvent systems, reducing the need for hazardous organic solvents and minimizing volatile organic compound (VOC) emissions. This approach aligns with green chemistry principles by reducing hazards and improving process safety profiles.
Energy efficiency considerations also factor prominently in environmental assessments of Lewis acid-catalyzed fluorination. Many traditional processes require elevated temperatures and pressures, resulting in substantial energy consumption. Newer Lewis acid systems that operate under milder conditions offer significant energy savings and reduced carbon footprints, contributing to overall sustainability goals.
Life cycle assessment (LCA) studies comparing conventional and Lewis acid-catalyzed fluorination processes reveal complex environmental trade-offs. While catalytic approaches typically reduce direct emissions and waste generation, the environmental burden may shift to catalyst production and recovery stages. Comprehensive LCA frameworks are essential for making informed decisions about process selection and optimization from an environmental perspective.
Regulatory frameworks increasingly influence the adoption of greener fluorination technologies. Stringent regulations on persistent organic pollutants, heavy metal emissions, and industrial waste have accelerated research into environmentally benign Lewis acid catalysts. Future developments will likely focus on bio-derived catalysts, non-metal alternatives, and continuous flow processes that further minimize environmental impacts while maintaining or enhancing reaction efficiency.
Metal-based Lewis acids commonly used in fluorination processes, such as aluminum chloride (AlCl₃), boron trifluoride (BF₃), and antimony pentafluoride (SbF₅), present toxicity concerns and waste management challenges. These catalysts often cannot be easily recovered after reaction, resulting in substantial waste streams containing heavy metals that require specialized disposal procedures to prevent environmental contamination.
Green chemistry principles offer promising pathways to mitigate these environmental impacts. The development of recyclable and recoverable Lewis acid catalysts represents a significant advancement in sustainable fluorination technology. Heterogeneous catalysts supported on solid matrices allow for easier separation and potential reuse, substantially reducing waste generation and resource consumption compared to traditional homogeneous systems.
Water-tolerant Lewis acids have emerged as environmentally preferable alternatives to conventional moisture-sensitive catalysts. These innovative catalysts can operate in aqueous or mixed solvent systems, reducing the need for hazardous organic solvents and minimizing volatile organic compound (VOC) emissions. This approach aligns with green chemistry principles by reducing hazards and improving process safety profiles.
Energy efficiency considerations also factor prominently in environmental assessments of Lewis acid-catalyzed fluorination. Many traditional processes require elevated temperatures and pressures, resulting in substantial energy consumption. Newer Lewis acid systems that operate under milder conditions offer significant energy savings and reduced carbon footprints, contributing to overall sustainability goals.
Life cycle assessment (LCA) studies comparing conventional and Lewis acid-catalyzed fluorination processes reveal complex environmental trade-offs. While catalytic approaches typically reduce direct emissions and waste generation, the environmental burden may shift to catalyst production and recovery stages. Comprehensive LCA frameworks are essential for making informed decisions about process selection and optimization from an environmental perspective.
Regulatory frameworks increasingly influence the adoption of greener fluorination technologies. Stringent regulations on persistent organic pollutants, heavy metal emissions, and industrial waste have accelerated research into environmentally benign Lewis acid catalysts. Future developments will likely focus on bio-derived catalysts, non-metal alternatives, and continuous flow processes that further minimize environmental impacts while maintaining or enhancing reaction efficiency.
Scale-up and Industrial Application Feasibility
The transition from laboratory-scale fluorination processes to industrial production presents significant engineering challenges that must be addressed systematically. Current industrial applications of Lewis acid-catalyzed fluorination processes are primarily concentrated in pharmaceutical, agrochemical, and specialty chemical sectors, with production scales ranging from hundreds of kilograms to several tons annually. The capital investment required for establishing commercial-scale fluorination facilities utilizing Lewis acid catalysts typically ranges from $5-20 million, depending on production capacity and process complexity.
Process safety considerations become paramount during scale-up, as many Lewis acids used in fluorination reactions (particularly AlCl₃, BF₃, and SbF₅) are highly reactive and moisture-sensitive. Industrial implementations require specialized corrosion-resistant equipment constructed from materials such as Hastelloy, Inconel, or fluoropolymer-lined vessels. Continuous flow reactors have demonstrated superior performance over batch processes for many Lewis acid-catalyzed fluorinations, offering better heat management, improved mixing, and reduced catalyst consumption.
Economic feasibility analysis indicates that Lewis acid-catalyzed fluorination processes become commercially viable when the target fluorinated compounds command market prices exceeding $50-100/kg, with process yields above 85% and catalyst recycling rates of at least 70%. The environmental impact assessment must account for waste streams containing spent Lewis acids, which require specialized neutralization and disposal protocols to comply with increasingly stringent regulations.
Recent industrial innovations have focused on immobilized Lewis acid catalysts, which facilitate easier separation and recycling while minimizing equipment corrosion. Companies including Honeywell, Solvay, and Arkema have successfully implemented such technologies in commercial operations, achieving significant reductions in catalyst consumption and waste generation.
Energy requirements for industrial-scale Lewis acid fluorination processes typically range from 2-5 kWh per kilogram of product, with cooling demands often exceeding heating requirements due to the exothermic nature of many fluorination reactions. Process intensification strategies, including microreactor technology and ultrasonic assistance, have demonstrated potential for reducing energy consumption by 20-30% while improving conversion rates.
The regulatory landscape for industrial fluorination processes varies significantly by region, with particularly stringent requirements in the European Union under REACH regulations. Compliance costs can represent 5-15% of total production expenses, necessitating thorough planning during scale-up design phases.
Process safety considerations become paramount during scale-up, as many Lewis acids used in fluorination reactions (particularly AlCl₃, BF₃, and SbF₅) are highly reactive and moisture-sensitive. Industrial implementations require specialized corrosion-resistant equipment constructed from materials such as Hastelloy, Inconel, or fluoropolymer-lined vessels. Continuous flow reactors have demonstrated superior performance over batch processes for many Lewis acid-catalyzed fluorinations, offering better heat management, improved mixing, and reduced catalyst consumption.
Economic feasibility analysis indicates that Lewis acid-catalyzed fluorination processes become commercially viable when the target fluorinated compounds command market prices exceeding $50-100/kg, with process yields above 85% and catalyst recycling rates of at least 70%. The environmental impact assessment must account for waste streams containing spent Lewis acids, which require specialized neutralization and disposal protocols to comply with increasingly stringent regulations.
Recent industrial innovations have focused on immobilized Lewis acid catalysts, which facilitate easier separation and recycling while minimizing equipment corrosion. Companies including Honeywell, Solvay, and Arkema have successfully implemented such technologies in commercial operations, achieving significant reductions in catalyst consumption and waste generation.
Energy requirements for industrial-scale Lewis acid fluorination processes typically range from 2-5 kWh per kilogram of product, with cooling demands often exceeding heating requirements due to the exothermic nature of many fluorination reactions. Process intensification strategies, including microreactor technology and ultrasonic assistance, have demonstrated potential for reducing energy consumption by 20-30% while improving conversion rates.
The regulatory landscape for industrial fluorination processes varies significantly by region, with particularly stringent requirements in the European Union under REACH regulations. Compliance costs can represent 5-15% of total production expenses, necessitating thorough planning during scale-up design phases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!