How to Analyze Reaction Profiles in Lewis Acid Chemistry?
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Chemistry Background and Research Objectives
Lewis acid chemistry represents a cornerstone of modern chemical reactions, dating back to Gilbert N. Lewis's groundbreaking definition in 1923. These electron pair acceptors have evolved from simple conceptual frameworks to sophisticated catalytic systems driving numerous industrial processes and pharmaceutical syntheses. The field has witnessed remarkable growth, transitioning from classical Lewis acids like AlCl₃ and BF₃ to designer catalysts featuring transition metals and main group elements with precisely tuned electronic properties.
The analysis of reaction profiles in Lewis acid chemistry has become increasingly sophisticated over the past decades, incorporating advanced computational methods alongside traditional experimental techniques. Understanding these reaction pathways is crucial for rational catalyst design and process optimization across multiple industries, from petrochemicals to fine chemical synthesis.
Current technological advancements in spectroscopic methods, particularly in-situ NMR, IR, and mass spectrometry, have revolutionized our ability to monitor Lewis acid-mediated reactions in real-time. These developments allow for unprecedented insights into reaction intermediates and transition states that were previously only theoretical constructs. Computational chemistry has similarly advanced, with density functional theory (DFT) calculations now capable of modeling complex Lewis acid interactions with remarkable accuracy.
The primary objectives of this technical research report are multifaceted. First, we aim to comprehensively evaluate existing methodologies for analyzing Lewis acid reaction profiles, identifying their respective strengths and limitations. Second, we seek to explore emerging technologies that may enhance our understanding of reaction mechanisms, particularly focusing on transient intermediates and rate-determining steps.
Additionally, this report will investigate the correlation between Lewis acid strength measurements and catalytic performance across various reaction types. Understanding this relationship is essential for developing predictive models that can accelerate catalyst discovery and optimization. We will also examine how structural modifications to Lewis acids influence reaction pathways and selectivity patterns.
The long-term technological trajectory suggests increasing integration of machine learning approaches with experimental data to develop predictive models for Lewis acid reactivity. These models promise to revolutionize catalyst design by enabling rapid virtual screening of potential Lewis acid catalysts before experimental validation, significantly reducing development timelines and costs.
By establishing a comprehensive framework for analyzing reaction profiles in Lewis acid chemistry, this research aims to provide valuable insights for both fundamental research and industrial applications, ultimately contributing to more efficient and sustainable chemical processes.
The analysis of reaction profiles in Lewis acid chemistry has become increasingly sophisticated over the past decades, incorporating advanced computational methods alongside traditional experimental techniques. Understanding these reaction pathways is crucial for rational catalyst design and process optimization across multiple industries, from petrochemicals to fine chemical synthesis.
Current technological advancements in spectroscopic methods, particularly in-situ NMR, IR, and mass spectrometry, have revolutionized our ability to monitor Lewis acid-mediated reactions in real-time. These developments allow for unprecedented insights into reaction intermediates and transition states that were previously only theoretical constructs. Computational chemistry has similarly advanced, with density functional theory (DFT) calculations now capable of modeling complex Lewis acid interactions with remarkable accuracy.
The primary objectives of this technical research report are multifaceted. First, we aim to comprehensively evaluate existing methodologies for analyzing Lewis acid reaction profiles, identifying their respective strengths and limitations. Second, we seek to explore emerging technologies that may enhance our understanding of reaction mechanisms, particularly focusing on transient intermediates and rate-determining steps.
Additionally, this report will investigate the correlation between Lewis acid strength measurements and catalytic performance across various reaction types. Understanding this relationship is essential for developing predictive models that can accelerate catalyst discovery and optimization. We will also examine how structural modifications to Lewis acids influence reaction pathways and selectivity patterns.
The long-term technological trajectory suggests increasing integration of machine learning approaches with experimental data to develop predictive models for Lewis acid reactivity. These models promise to revolutionize catalyst design by enabling rapid virtual screening of potential Lewis acid catalysts before experimental validation, significantly reducing development timelines and costs.
By establishing a comprehensive framework for analyzing reaction profiles in Lewis acid chemistry, this research aims to provide valuable insights for both fundamental research and industrial applications, ultimately contributing to more efficient and sustainable chemical processes.
Market Applications and Demand for Lewis Acid Reactions
Lewis acid chemistry has witnessed significant market growth across multiple industries due to its versatile applications in catalysis and synthesis processes. The pharmaceutical sector represents the largest market segment, with Lewis acid-catalyzed reactions being instrumental in the synthesis of active pharmaceutical ingredients (APIs) and complex drug molecules. This sector's demand is primarily driven by the need for efficient, selective, and environmentally friendly synthetic routes that can reduce production costs while maintaining high product quality.
The fine chemicals industry constitutes another substantial market, where Lewis acid reactions enable the production of specialty chemicals, fragrances, flavors, and agricultural compounds. Companies in this space increasingly seek reaction profile analysis tools to optimize yields and minimize waste generation, particularly as sustainability concerns grow among consumers and regulatory bodies.
Polymer manufacturing represents a rapidly expanding application area, with Lewis acid catalysts facilitating polymerization processes for materials used in electronics, automotive components, and consumer goods. The ability to precisely control reaction kinetics through advanced profile analysis directly impacts product properties such as molecular weight distribution and mechanical characteristics.
The petrochemical industry utilizes Lewis acid chemistry in various refining and conversion processes. Enhanced reaction profile analysis capabilities allow for more efficient catalyst utilization, improved process economics, and reduced environmental impact. This sector's demand is particularly sensitive to energy efficiency improvements that can be achieved through optimized reaction conditions.
Academic and research institutions form a distinct market segment with growing demand for sophisticated reaction profile analysis tools. This demand is fueled by the expanding research into novel catalytic systems, green chemistry initiatives, and fundamental studies of reaction mechanisms.
Market analysis indicates a compound annual growth rate of approximately 6.8% for Lewis acid chemistry applications across all sectors, with particularly strong growth in regions with expanding pharmaceutical and specialty chemical manufacturing capabilities, such as India, China, and Southeast Asia.
The demand for advanced analytical techniques is further driven by increasingly stringent regulatory requirements regarding product purity and process documentation, particularly in pharmaceutical and food-related applications. Companies must demonstrate thorough understanding of reaction profiles to satisfy quality assurance standards and regulatory compliance requirements.
Emerging trends indicate growing interest in real-time monitoring solutions that can provide immediate feedback for process control, reducing the time and resources required for reaction optimization while improving consistency in production environments.
The fine chemicals industry constitutes another substantial market, where Lewis acid reactions enable the production of specialty chemicals, fragrances, flavors, and agricultural compounds. Companies in this space increasingly seek reaction profile analysis tools to optimize yields and minimize waste generation, particularly as sustainability concerns grow among consumers and regulatory bodies.
Polymer manufacturing represents a rapidly expanding application area, with Lewis acid catalysts facilitating polymerization processes for materials used in electronics, automotive components, and consumer goods. The ability to precisely control reaction kinetics through advanced profile analysis directly impacts product properties such as molecular weight distribution and mechanical characteristics.
The petrochemical industry utilizes Lewis acid chemistry in various refining and conversion processes. Enhanced reaction profile analysis capabilities allow for more efficient catalyst utilization, improved process economics, and reduced environmental impact. This sector's demand is particularly sensitive to energy efficiency improvements that can be achieved through optimized reaction conditions.
Academic and research institutions form a distinct market segment with growing demand for sophisticated reaction profile analysis tools. This demand is fueled by the expanding research into novel catalytic systems, green chemistry initiatives, and fundamental studies of reaction mechanisms.
Market analysis indicates a compound annual growth rate of approximately 6.8% for Lewis acid chemistry applications across all sectors, with particularly strong growth in regions with expanding pharmaceutical and specialty chemical manufacturing capabilities, such as India, China, and Southeast Asia.
The demand for advanced analytical techniques is further driven by increasingly stringent regulatory requirements regarding product purity and process documentation, particularly in pharmaceutical and food-related applications. Companies must demonstrate thorough understanding of reaction profiles to satisfy quality assurance standards and regulatory compliance requirements.
Emerging trends indicate growing interest in real-time monitoring solutions that can provide immediate feedback for process control, reducing the time and resources required for reaction optimization while improving consistency in production environments.
Current Analytical Methods and Technical Limitations
The analysis of reaction profiles in Lewis acid chemistry currently relies on a combination of spectroscopic, chromatographic, and computational methods. Nuclear Magnetic Resonance (NMR) spectroscopy stands as the cornerstone technique, offering detailed structural information about Lewis acid-base adducts and reaction intermediates. Both 1H and 13C NMR provide valuable insights, while multinuclear NMR (including 11B, 19F, and 27Al) has become increasingly important for tracking Lewis acid behavior specifically.
Infrared (IR) spectroscopy complements NMR by monitoring characteristic vibrational changes during Lewis acid-base interactions. The shifts in carbonyl stretching frequencies, for instance, serve as reliable indicators of coordination strength and electronic effects. UV-Visible spectroscopy further extends analytical capabilities by tracking electronic transitions that occur during complexation events.
Mass spectrometry techniques, particularly ESI-MS (Electrospray Ionization Mass Spectrometry), have revolutionized the field by enabling direct observation of reaction intermediates under mild conditions. This approach has proven invaluable for capturing transient species in Lewis acid catalysis that would otherwise remain undetected.
Chromatographic methods, including HPLC and GC, provide crucial kinetic data by monitoring reaction progress and product distribution. When coupled with mass spectrometry (LC-MS, GC-MS), these techniques offer powerful tools for comprehensive reaction profiling.
Despite these advances, significant technical limitations persist. The high sensitivity of many Lewis acid systems to moisture and oxygen necessitates specialized handling techniques and equipment, often complicating analysis. The transient nature of key intermediates presents another major challenge, as many exist only briefly before undergoing further transformations, making their direct observation exceptionally difficult.
Structural complexity introduces additional complications, particularly when multiple coordination sites or competing equilibria are involved. Current analytical methods often struggle to differentiate between similar Lewis acid species or to quantify their relative contributions to overall reactivity.
Computational limitations also hinder progress, as accurate modeling of Lewis acid interactions requires sophisticated quantum mechanical calculations that balance accuracy with computational feasibility. The development of reliable force fields for molecular dynamics simulations of Lewis acid systems remains an ongoing challenge.
Standardization issues further complicate the field, with varying experimental conditions making direct comparisons between different studies problematic. The lack of universal benchmarks for Lewis acidity measurements continues to impede systematic analysis and categorization of Lewis acid strength and selectivity.
Infrared (IR) spectroscopy complements NMR by monitoring characteristic vibrational changes during Lewis acid-base interactions. The shifts in carbonyl stretching frequencies, for instance, serve as reliable indicators of coordination strength and electronic effects. UV-Visible spectroscopy further extends analytical capabilities by tracking electronic transitions that occur during complexation events.
Mass spectrometry techniques, particularly ESI-MS (Electrospray Ionization Mass Spectrometry), have revolutionized the field by enabling direct observation of reaction intermediates under mild conditions. This approach has proven invaluable for capturing transient species in Lewis acid catalysis that would otherwise remain undetected.
Chromatographic methods, including HPLC and GC, provide crucial kinetic data by monitoring reaction progress and product distribution. When coupled with mass spectrometry (LC-MS, GC-MS), these techniques offer powerful tools for comprehensive reaction profiling.
Despite these advances, significant technical limitations persist. The high sensitivity of many Lewis acid systems to moisture and oxygen necessitates specialized handling techniques and equipment, often complicating analysis. The transient nature of key intermediates presents another major challenge, as many exist only briefly before undergoing further transformations, making their direct observation exceptionally difficult.
Structural complexity introduces additional complications, particularly when multiple coordination sites or competing equilibria are involved. Current analytical methods often struggle to differentiate between similar Lewis acid species or to quantify their relative contributions to overall reactivity.
Computational limitations also hinder progress, as accurate modeling of Lewis acid interactions requires sophisticated quantum mechanical calculations that balance accuracy with computational feasibility. The development of reliable force fields for molecular dynamics simulations of Lewis acid systems remains an ongoing challenge.
Standardization issues further complicate the field, with varying experimental conditions making direct comparisons between different studies problematic. The lack of universal benchmarks for Lewis acidity measurements continues to impede systematic analysis and categorization of Lewis acid strength and selectivity.
Established Reaction Profile Analysis Techniques
01 Lewis acid catalyzed organic synthesis reactions
Lewis acids are widely used as catalysts in organic synthesis reactions to facilitate bond formation and cleavage. These catalysts work by accepting electron pairs from reactants, thereby activating them for nucleophilic attack. Common reactions include alkylation, acylation, and various carbon-carbon bond forming processes. The reaction profiles typically show enhanced reaction rates and improved selectivity compared to non-catalyzed alternatives, with the Lewis acid lowering activation energy barriers through coordination with functional groups.- Lewis acid catalyzed organic synthesis reactions: Lewis acids serve as catalysts in various organic synthesis reactions by accepting electron pairs from substrates. These reactions include alkylation, acylation, and cyclization processes. The Lewis acid interacts with functional groups containing lone pairs of electrons, activating them for nucleophilic attack. This catalytic approach enables more efficient reaction pathways with improved yields and selectivity compared to traditional methods.
- Lewis acid catalysts in polymerization reactions: Lewis acids play a crucial role in polymerization processes, particularly in the production of various polymers and copolymers. They function as initiators or co-catalysts that activate monomers for chain growth. The reaction profiles typically show distinct initiation, propagation, and termination phases. By controlling the Lewis acid concentration and reaction conditions, polymer properties such as molecular weight, distribution, and stereochemistry can be precisely tuned.
- Metal-based Lewis acid catalytic systems: Various metal compounds function as Lewis acids in chemical reactions, with different metals providing unique reactivity profiles. Transition metals, lanthanides, and main group metals can be incorporated into catalytic systems where the metal center acts as the Lewis acidic site. These catalysts often exhibit distinctive reaction kinetics, selectivity patterns, and tolerance to functional groups. The metal's electronic properties and coordination environment can be modified to optimize catalytic performance for specific transformations.
- Lewis acid-mediated reaction mechanisms and energy profiles: The reaction profiles of Lewis acid-mediated transformations typically show altered energy landscapes compared to uncatalyzed processes. Lewis acids lower activation barriers by stabilizing transition states and intermediates through coordination. Computational and experimental studies reveal that these catalysts can change reaction pathways, influence stereochemical outcomes, and enable otherwise unfavorable transformations. Understanding these energy profiles helps in designing more efficient catalytic systems with improved selectivity and reactivity.
- Industrial applications of Lewis acid chemistry: Lewis acid chemistry has significant industrial applications across various sectors including petrochemicals, pharmaceuticals, and materials science. These catalysts enable large-scale processes with improved efficiency, selectivity, and environmental profile. Industrial reaction profiles often focus on optimizing catalyst loading, recycling, and stability under process conditions. Recent developments include heterogeneous Lewis acid catalysts that combine high activity with ease of separation and reuse, addressing sustainability concerns in chemical manufacturing.
02 Lewis acid-mediated polymerization processes
Lewis acids play a crucial role in various polymerization reactions, particularly in the production of specialty polymers. They function as initiators or co-catalysts in cationic polymerization, ring-opening polymerization, and coordination polymerization processes. The reaction profiles of these systems typically exhibit induction periods followed by propagation phases with characteristic kinetics. The molecular weight distribution and polymer architecture can be controlled by adjusting the Lewis acid strength, concentration, and reaction conditions.Expand Specific Solutions03 Lewis acid complexes in transition metal catalysis
The combination of Lewis acids with transition metal catalysts creates powerful catalytic systems with unique reaction profiles. These hybrid catalysts often show synergistic effects, where the Lewis acid enhances the activity or selectivity of the transition metal center. Such systems are employed in hydrogenation, oxidation, cross-coupling, and metathesis reactions. The reaction profiles typically show modified induction periods, altered reaction rates, and sometimes completely different product distributions compared to the individual catalytic components.Expand Specific Solutions04 Reaction kinetics and mechanistic studies of Lewis acid catalysis
Studies on the kinetics and mechanisms of Lewis acid-catalyzed reactions provide insights into reaction pathways and energy profiles. These investigations utilize spectroscopic techniques, computational methods, and kinetic measurements to elucidate the role of Lewis acids in reaction intermediates and transition states. The reaction profiles often reveal multi-step processes with rate-determining steps influenced by Lewis acid strength, solvent effects, and substrate electronic properties. Understanding these profiles enables optimization of reaction conditions and catalyst design.Expand Specific Solutions05 Industrial applications of Lewis acid chemistry
Lewis acid chemistry has significant applications in industrial processes, particularly in petrochemical, pharmaceutical, and materials manufacturing. These applications leverage the unique reaction profiles of Lewis acid systems to achieve efficient transformations at commercial scale. Industrial processes often employ heterogeneous Lewis acid catalysts for ease of separation and recycling. The reaction profiles in industrial settings are optimized for factors such as conversion rates, selectivity, catalyst lifetime, and process economics, often differing from laboratory-scale profiles due to heat and mass transfer considerations.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid chemistry reaction profile analysis market is in a growth phase, characterized by increasing demand for advanced analytical techniques in pharmaceutical and chemical industries. The market size is expanding due to rising applications in drug discovery and materials science, with an estimated annual growth rate of 7-8%. Technologically, the field is moderately mature but evolving rapidly with innovations in computational methods and spectroscopic techniques. Leading players include established pharmaceutical companies like Takeda, Pfizer, and Sanofi, alongside specialized research institutions such as Shanghai Institute of Organic Chemistry and Japan Science & Technology Agency. ExxonMobil Chemical and China Petroleum & Chemical Corp represent significant industrial applications, while academic institutions like Zhejiang University and Brown University contribute cutting-edge research methodologies.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed sophisticated analytical platforms for Lewis acid chemistry reaction profiling, particularly in catalytic processes relevant to petrochemical production. Their approach combines in-situ spectroscopic techniques (IR, NMR, and Raman) with computational modeling to monitor Lewis acid-base interactions in real-time. They've pioneered the use of solid-state NMR to characterize Lewis acid sites on heterogeneous catalysts and track reaction intermediates. Their proprietary "Reaction Profile Analysis System" integrates temperature-controlled reaction vessels with automated sampling and multi-detector analysis to generate comprehensive kinetic and thermodynamic profiles of Lewis acid-catalyzed reactions. This system enables precise control of reaction parameters while simultaneously collecting spectroscopic and chromatographic data to elucidate reaction mechanisms and optimize catalytic performance.
Strengths: Exceptional integration of multiple analytical techniques providing comprehensive reaction profiles; advanced computational capabilities for mechanistic interpretation; extensive experience with industrial-scale Lewis acid catalysis. Weaknesses: Systems primarily optimized for petrochemical applications; proprietary nature limits broader scientific accessibility; high capital investment requirements for implementation.
Shanghai Institute of Organic Chemistry
Technical Solution: The Shanghai Institute of Organic Chemistry (SIOC) has developed sophisticated methodologies for analyzing Lewis acid reaction profiles, particularly in asymmetric synthesis and complex natural product chemistry. Their approach integrates advanced spectroscopic techniques with computational modeling to elucidate reaction mechanisms and transition states. SIOC's "Reaction Mechanism Analysis Platform" combines time-resolved spectroscopy (NMR, IR, and UV-Vis) with theoretical calculations to track reaction intermediates and energy profiles. They've pioneered the use of paramagnetic NMR techniques to study Lewis acid-substrate complexation dynamics and stereochemical outcomes. Their methodology incorporates isotopic labeling strategies to track atom movement throughout reaction pathways, providing detailed mechanistic insights. SIOC researchers have developed novel probe molecules specifically designed to characterize Lewis acid strength and selectivity profiles across different catalyst systems.
Strengths: Exceptional integration of experimental and theoretical approaches; specialized expertise in asymmetric catalysis; strong focus on mechanistic understanding rather than just reaction outcomes. Weaknesses: Systems primarily optimized for research rather than industrial applications; requires highly specialized expertise to implement effectively.
Key Spectroscopic and Computational Approaches
Ionic liquid, adduct and methods thereof
PatentWO2016005935A1
Innovation
- A process that reacts at least one electron-pair acceptor with at least one electron-pair donor to form an adduct, which is then further reacted with an electron-pair acceptor to produce the ionic liquid without the need for heating, using a method that involves contacting the reactants in the presence or absence of solvents and under inert atmospheres to obtain the ionic liquid.
Pyridinone analogs
PatentInactiveUS20110112086A1
Innovation
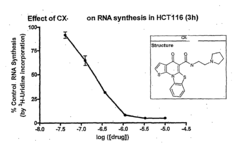
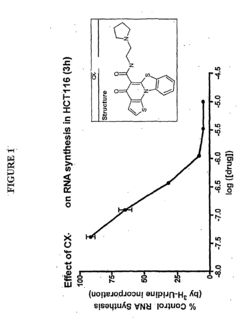
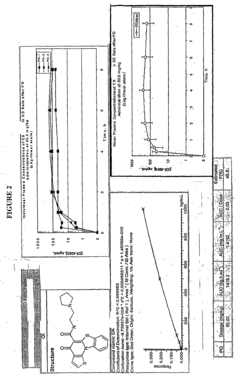
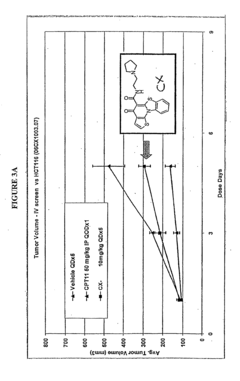
- Development of pyridinone analogs that inhibit cell proliferation and induce apoptosis by targeting ribosomal RNA biogenesis, interacting with quadruplex-forming regions of nucleic acids and modulating the activity of proteins like nucleolin, thereby reducing cell proliferation and inducing cell death.
Environmental Impact and Green Chemistry Considerations
The environmental impact of Lewis acid chemistry has become increasingly significant as industries and research institutions face growing pressure to adopt sustainable practices. Traditional Lewis acid catalysts often involve metal compounds that pose substantial environmental risks through their production, use, and disposal cycles. Heavy metals such as aluminum, tin, and boron derivatives commonly used in Lewis acid reactions can persist in ecosystems, potentially causing long-term contamination of soil and water resources when improperly managed.
Green chemistry principles offer a framework for mitigating these environmental concerns while maintaining reaction efficiency. The development of recyclable Lewis acid catalysts represents a major advancement in this field. Heterogeneous catalysts supported on inert materials allow for easier separation and recovery, significantly reducing waste generation compared to homogeneous systems. Recent innovations include magnetic nanoparticle-supported Lewis acids that can be recovered using external magnetic fields, achieving recovery rates exceeding 95% while maintaining catalytic activity through multiple cycles.
Water-compatible Lewis acid systems have emerged as another environmentally beneficial approach. These catalysts function effectively in aqueous media, eliminating the need for hazardous organic solvents that traditionally dominate Lewis acid chemistry. Lanthanide triflates exemplify this category, demonstrating remarkable stability and activity in water while facilitating simpler reaction workups and reduced organic waste streams.
Reaction profile analysis plays a crucial role in quantifying environmental benefits. Life cycle assessment (LCA) methodologies applied to Lewis acid reactions provide comprehensive evaluations of environmental impacts across reaction parameters. These assessments typically reveal that catalyst recovery efficiency and solvent selection represent the most significant factors affecting the overall environmental footprint of a Lewis acid-mediated process.
Atom economy metrics offer valuable insights when analyzing reaction profiles from a green chemistry perspective. Lewis acid reactions with high atom utilization minimize waste generation intrinsically. Modern analytical techniques such as real-time reaction monitoring through in-situ spectroscopy enable researchers to optimize reaction conditions for maximum atom economy while maintaining yield and selectivity parameters.
Regulatory frameworks increasingly influence Lewis acid chemistry practices globally. The implementation of REACH regulations in Europe and similar initiatives worldwide has accelerated the transition toward greener Lewis acid alternatives. Reaction profile analysis must therefore incorporate regulatory compliance assessments to ensure developed methodologies remain viable in evolving regulatory landscapes.
Green chemistry principles offer a framework for mitigating these environmental concerns while maintaining reaction efficiency. The development of recyclable Lewis acid catalysts represents a major advancement in this field. Heterogeneous catalysts supported on inert materials allow for easier separation and recovery, significantly reducing waste generation compared to homogeneous systems. Recent innovations include magnetic nanoparticle-supported Lewis acids that can be recovered using external magnetic fields, achieving recovery rates exceeding 95% while maintaining catalytic activity through multiple cycles.
Water-compatible Lewis acid systems have emerged as another environmentally beneficial approach. These catalysts function effectively in aqueous media, eliminating the need for hazardous organic solvents that traditionally dominate Lewis acid chemistry. Lanthanide triflates exemplify this category, demonstrating remarkable stability and activity in water while facilitating simpler reaction workups and reduced organic waste streams.
Reaction profile analysis plays a crucial role in quantifying environmental benefits. Life cycle assessment (LCA) methodologies applied to Lewis acid reactions provide comprehensive evaluations of environmental impacts across reaction parameters. These assessments typically reveal that catalyst recovery efficiency and solvent selection represent the most significant factors affecting the overall environmental footprint of a Lewis acid-mediated process.
Atom economy metrics offer valuable insights when analyzing reaction profiles from a green chemistry perspective. Lewis acid reactions with high atom utilization minimize waste generation intrinsically. Modern analytical techniques such as real-time reaction monitoring through in-situ spectroscopy enable researchers to optimize reaction conditions for maximum atom economy while maintaining yield and selectivity parameters.
Regulatory frameworks increasingly influence Lewis acid chemistry practices globally. The implementation of REACH regulations in Europe and similar initiatives worldwide has accelerated the transition toward greener Lewis acid alternatives. Reaction profile analysis must therefore incorporate regulatory compliance assessments to ensure developed methodologies remain viable in evolving regulatory landscapes.
Scalability and Industrial Implementation Challenges
Scaling Lewis acid chemistry reactions from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. The exothermic nature of many Lewis acid-catalyzed reactions requires sophisticated heat management systems when implemented at scale. Temperature control becomes increasingly difficult as reaction volumes expand, potentially leading to runaway reactions, reduced selectivity, and formation of unwanted by-products. Industrial implementation necessitates the development of specialized cooling systems and reactor designs capable of maintaining precise temperature profiles throughout the reaction process.
Material handling poses another substantial challenge, particularly with moisture-sensitive Lewis acids such as aluminum chloride and boron trifluoride. Industrial-scale operations require specialized equipment for storage, transfer, and recovery of these reagents, along with robust protocols for managing their reactivity with atmospheric moisture. The corrosive nature of many Lewis acids further complicates equipment selection, often necessitating expensive corrosion-resistant materials that significantly impact capital expenditure.
Reaction monitoring and profile analysis face considerable obstacles during scale-up. While laboratory-scale reactions can be monitored using sophisticated analytical techniques like in-situ NMR or IR spectroscopy, these methods become impractical at industrial scale. Companies must develop alternative monitoring strategies, such as sampling systems coupled with rapid analysis methods or the implementation of process analytical technology (PAT) tools that can withstand harsh reaction conditions while providing real-time data on reaction progress.
Waste management represents a critical consideration for industrial implementation. Lewis acid chemistry often generates substantial quantities of metal-containing waste streams that require specialized treatment before disposal. Developing economically viable recovery and recycling processes for Lewis acids becomes essential for sustainable manufacturing, though these processes add complexity and cost to production operations.
Regulatory compliance adds another layer of complexity to industrial implementation. Many Lewis acids are classified as hazardous substances, subject to strict handling, storage, and transportation regulations. Companies must navigate these regulatory frameworks while designing manufacturing processes that minimize worker exposure and environmental impact, often necessitating significant investments in containment systems, monitoring equipment, and personnel training.
The economic viability of scaled processes ultimately depends on balancing these technical challenges against production costs. Successful industrial implementation requires holistic process development that addresses reaction engineering, materials handling, analytical monitoring, waste management, and regulatory compliance simultaneously, while maintaining product quality and economic competitiveness.
Material handling poses another substantial challenge, particularly with moisture-sensitive Lewis acids such as aluminum chloride and boron trifluoride. Industrial-scale operations require specialized equipment for storage, transfer, and recovery of these reagents, along with robust protocols for managing their reactivity with atmospheric moisture. The corrosive nature of many Lewis acids further complicates equipment selection, often necessitating expensive corrosion-resistant materials that significantly impact capital expenditure.
Reaction monitoring and profile analysis face considerable obstacles during scale-up. While laboratory-scale reactions can be monitored using sophisticated analytical techniques like in-situ NMR or IR spectroscopy, these methods become impractical at industrial scale. Companies must develop alternative monitoring strategies, such as sampling systems coupled with rapid analysis methods or the implementation of process analytical technology (PAT) tools that can withstand harsh reaction conditions while providing real-time data on reaction progress.
Waste management represents a critical consideration for industrial implementation. Lewis acid chemistry often generates substantial quantities of metal-containing waste streams that require specialized treatment before disposal. Developing economically viable recovery and recycling processes for Lewis acids becomes essential for sustainable manufacturing, though these processes add complexity and cost to production operations.
Regulatory compliance adds another layer of complexity to industrial implementation. Many Lewis acids are classified as hazardous substances, subject to strict handling, storage, and transportation regulations. Companies must navigate these regulatory frameworks while designing manufacturing processes that minimize worker exposure and environmental impact, often necessitating significant investments in containment systems, monitoring equipment, and personnel training.
The economic viability of scaled processes ultimately depends on balancing these technical challenges against production costs. Successful industrial implementation requires holistic process development that addresses reaction engineering, materials handling, analytical monitoring, waste management, and regulatory compliance simultaneously, while maintaining product quality and economic competitiveness.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!