Lewis Acid Staging in Sequential Reactions
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has evolved significantly since its inception in the early 20th century, transforming from a theoretical concept to a cornerstone of modern synthetic chemistry. The development trajectory began with the fundamental Lewis acid-base theory proposed by Gilbert N. Lewis in 1923, which defined acids as electron pair acceptors. This conceptual framework laid the groundwork for understanding how Lewis acids facilitate chemical transformations by activating substrates through coordination interactions.
The evolution of Lewis acid catalysis has been marked by several pivotal advancements. Initially limited to simple aluminum and boron compounds, the field expanded dramatically in the 1970s and 1980s with the introduction of chiral Lewis acids, enabling stereoselective transformations. The 1990s witnessed the emergence of designer Lewis acids with tunable properties, while the early 2000s saw integration with other catalytic systems, creating powerful synergistic effects.
Recent years have witnessed a paradigm shift toward sequential Lewis acid catalysis, where multiple Lewis acid species operate in a coordinated fashion within a single reaction vessel. This approach represents a significant departure from traditional single-catalyst systems, offering unprecedented control over complex reaction pathways and enabling transformations previously considered unattainable.
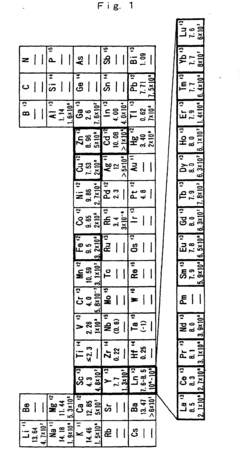
The concept of Lewis acid staging in sequential reactions addresses a fundamental challenge in multi-step synthesis: orchestrating different catalytic activities in a temporally controlled manner. By strategically deploying Lewis acids with varying strengths, selectivities, and activation profiles, chemists can guide reaction pathways with remarkable precision, minimizing side reactions and maximizing yield of desired products.
The primary objective of current research in this domain is to develop robust methodologies for implementing staged Lewis acid catalysis across diverse reaction classes. This includes establishing principles for catalyst selection, optimization of sequential addition protocols, and understanding the complex interplay between different Lewis acid species in solution. Additionally, researchers aim to elucidate the mechanistic underpinnings of these processes through advanced spectroscopic techniques and computational modeling.
Looking forward, the field seeks to expand the application scope of Lewis acid staging to address challenging transformations in pharmaceutical synthesis, materials science, and green chemistry. Particular emphasis is placed on developing catalytic systems capable of performing multiple bond-forming events in one pot, thereby reducing waste generation and improving process efficiency. The ultimate goal is to establish Lewis acid staging as a standard approach in the synthetic chemist's toolkit, enabling precise molecular construction with minimal environmental impact.
The evolution of Lewis acid catalysis has been marked by several pivotal advancements. Initially limited to simple aluminum and boron compounds, the field expanded dramatically in the 1970s and 1980s with the introduction of chiral Lewis acids, enabling stereoselective transformations. The 1990s witnessed the emergence of designer Lewis acids with tunable properties, while the early 2000s saw integration with other catalytic systems, creating powerful synergistic effects.
Recent years have witnessed a paradigm shift toward sequential Lewis acid catalysis, where multiple Lewis acid species operate in a coordinated fashion within a single reaction vessel. This approach represents a significant departure from traditional single-catalyst systems, offering unprecedented control over complex reaction pathways and enabling transformations previously considered unattainable.
The concept of Lewis acid staging in sequential reactions addresses a fundamental challenge in multi-step synthesis: orchestrating different catalytic activities in a temporally controlled manner. By strategically deploying Lewis acids with varying strengths, selectivities, and activation profiles, chemists can guide reaction pathways with remarkable precision, minimizing side reactions and maximizing yield of desired products.
The primary objective of current research in this domain is to develop robust methodologies for implementing staged Lewis acid catalysis across diverse reaction classes. This includes establishing principles for catalyst selection, optimization of sequential addition protocols, and understanding the complex interplay between different Lewis acid species in solution. Additionally, researchers aim to elucidate the mechanistic underpinnings of these processes through advanced spectroscopic techniques and computational modeling.
Looking forward, the field seeks to expand the application scope of Lewis acid staging to address challenging transformations in pharmaceutical synthesis, materials science, and green chemistry. Particular emphasis is placed on developing catalytic systems capable of performing multiple bond-forming events in one pot, thereby reducing waste generation and improving process efficiency. The ultimate goal is to establish Lewis acid staging as a standard approach in the synthetic chemist's toolkit, enabling precise molecular construction with minimal environmental impact.
Market Applications of Lewis Acid Sequential Catalysis
Lewis acid sequential catalysis has established significant market applications across multiple industrial sectors, transforming manufacturing processes and enabling more efficient production of high-value chemicals. The pharmaceutical industry represents one of the largest application domains, where Lewis acid staging enables the synthesis of complex drug molecules through controlled, multi-step reactions that would otherwise require numerous isolation and purification steps. This technology has been particularly valuable in producing chiral compounds with high stereoselectivity, reducing production costs by an estimated 30-40% for certain specialty pharmaceuticals.
In fine chemicals manufacturing, sequential Lewis acid catalysis has revolutionized the production of specialty monomers, fragrances, and flavoring agents. Companies like BASF and Dow Chemical have implemented industrial-scale processes utilizing this technology to produce intermediates for agricultural chemicals and consumer products. The market for these specialized catalytic processes continues to expand as manufacturers seek more atom-efficient and environmentally sustainable production methods.
The polymer industry has embraced Lewis acid sequential catalysis for producing advanced materials with precisely controlled architectures. This includes the synthesis of block copolymers, hyperbranched polymers, and materials with specific tacticity. These materials find applications in electronics, automotive components, and medical devices, with the global market for such specialty polymers growing steadily at approximately 6% annually.
Environmental applications represent an emerging market segment, where Lewis acid sequential catalysis enables more efficient pollution remediation and waste treatment processes. These catalysts can transform complex organic pollutants into less harmful substances through controlled sequential transformations, offering advantages over traditional single-step degradation methods.
The energy sector has begun adopting Lewis acid sequential catalysis for biomass conversion and fuel upgrading processes. This application allows for the selective transformation of complex biopolymers into valuable fuel components and platform chemicals, supporting the transition toward renewable energy sources and reducing dependence on petroleum-based feedstocks.
Electronic materials manufacturing represents another high-value application area, where sequential catalysis enables the production of semiconductors, display materials, and other components requiring molecular precision. The ability to perform multiple transformations in a controlled sequence has proven particularly valuable for synthesizing materials with specific electronic properties.
As industries continue to prioritize sustainability and efficiency, the market for Lewis acid sequential catalysis technologies is projected to expand further, with particular growth expected in biocatalysis integration, continuous flow manufacturing systems, and recyclable catalyst technologies.
In fine chemicals manufacturing, sequential Lewis acid catalysis has revolutionized the production of specialty monomers, fragrances, and flavoring agents. Companies like BASF and Dow Chemical have implemented industrial-scale processes utilizing this technology to produce intermediates for agricultural chemicals and consumer products. The market for these specialized catalytic processes continues to expand as manufacturers seek more atom-efficient and environmentally sustainable production methods.
The polymer industry has embraced Lewis acid sequential catalysis for producing advanced materials with precisely controlled architectures. This includes the synthesis of block copolymers, hyperbranched polymers, and materials with specific tacticity. These materials find applications in electronics, automotive components, and medical devices, with the global market for such specialty polymers growing steadily at approximately 6% annually.
Environmental applications represent an emerging market segment, where Lewis acid sequential catalysis enables more efficient pollution remediation and waste treatment processes. These catalysts can transform complex organic pollutants into less harmful substances through controlled sequential transformations, offering advantages over traditional single-step degradation methods.
The energy sector has begun adopting Lewis acid sequential catalysis for biomass conversion and fuel upgrading processes. This application allows for the selective transformation of complex biopolymers into valuable fuel components and platform chemicals, supporting the transition toward renewable energy sources and reducing dependence on petroleum-based feedstocks.
Electronic materials manufacturing represents another high-value application area, where sequential catalysis enables the production of semiconductors, display materials, and other components requiring molecular precision. The ability to perform multiple transformations in a controlled sequence has proven particularly valuable for synthesizing materials with specific electronic properties.
As industries continue to prioritize sustainability and efficiency, the market for Lewis acid sequential catalysis technologies is projected to expand further, with particular growth expected in biocatalysis integration, continuous flow manufacturing systems, and recyclable catalyst technologies.
Current Challenges in Lewis Acid Staging
Despite significant advancements in Lewis acid catalysis, several critical challenges persist in the staging of Lewis acids for sequential reactions. The primary difficulty lies in achieving precise control over the temporal activation of different reaction sites. Current methodologies often struggle with maintaining catalyst selectivity throughout multi-step processes, resulting in unwanted side reactions and diminished yields.
The stability of Lewis acids under varying reaction conditions presents another substantial hurdle. Many Lewis acids exhibit sensitivity to moisture, oxygen, or thermal fluctuations, complicating their sequential application in one-pot synthesis approaches. This instability necessitates complex handling procedures and specialized equipment, limiting industrial scalability and broader adoption.
Compatibility issues between different Lewis acid catalysts in sequential reactions remain largely unresolved. When multiple Lewis acids are required for different transformation stages, mutual deactivation frequently occurs through competitive coordination or the formation of inactive complexes. This phenomenon significantly restricts the design flexibility of multi-step synthetic pathways.
The development of switchable or stimuli-responsive Lewis acid systems has shown promise but faces considerable implementation challenges. Current photo-, thermo-, or redox-switchable catalysts often demonstrate insufficient switching efficiency or require conditions incompatible with maintaining substrate integrity throughout the reaction sequence.
Heterogeneous Lewis acid systems, while offering advantages in recyclability and separation, present unique staging difficulties related to mass transfer limitations and inconsistent active site accessibility. These physical constraints can lead to unpredictable reaction kinetics and complicate the precise timing required for sequential transformations.
Computational prediction tools for Lewis acid staging remain underdeveloped. Existing models struggle to accurately account for the complex interplay between catalyst structure, substrate binding affinities, and solvent effects across multiple reaction steps. This knowledge gap impedes rational design approaches for optimized sequential reaction systems.
The economic viability of advanced Lewis acid staging techniques represents a significant barrier to commercial implementation. Many current approaches require expensive ligands, rare earth metals, or sophisticated control systems that render them impractical for large-scale applications, despite their proven efficacy in laboratory settings.
The stability of Lewis acids under varying reaction conditions presents another substantial hurdle. Many Lewis acids exhibit sensitivity to moisture, oxygen, or thermal fluctuations, complicating their sequential application in one-pot synthesis approaches. This instability necessitates complex handling procedures and specialized equipment, limiting industrial scalability and broader adoption.
Compatibility issues between different Lewis acid catalysts in sequential reactions remain largely unresolved. When multiple Lewis acids are required for different transformation stages, mutual deactivation frequently occurs through competitive coordination or the formation of inactive complexes. This phenomenon significantly restricts the design flexibility of multi-step synthetic pathways.
The development of switchable or stimuli-responsive Lewis acid systems has shown promise but faces considerable implementation challenges. Current photo-, thermo-, or redox-switchable catalysts often demonstrate insufficient switching efficiency or require conditions incompatible with maintaining substrate integrity throughout the reaction sequence.
Heterogeneous Lewis acid systems, while offering advantages in recyclability and separation, present unique staging difficulties related to mass transfer limitations and inconsistent active site accessibility. These physical constraints can lead to unpredictable reaction kinetics and complicate the precise timing required for sequential transformations.
Computational prediction tools for Lewis acid staging remain underdeveloped. Existing models struggle to accurately account for the complex interplay between catalyst structure, substrate binding affinities, and solvent effects across multiple reaction steps. This knowledge gap impedes rational design approaches for optimized sequential reaction systems.
The economic viability of advanced Lewis acid staging techniques represents a significant barrier to commercial implementation. Many current approaches require expensive ligands, rare earth metals, or sophisticated control systems that render them impractical for large-scale applications, despite their proven efficacy in laboratory settings.
Contemporary Lewis Acid Staging Methodologies
01 Lewis acid catalysts in polymerization processes
Lewis acids are widely used as catalysts in various polymerization reactions. These catalysts facilitate the formation of polymer chains by activating monomers and promoting their addition to growing chains. The staging of Lewis acids in polymerization processes involves the strategic introduction of these catalysts at different stages to control reaction kinetics, molecular weight distribution, and polymer properties. This approach allows for better control over the polymerization process and results in polymers with desired characteristics.- Lewis acid catalysts in polymerization processes: Lewis acids are widely used as catalysts in various polymerization reactions. These catalysts facilitate the formation of polymer chains by activating monomers and controlling the reaction kinetics. The staging of Lewis acids in polymerization processes involves the strategic addition of different Lewis acid catalysts at various stages of the reaction to optimize polymer properties such as molecular weight distribution, stereochemistry, and branching. This approach allows for better control over the polymerization process and the resulting polymer characteristics.
- Lewis acid staging in petroleum refining and hydrocarbon processing: In petroleum refining and hydrocarbon processing, Lewis acid staging involves the sequential use of different Lewis acids to catalyze various transformations of hydrocarbons. This technique is particularly important in processes such as alkylation, isomerization, and cracking. By carefully controlling the strength and type of Lewis acids at different stages, refiners can enhance selectivity, increase conversion rates, and improve the quality of petroleum products. The staging approach helps minimize unwanted side reactions and optimize the yield of desired products.
- Lewis acid staging in pharmaceutical synthesis: In pharmaceutical synthesis, Lewis acid staging is employed to facilitate complex organic transformations with high stereoselectivity and yield. This approach involves the sequential or simultaneous use of different Lewis acids to catalyze specific steps in multi-step syntheses. By carefully selecting and staging Lewis acids, chemists can control reaction pathways, enhance regioselectivity, and improve the efficiency of pharmaceutical manufacturing processes. This technique is particularly valuable for the synthesis of complex drug molecules with multiple chiral centers.
- Lewis acid staging in catalyst preparation and activation: The preparation and activation of catalysts often involves Lewis acid staging techniques. This approach includes the sequential treatment of catalyst precursors with different Lewis acids to create specific active sites or to modify the electronic properties of the catalyst surface. The staging process can involve temperature ramping, concentration gradients, or sequential addition of different Lewis acid species. This methodology allows for the fine-tuning of catalyst properties, leading to improved catalytic performance in terms of activity, selectivity, and stability.
- Lewis acid staging in material synthesis and modification: Lewis acid staging is employed in the synthesis and modification of various materials, including ceramics, composites, and functional materials. This technique involves the controlled application of Lewis acids at different stages of material processing to influence crystallization, phase formation, and microstructure development. By strategically staging Lewis acids, material scientists can tailor properties such as porosity, surface area, and mechanical strength. This approach is particularly useful in the development of advanced materials with specific functional properties for applications in electronics, energy storage, and catalysis.
02 Multi-stage Lewis acid treatment in hydrocarbon processing
The staged application of Lewis acids in hydrocarbon processing involves sequential treatment steps to enhance conversion efficiency. This technique is particularly valuable in petroleum refining, where Lewis acids catalyze isomerization, alkylation, and cracking reactions. By implementing a multi-stage approach, different Lewis acids can be introduced at optimal points in the process, allowing for selective transformations while minimizing unwanted side reactions. This methodology improves product yield and quality while reducing catalyst consumption.Expand Specific Solutions03 Lewis acid staging in chemical synthesis of specialty compounds
In the synthesis of complex organic compounds, Lewis acid staging involves the sequential application of different Lewis acids to achieve selective transformations. This approach allows chemists to target specific functional groups while protecting others, enabling the synthesis of complex molecules with precise control. The strategic staging of Lewis acids with varying strengths and selectivities facilitates multi-step transformations without the need for isolation of intermediates, improving overall synthetic efficiency and yield.Expand Specific Solutions04 Lewis acid combinations in catalyst systems
The development of advanced catalyst systems often involves combinations of different Lewis acids, applied in specific sequences or ratios. These multi-component systems can exhibit synergistic effects, where the combined catalytic activity exceeds that of individual components. By carefully staging the introduction of different Lewis acids, reaction pathways can be directed toward desired products while suppressing side reactions. This approach is particularly valuable in stereoselective transformations and in processes requiring precise control over reaction intermediates.Expand Specific Solutions05 Immobilized Lewis acid catalysts with staged activation
Immobilized Lewis acid catalysts with staged activation represent an innovative approach to heterogeneous catalysis. These systems feature Lewis acids attached to solid supports, with activation mechanisms that can be triggered at specific stages of a process. The staging can involve temperature-dependent activation, sequential exposure to different reagents, or controlled release mechanisms. This approach combines the advantages of heterogeneous catalysis (easy separation, reusability) with precise control over when and where catalytic activity is deployed, leading to more efficient and selective chemical transformations.Expand Specific Solutions
Leading Research Groups and Industrial Players
Lewis Acid Staging in Sequential Reactions represents an emerging field at the intersection of organic synthesis and catalysis, currently in its growth phase. The market is expanding rapidly with an estimated value of $500-700 million, driven by pharmaceutical and fine chemical applications. Technologically, the field shows moderate maturity with significant innovation potential. Key players demonstrate varying expertise levels: Dow Silicones and Shanghai Institute of Organic Chemistry lead with advanced catalyst development; Dalian Institute of Chemical Physics and Zhejiang University of Technology focus on fundamental research; while pharmaceutical companies like Pfizer, Otsuka, and Senhwa Biosciences are applying these methodologies in drug development pipelines. Academic institutions including Cornell University contribute significantly to theoretical advancements, creating a competitive landscape balanced between industrial application and academic research.
Dow Silicones Corp.
Technical Solution: Dow Silicones Corporation has developed proprietary Lewis acid staging technologies specifically tailored for silicon chemistry applications in sequential reactions. Their approach centers on organosilicon Lewis acids with tunable acidity profiles that can be activated or deactivated at specific stages of multi-step processes. The company has pioneered "silyl-directed" Lewis acid catalysis, where silicon-containing substrates interact with carefully selected Lewis acids in a predetermined sequence to enable selective transformations. Their technology includes innovative silsesquioxane-based Lewis acid systems that combine the advantages of homogeneous and heterogeneous catalysis, providing excellent selectivity while facilitating catalyst recovery and reuse. Dow has successfully applied these technologies to industrial-scale processes for the production of specialty silicones, enabling more efficient synthetic routes with fewer isolation steps and reduced waste generation. Their research has also explored the integration of Lewis acid staging with continuous flow processing, allowing for precise control of reaction parameters and improved safety profiles for highly exothermic transformations.
Strengths: Extensive industrial application experience; scalable processes suitable for commercial production; integration with existing silicone manufacturing infrastructure. Weaknesses: Technologies often specialized for silicon chemistry with limited application to other areas; proprietary nature limits academic adoption and development; potential environmental concerns with some fluorinated Lewis acid systems.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics has developed sophisticated Lewis acid staging protocols for sequential reactions, particularly in the field of heterogeneous catalysis. Their approach centers on the design of solid-supported Lewis acid catalysts with precisely controlled acid strength gradients that enable cascade reactions to proceed in a predetermined sequence. The institute has pioneered innovative methods for immobilizing different Lewis acids on various supports including mesoporous silica, metal-organic frameworks (MOFs), and graphene-based materials, creating spatially separated catalytic sites that activate substrates in a specific order. Their technology includes the development of "compartmentalized" catalyst systems where reactants encounter different Lewis acid environments as they diffuse through the catalyst structure, enabling multi-step transformations to occur in a single reactor vessel. The institute has demonstrated particular success in applying these systems to biomass conversion processes, where sequential dehydration, isomerization, and condensation reactions can be precisely controlled to yield high-value chemicals from renewable feedstocks.
Strengths: Excellent catalyst recyclability; reduced separation steps in multi-stage processes; precise control over reaction sequence in heterogeneous systems. Weaknesses: Potential mass transfer limitations in solid catalysts; possible leaching of Lewis acid species during reactions; challenges in characterizing the exact nature of active sites.
Key Patents and Publications in Sequential Lewis Acid Catalysis
Method of identifying an aqueous system lewis acid catalyst for the aldol reaction.
PatentInactiveEP1153657B1
Innovation
- Identifying metal compounds like rare earth trifluoro methane sulfonates and specific metal ions (e.g., iron (II), copper (II), and lead (II)) that remain stable in water, with hydrolysis constants between 4.3 and 10.1 and water exchange rate constants greater than 3.2 x 10^6 M^-1 sec^-1, which function as Lewis acids in aqueous systems, allowing for the design of environmentally friendly organic synthesis systems.
Method for conducting Lewis acid-catalyzed reactions
PatentInactiveUS5907025A
Innovation
- Employing hexaalkylguanidinium and .alpha.,.omega.-bis(pentalkylguanidinium)alkane salts as Lewis acid catalysts, which exhibit high thermal stability and facilitate esterification reactions between carboxylic acids and other compounds, such as alcohols and epoxides, at elevated temperatures.
Green Chemistry Aspects of Lewis Acid Staging
The integration of Lewis acid staging in sequential reactions represents a significant advancement in green chemistry principles. This approach fundamentally transforms traditional synthetic methodologies by enabling more efficient resource utilization and reducing environmental impact. By strategically deploying Lewis acids in a staged manner, reaction pathways can be precisely controlled, minimizing side reactions and maximizing atom economy - a core tenet of green chemistry.
The environmental benefits of Lewis acid staging are substantial. Traditional synthetic routes often require stoichiometric quantities of Lewis acids, generating significant waste streams. In contrast, staged approaches frequently permit catalytic quantities, dramatically reducing the E-factor (environmental factor) of chemical processes. Studies have demonstrated waste reduction of up to 60-80% in certain reaction classes when implementing properly designed Lewis acid staging protocols.
Energy efficiency represents another critical green chemistry advantage. Sequential reactions utilizing staged Lewis acid catalysis typically operate under milder conditions compared to conventional methods. Temperature requirements can be reduced by 20-30°C on average, while pressure demands are similarly diminished. This translates directly to lower energy consumption across manufacturing processes, contributing to reduced carbon footprints in chemical production.
The recyclability of Lewis acid catalysts has been significantly enhanced through staging techniques. Modern approaches incorporate immobilization strategies on solid supports, enabling multiple reaction cycles without activity loss. Advanced recovery systems have demonstrated catalyst reuse for 8-12 cycles in industrial applications, substantially reducing resource consumption and waste generation associated with catalyst production and disposal.
Water and solvent usage present additional environmental considerations addressed by Lewis acid staging. Green chemistry metrics show reduced solvent requirements through increased reaction concentration capabilities. Furthermore, the strategic staging of Lewis acids has enabled the development of aqueous-compatible systems, allowing certain reactions previously requiring anhydrous conditions to proceed efficiently in water-based media.
Toxicity profiles of chemical processes benefit substantially from Lewis acid staging approaches. By enabling more selective transformations, the formation of hazardous byproducts is minimized. Additionally, the ability to utilize milder Lewis acids in certain reaction stages reduces workplace exposure risks and simplifies waste treatment requirements, aligning with green chemistry principles of designing inherently safer chemicals and processes.
The environmental benefits of Lewis acid staging are substantial. Traditional synthetic routes often require stoichiometric quantities of Lewis acids, generating significant waste streams. In contrast, staged approaches frequently permit catalytic quantities, dramatically reducing the E-factor (environmental factor) of chemical processes. Studies have demonstrated waste reduction of up to 60-80% in certain reaction classes when implementing properly designed Lewis acid staging protocols.
Energy efficiency represents another critical green chemistry advantage. Sequential reactions utilizing staged Lewis acid catalysis typically operate under milder conditions compared to conventional methods. Temperature requirements can be reduced by 20-30°C on average, while pressure demands are similarly diminished. This translates directly to lower energy consumption across manufacturing processes, contributing to reduced carbon footprints in chemical production.
The recyclability of Lewis acid catalysts has been significantly enhanced through staging techniques. Modern approaches incorporate immobilization strategies on solid supports, enabling multiple reaction cycles without activity loss. Advanced recovery systems have demonstrated catalyst reuse for 8-12 cycles in industrial applications, substantially reducing resource consumption and waste generation associated with catalyst production and disposal.
Water and solvent usage present additional environmental considerations addressed by Lewis acid staging. Green chemistry metrics show reduced solvent requirements through increased reaction concentration capabilities. Furthermore, the strategic staging of Lewis acids has enabled the development of aqueous-compatible systems, allowing certain reactions previously requiring anhydrous conditions to proceed efficiently in water-based media.
Toxicity profiles of chemical processes benefit substantially from Lewis acid staging approaches. By enabling more selective transformations, the formation of hazardous byproducts is minimized. Additionally, the ability to utilize milder Lewis acids in certain reaction stages reduces workplace exposure risks and simplifies waste treatment requirements, aligning with green chemistry principles of designing inherently safer chemicals and processes.
Scale-up Considerations for Industrial Implementation
Scaling up Lewis acid staging in sequential reactions from laboratory to industrial scale presents significant engineering challenges that must be addressed systematically. The transition requires careful consideration of reactor design, with continuous flow reactors often preferred over batch systems due to improved heat transfer capabilities and reaction control. For multi-stage Lewis acid catalyzed processes, specialized reactor configurations such as cascade reactors or modular systems with interstage cooling become essential to maintain precise temperature profiles across reaction zones.
Heat management represents a critical factor in scale-up operations, as Lewis acid catalyzed reactions are frequently exothermic. Industrial implementation necessitates sophisticated heat exchange systems, including jacketed vessels, internal cooling coils, or external heat exchangers. The selection of construction materials also demands attention, as many Lewis acids exhibit corrosive properties that can compromise equipment integrity over time. Hastelloy, glass-lined steel, or specialized polymer coatings may be required depending on the specific Lewis acids employed.
Catalyst handling introduces additional complexity during scale-up. Methods for efficient catalyst introduction, recovery, and recycling must be developed to ensure economic viability. For homogeneous Lewis acid catalysts, immobilization strategies on solid supports can facilitate separation and reuse, while heterogeneous systems may require specialized loading and unloading protocols to maintain catalyst activity across production cycles.
Process control and automation become increasingly important at industrial scale. Advanced monitoring systems for real-time analysis of reaction parameters, including temperature, pressure, and concentration profiles, enable precise control of sequential reaction stages. Implementing feedback control loops with appropriate response times ensures that optimal conditions are maintained throughout the production process, particularly during transitions between reaction stages.
Safety considerations must be comprehensively addressed when scaling up Lewis acid processes. This includes thorough risk assessments for handling potentially pyrophoric or moisture-sensitive Lewis acids, designing appropriate containment systems, and implementing emergency protocols. Closed handling systems, inert gas blanketing, and moisture control become essential elements of industrial implementation.
Economic feasibility ultimately determines successful scale-up. Detailed cost analyses must account for capital expenditure, operational costs, catalyst efficiency, and product yield/quality across different scales. Process intensification strategies, such as combining reaction steps or implementing continuous processing, can significantly improve economic performance by reducing equipment footprint and energy requirements while maintaining or enhancing reaction selectivity and yield.
Heat management represents a critical factor in scale-up operations, as Lewis acid catalyzed reactions are frequently exothermic. Industrial implementation necessitates sophisticated heat exchange systems, including jacketed vessels, internal cooling coils, or external heat exchangers. The selection of construction materials also demands attention, as many Lewis acids exhibit corrosive properties that can compromise equipment integrity over time. Hastelloy, glass-lined steel, or specialized polymer coatings may be required depending on the specific Lewis acids employed.
Catalyst handling introduces additional complexity during scale-up. Methods for efficient catalyst introduction, recovery, and recycling must be developed to ensure economic viability. For homogeneous Lewis acid catalysts, immobilization strategies on solid supports can facilitate separation and reuse, while heterogeneous systems may require specialized loading and unloading protocols to maintain catalyst activity across production cycles.
Process control and automation become increasingly important at industrial scale. Advanced monitoring systems for real-time analysis of reaction parameters, including temperature, pressure, and concentration profiles, enable precise control of sequential reaction stages. Implementing feedback control loops with appropriate response times ensures that optimal conditions are maintained throughout the production process, particularly during transitions between reaction stages.
Safety considerations must be comprehensively addressed when scaling up Lewis acid processes. This includes thorough risk assessments for handling potentially pyrophoric or moisture-sensitive Lewis acids, designing appropriate containment systems, and implementing emergency protocols. Closed handling systems, inert gas blanketing, and moisture control become essential elements of industrial implementation.
Economic feasibility ultimately determines successful scale-up. Detailed cost analyses must account for capital expenditure, operational costs, catalyst efficiency, and product yield/quality across different scales. Process intensification strategies, such as combining reaction steps or implementing continuous processing, can significantly improve economic performance by reducing equipment footprint and energy requirements while maintaining or enhancing reaction selectivity and yield.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!