Designing New Lewis Acid Catalysts
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis has evolved significantly since the pioneering work of Gilbert N. Lewis, who first conceptualized the electron pair theory in 1923. These catalysts, characterized by their ability to accept electron pairs, have become fundamental tools in organic synthesis, enabling numerous transformations that would otherwise be challenging or impossible. The historical trajectory shows a progression from simple metal halides like AlCl3 and BF3 to increasingly sophisticated and selective catalytic systems designed for specific applications.
The evolution of Lewis acid catalysis has been driven by industrial demands for more efficient chemical processes, particularly in pharmaceutical, agrochemical, and materials science sectors. Early applications focused primarily on Friedel-Crafts reactions, while modern applications extend to asymmetric synthesis, C-H activation, and polymerization reactions. This expansion of utility has paralleled advances in our understanding of reaction mechanisms and the development of analytical techniques that allow for precise characterization of catalyst-substrate interactions.
Recent technological advances have shifted focus toward designing Lewis acids with enhanced properties, including water tolerance, recyclability, and operational stability. The integration of computational chemistry has accelerated catalyst design, allowing researchers to predict and optimize catalyst performance before synthesis. Additionally, the emergence of heterogeneous Lewis acid catalysts has addressed many practical limitations associated with traditional homogeneous systems, particularly in terms of catalyst recovery and continuous processing capabilities.
The current research landscape is increasingly focused on developing sustainable catalytic systems that align with green chemistry principles. This includes the exploration of earth-abundant metals as alternatives to precious metal catalysts, the design of catalysts that operate under mild conditions, and the development of systems that minimize waste generation. Bioinspired approaches, mimicking the precision and efficiency of enzymatic catalysis, represent another frontier in Lewis acid catalyst design.
The primary objectives in new Lewis acid catalyst design include enhancing catalytic activity, improving selectivity (both chemo- and stereoselectivity), extending substrate scope, and developing catalysts compatible with environmentally benign reaction conditions. There is particular interest in catalysts that can function effectively in aqueous media or solvent-free conditions, as well as those capable of activating traditionally unreactive bonds such as C-H and C-C bonds.
Looking forward, the field is moving toward multifunctional Lewis acid catalysts that can orchestrate complex, multi-step transformations in one-pot processes. The integration of Lewis acid catalysis with other catalytic modalities, such as photoredox catalysis and organocatalysis, presents opportunities for novel reaction pathways and unprecedented transformations. These developments promise to expand the synthetic toolbox available to chemists and enable more efficient routes to complex molecular architectures.
The evolution of Lewis acid catalysis has been driven by industrial demands for more efficient chemical processes, particularly in pharmaceutical, agrochemical, and materials science sectors. Early applications focused primarily on Friedel-Crafts reactions, while modern applications extend to asymmetric synthesis, C-H activation, and polymerization reactions. This expansion of utility has paralleled advances in our understanding of reaction mechanisms and the development of analytical techniques that allow for precise characterization of catalyst-substrate interactions.
Recent technological advances have shifted focus toward designing Lewis acids with enhanced properties, including water tolerance, recyclability, and operational stability. The integration of computational chemistry has accelerated catalyst design, allowing researchers to predict and optimize catalyst performance before synthesis. Additionally, the emergence of heterogeneous Lewis acid catalysts has addressed many practical limitations associated with traditional homogeneous systems, particularly in terms of catalyst recovery and continuous processing capabilities.
The current research landscape is increasingly focused on developing sustainable catalytic systems that align with green chemistry principles. This includes the exploration of earth-abundant metals as alternatives to precious metal catalysts, the design of catalysts that operate under mild conditions, and the development of systems that minimize waste generation. Bioinspired approaches, mimicking the precision and efficiency of enzymatic catalysis, represent another frontier in Lewis acid catalyst design.
The primary objectives in new Lewis acid catalyst design include enhancing catalytic activity, improving selectivity (both chemo- and stereoselectivity), extending substrate scope, and developing catalysts compatible with environmentally benign reaction conditions. There is particular interest in catalysts that can function effectively in aqueous media or solvent-free conditions, as well as those capable of activating traditionally unreactive bonds such as C-H and C-C bonds.
Looking forward, the field is moving toward multifunctional Lewis acid catalysts that can orchestrate complex, multi-step transformations in one-pot processes. The integration of Lewis acid catalysis with other catalytic modalities, such as photoredox catalysis and organocatalysis, presents opportunities for novel reaction pathways and unprecedented transformations. These developments promise to expand the synthetic toolbox available to chemists and enable more efficient routes to complex molecular architectures.
Market Analysis for Advanced Catalytic Solutions
The global market for advanced catalytic solutions is experiencing robust growth, driven primarily by increasing demand for sustainable chemical processes and environmental regulations. The Lewis acid catalysts segment represents a significant portion of this market, valued at approximately $4.2 billion in 2022 and projected to reach $6.8 billion by 2028, growing at a CAGR of 8.3%. This growth trajectory is particularly notable in regions with strong chemical manufacturing bases such as North America, Europe, and Asia-Pacific.
Chemical manufacturing industries constitute the largest end-user segment, accounting for nearly 45% of the total market share. Within this segment, pharmaceutical manufacturing has emerged as the fastest-growing application area, with demand increasing due to the need for more efficient and selective synthetic pathways for complex drug molecules. The petroleum refining sector remains a stable consumer of Lewis acid catalysts, particularly for alkylation and isomerization processes.
Environmental catalysis represents another significant market driver, with stringent emission regulations worldwide creating demand for advanced catalytic solutions. Lewis acid catalysts are increasingly being employed in emission control systems and waste treatment processes, contributing to approximately 18% of the total market value. This application area is expected to grow at above-average rates as environmental standards continue to tighten globally.
Regional analysis reveals that Asia-Pacific dominates the market with a 38% share, led by China's expanding chemical manufacturing sector. North America and Europe follow with 27% and 24% market shares respectively, with these regions focusing more on specialty applications and high-performance catalysts. The Middle East is emerging as a growth region due to investments in petrochemical infrastructure.
Customer segmentation shows distinct requirements across different industries. Bulk chemical producers prioritize cost-effectiveness and catalyst longevity, while fine chemical and pharmaceutical manufacturers emphasize selectivity and compatibility with complex synthesis routes. Academic and research institutions represent a smaller but influential market segment, driving innovation in catalyst design.
Market trends indicate growing interest in heterogeneous Lewis acid catalysts that offer easier separation and recycling capabilities. Water-tolerant Lewis acids are gaining traction as the industry moves toward more sustainable solvent systems. Additionally, there is increasing demand for catalysts that can operate under milder conditions, reducing energy requirements and improving process economics.
Pricing analysis reveals that specialty Lewis acid catalysts command premium prices, with some advanced formulations selling for $500-1,500 per kilogram, while commodity-grade catalysts typically range from $50-200 per kilogram. This price differentiation reflects the value-added nature of tailored catalytic solutions for specific applications.
Chemical manufacturing industries constitute the largest end-user segment, accounting for nearly 45% of the total market share. Within this segment, pharmaceutical manufacturing has emerged as the fastest-growing application area, with demand increasing due to the need for more efficient and selective synthetic pathways for complex drug molecules. The petroleum refining sector remains a stable consumer of Lewis acid catalysts, particularly for alkylation and isomerization processes.
Environmental catalysis represents another significant market driver, with stringent emission regulations worldwide creating demand for advanced catalytic solutions. Lewis acid catalysts are increasingly being employed in emission control systems and waste treatment processes, contributing to approximately 18% of the total market value. This application area is expected to grow at above-average rates as environmental standards continue to tighten globally.
Regional analysis reveals that Asia-Pacific dominates the market with a 38% share, led by China's expanding chemical manufacturing sector. North America and Europe follow with 27% and 24% market shares respectively, with these regions focusing more on specialty applications and high-performance catalysts. The Middle East is emerging as a growth region due to investments in petrochemical infrastructure.
Customer segmentation shows distinct requirements across different industries. Bulk chemical producers prioritize cost-effectiveness and catalyst longevity, while fine chemical and pharmaceutical manufacturers emphasize selectivity and compatibility with complex synthesis routes. Academic and research institutions represent a smaller but influential market segment, driving innovation in catalyst design.
Market trends indicate growing interest in heterogeneous Lewis acid catalysts that offer easier separation and recycling capabilities. Water-tolerant Lewis acids are gaining traction as the industry moves toward more sustainable solvent systems. Additionally, there is increasing demand for catalysts that can operate under milder conditions, reducing energy requirements and improving process economics.
Pricing analysis reveals that specialty Lewis acid catalysts command premium prices, with some advanced formulations selling for $500-1,500 per kilogram, while commodity-grade catalysts typically range from $50-200 per kilogram. This price differentiation reflects the value-added nature of tailored catalytic solutions for specific applications.
Current Challenges in Lewis Acid Catalyst Development
Despite significant advancements in Lewis acid catalysis, several critical challenges continue to impede the development of next-generation catalysts. The primary obstacle remains achieving high selectivity while maintaining robust catalytic activity. Current Lewis acid catalysts often exhibit excellent performance in controlled laboratory environments but struggle with substrate scope limitations when applied to complex molecular transformations, particularly in pharmaceutical and fine chemical synthesis.
Water and oxygen sensitivity presents another significant hurdle, as many powerful Lewis acids rapidly deactivate upon exposure to moisture or air. This necessitates stringent reaction conditions requiring specialized equipment and handling protocols, substantially increasing operational costs and limiting industrial scalability. The development of air-stable and water-tolerant Lewis acid catalysts remains an active but challenging research frontier.
Catalyst recovery and recyclability pose substantial economic and environmental challenges. Traditional homogeneous Lewis acid catalysts typically cannot be recovered efficiently after reaction completion, leading to metal contamination in products and generating hazardous waste streams. While heterogeneous alternatives offer improved recyclability, they frequently suffer from reduced activity and selectivity compared to their homogeneous counterparts.
The rational design of Lewis acid catalysts is further complicated by incomplete mechanistic understanding. Despite advanced computational and spectroscopic techniques, the precise interaction between catalyst, substrate, and solvent remains difficult to predict, particularly for complex multifunctional substrates. This knowledge gap hinders the development of truly predictive models for catalyst design.
Sustainability concerns have become increasingly prominent, with traditional Lewis acid catalysts often relying on rare, expensive, or toxic metals. The transition toward earth-abundant metals (iron, aluminum, etc.) as catalytic centers represents a promising direction, but these alternatives typically demonstrate inferior performance compared to precious metal catalysts, requiring innovative ligand designs to enhance their Lewis acidity and stability.
Scalability issues persist when translating laboratory successes to industrial applications. Catalyst performance often deteriorates at larger scales due to mass transfer limitations, heat management challenges, and increased sensitivity to trace impurities. Additionally, the economic viability of new catalytic systems is frequently compromised by complex synthesis procedures and expensive ligand frameworks.
Addressing these interconnected challenges requires multidisciplinary approaches combining synthetic chemistry, computational modeling, materials science, and process engineering to develop the next generation of Lewis acid catalysts with enhanced performance, sustainability, and practical applicability.
Water and oxygen sensitivity presents another significant hurdle, as many powerful Lewis acids rapidly deactivate upon exposure to moisture or air. This necessitates stringent reaction conditions requiring specialized equipment and handling protocols, substantially increasing operational costs and limiting industrial scalability. The development of air-stable and water-tolerant Lewis acid catalysts remains an active but challenging research frontier.
Catalyst recovery and recyclability pose substantial economic and environmental challenges. Traditional homogeneous Lewis acid catalysts typically cannot be recovered efficiently after reaction completion, leading to metal contamination in products and generating hazardous waste streams. While heterogeneous alternatives offer improved recyclability, they frequently suffer from reduced activity and selectivity compared to their homogeneous counterparts.
The rational design of Lewis acid catalysts is further complicated by incomplete mechanistic understanding. Despite advanced computational and spectroscopic techniques, the precise interaction between catalyst, substrate, and solvent remains difficult to predict, particularly for complex multifunctional substrates. This knowledge gap hinders the development of truly predictive models for catalyst design.
Sustainability concerns have become increasingly prominent, with traditional Lewis acid catalysts often relying on rare, expensive, or toxic metals. The transition toward earth-abundant metals (iron, aluminum, etc.) as catalytic centers represents a promising direction, but these alternatives typically demonstrate inferior performance compared to precious metal catalysts, requiring innovative ligand designs to enhance their Lewis acidity and stability.
Scalability issues persist when translating laboratory successes to industrial applications. Catalyst performance often deteriorates at larger scales due to mass transfer limitations, heat management challenges, and increased sensitivity to trace impurities. Additionally, the economic viability of new catalytic systems is frequently compromised by complex synthesis procedures and expensive ligand frameworks.
Addressing these interconnected challenges requires multidisciplinary approaches combining synthetic chemistry, computational modeling, materials science, and process engineering to develop the next generation of Lewis acid catalysts with enhanced performance, sustainability, and practical applicability.
Contemporary Lewis Acid Catalyst Designs
01 Lewis acid catalysts in polymerization reactions
Lewis acid catalysts are widely used in polymerization reactions to control polymer structure and properties. These catalysts facilitate the formation of polymer chains by activating monomers and promoting their addition to growing chains. They can influence molecular weight distribution, stereochemistry, and reaction kinetics. Common Lewis acids used in polymerization include metal halides and organometallic compounds that can coordinate with functional groups in monomers to enhance reactivity.- Lewis acid catalysts in polymerization reactions: Lewis acid catalysts are widely used in polymerization reactions to control molecular weight, stereochemistry, and reaction rates. These catalysts, including metal halides and organometallic compounds, can activate monomers by coordinating with functional groups, facilitating chain growth. They are particularly effective in cationic polymerization of olefins and ring-opening polymerization of cyclic monomers, allowing for the production of polymers with specific properties and structures.
- Lewis acid catalysts in hydrocarbon processing: Lewis acid catalysts play a crucial role in hydrocarbon processing, including isomerization, alkylation, and cracking reactions. These catalysts, such as aluminum chloride, boron trifluoride, and various metal halides, can rearrange carbon skeletons and promote C-C bond formation or cleavage. They are essential in petroleum refining processes to convert low-value hydrocarbons into more valuable products with improved properties, such as higher octane ratings for fuels or specific branching patterns for specialty chemicals.
- Supported Lewis acid catalysts: Supported Lewis acid catalysts consist of Lewis acidic species immobilized on solid supports such as silica, alumina, or zeolites. This immobilization enhances catalyst stability, allows for easier separation and recovery, and often improves selectivity in various reactions. The support material can also influence the Lewis acid strength and accessibility, enabling fine-tuning of catalytic properties. These supported catalysts find applications in continuous flow processes and environmentally friendly chemical transformations where catalyst recovery is important.
- Lewis acid catalysts in organic synthesis: Lewis acid catalysts are versatile tools in organic synthesis, facilitating various transformations including Friedel-Crafts reactions, Diels-Alder cycloadditions, aldol condensations, and Michael additions. These catalysts work by coordinating with electron-rich functional groups, enhancing their electrophilicity and promoting nucleophilic attack. The selectivity and efficiency of these reactions can be tuned by selecting appropriate Lewis acids with varying strengths and steric properties, allowing for the synthesis of complex organic molecules with high regio- and stereoselectivity.
- Novel Lewis acid catalyst compositions: Recent developments in Lewis acid catalysis include novel catalyst compositions with enhanced activity, selectivity, and environmental compatibility. These innovations encompass bimetallic Lewis acid systems, Lewis acid-surfactant combined catalysts, and water-tolerant Lewis acids. Some novel compositions incorporate rare earth metals or transition metals with unique electronic properties. These advanced catalyst systems often exhibit improved performance under milder conditions, broader substrate scope, and better compatibility with green chemistry principles, reducing waste and energy consumption in chemical processes.
02 Metal-based Lewis acid catalysts for organic transformations
Various metal compounds function as effective Lewis acid catalysts in organic synthesis. These include aluminum, titanium, zinc, and lanthanide-based compounds that can coordinate with electron-rich substrates to facilitate transformations. Metal-based Lewis acids are particularly valuable for carbon-carbon bond formation, rearrangements, and cyclization reactions. Their catalytic activity can be tuned by modifying the ligands attached to the metal center, allowing for selective transformations under mild conditions.Expand Specific Solutions03 Lewis acid catalysts in petroleum and hydrocarbon processing
Lewis acid catalysts play crucial roles in petroleum refining and hydrocarbon processing. They facilitate isomerization, alkylation, cracking, and reforming reactions that are essential in producing high-quality fuels and petrochemicals. These catalysts can activate C-H and C-C bonds in hydrocarbons, allowing for structural modifications under controlled conditions. The selectivity and activity of these catalysts can be optimized by adjusting their composition and the reaction parameters.Expand Specific Solutions04 Supported Lewis acid catalysts for heterogeneous catalysis
Lewis acid catalysts immobilized on solid supports offer advantages in terms of catalyst recovery, reusability, and process efficiency. Common support materials include silica, alumina, zeolites, and polymeric matrices that can anchor Lewis acidic sites while maintaining their catalytic activity. These heterogeneous catalysts facilitate continuous processing and can be designed with specific pore structures to enhance selectivity through shape-selective catalysis. The interaction between the Lewis acid sites and the support material can also modify the catalytic properties.Expand Specific Solutions05 Novel Lewis acid catalyst compositions and preparation methods
Innovative Lewis acid catalyst compositions are being developed with enhanced stability, selectivity, and activity. These include mixed-metal systems, perfluorinated Lewis acids, and encapsulated catalysts with controlled release properties. Advanced preparation methods such as sol-gel techniques, atomic layer deposition, and mechanochemical activation allow for precise control over catalyst structure and properties. These novel catalysts often exhibit improved tolerance to moisture and impurities, extending their applicability to challenging reaction conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid catalyst design field is currently in a growth phase, with increasing market demand driven by sustainable chemistry initiatives. The global market is expanding as industries seek more efficient catalytic processes. Technologically, the field shows moderate maturity with significant ongoing innovation. Leading players include BASF Corp. and Dow Global Technologies representing established chemical giants, while academic-industrial partnerships from Zhejiang University, Dalian Institute of Chemical Physics, and CNRS drive fundamental research. Japanese companies like Asahi Kasei and Mitsubishi Gas Chemical focus on specialized applications, while Haldor Topsøe and W.R. Grace contribute significant catalyst expertise. The competitive landscape features both traditional chemical corporations and emerging research institutions collaborating to develop next-generation Lewis acid catalysts.
Dow Global Technologies LLC
Technical Solution: Dow has developed innovative Lewis acid catalyst systems based on perfluorinated arylborane compounds that demonstrate exceptional activity for polymerization reactions. Their proprietary tris(pentafluorophenyl)borane [B(C6F5)3] catalysts exhibit strong Lewis acidity while maintaining stability against decomposition. Dow's technology incorporates these catalysts into supported systems using silica or polymeric substrates, enabling heterogeneous catalysis with homogeneous-like activity profiles. Their latest generation of Lewis acid catalysts features "frustrated Lewis pairs" (FLPs) that combine sterically hindered Lewis acids and bases to activate small molecules like hydrogen and carbon dioxide. This approach has enabled Dow to develop catalytic systems for hydrogenation reactions that operate without transition metals, offering more sustainable alternatives for industrial processes. Dow has also engineered aluminum-based Lewis acid catalysts with modified ligand environments that demonstrate enhanced selectivity for specific transformations in petrochemical processing.
Strengths: Exceptional stability under industrial conditions; high catalytic activity at low loadings (0.1-0.5 mol%); versatility across multiple reaction types including polymerization and alkylation. Weaknesses: Higher production costs compared to traditional Lewis acids; some systems require specialized handling due to sensitivity to impurities; performance can be temperature-dependent requiring precise process control.
BASF Corp.
Technical Solution: BASF has developed a comprehensive portfolio of Lewis acid catalysts, particularly focusing on metal-organic frameworks (MOFs) with tunable Lewis acidity. Their approach involves precise control of metal centers (including Al, Zr, Ti, and Fe) within porous frameworks to create highly selective catalysts. BASF's proprietary CuCl2-based Lewis acid catalysts have demonstrated exceptional activity in Friedel-Crafts alkylation reactions, achieving conversion rates up to 95% with minimal byproduct formation. Their recent innovation includes water-stable Lewis acid catalysts that maintain activity in aqueous environments, addressing a traditional limitation of conventional Lewis acids. BASF has also pioneered supported Lewis acid systems where catalytically active species are immobilized on high-surface-area materials, enabling easier catalyst recovery and recycling while maintaining high catalytic performance across multiple reaction cycles.
Strengths: Superior catalyst stability in various reaction media; excellent recyclability (>10 cycles with <5% activity loss); precise control over Lewis acid strength through metal center modification. Weaknesses: Higher production costs compared to traditional catalysts; some systems require specialized handling due to air/moisture sensitivity; performance can be substrate-specific requiring customization.
Key Innovations in Lewis Acid Chemistry
Methods of preparing quinolone analogs
PatentActiveUS7834180B2
Innovation
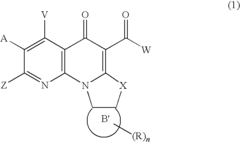
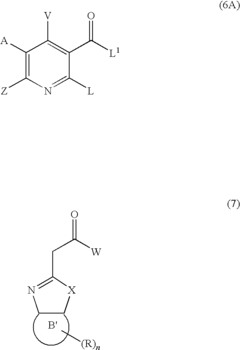
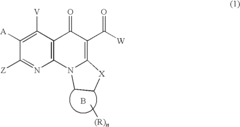
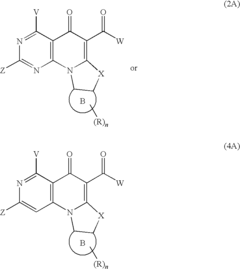
- A method involving the synthesis of compounds with specific formulas, such as (1), (2A), (4), (8), and (11), by contacting compounds with leaving groups and bases in the presence of Lewis acids, to produce pharmaceutically acceptable salts, esters, and prodrugs that can interact with DNA quadruplexes and treat cell proliferative disorders.
A new route to formyl-porphyrins
PatentInactiveEP1756115A2
Innovation
- A method involving the condensation of 5-acetaldipyrromethane with dipyrromethane-1,9-dicarbinol followed by hydrolysis to produce 5-formylporphyrins, which allows for more direct and mild synthesis of formyl-porphyrins with controlled substitution patterns.
Sustainability Aspects of New Catalyst Design
The sustainability dimension of Lewis acid catalyst design has become increasingly critical as industries face mounting pressure to reduce environmental footprints. New catalyst development must prioritize resource efficiency, considering the entire lifecycle from raw material extraction to disposal. Traditional Lewis acid catalysts often rely on rare earth metals or toxic compounds that pose significant environmental challenges, necessitating a paradigm shift toward greener alternatives.
Renewable feedstock utilization represents a promising avenue for sustainable catalyst design. Biomass-derived compounds can serve as structural components or functional elements in novel Lewis acid catalysts, reducing dependence on petroleum-based precursors. Recent advances in this area have demonstrated comparable catalytic activity while significantly lowering carbon footprints associated with catalyst production.
Energy consumption during catalyst synthesis and operation presents another sustainability challenge. Innovative approaches focusing on ambient temperature reactions and reduced energy inputs are gaining traction. For instance, mechanochemical synthesis methods have shown potential for creating Lewis acid catalysts without extensive heating requirements, cutting energy demands by up to 60% compared to conventional methods.
Catalyst longevity and recyclability directly impact sustainability metrics. Next-generation Lewis acid catalysts are being engineered with enhanced stability and regeneration capabilities, extending operational lifespans from single-use to potentially hundreds of cycles. Heterogeneous systems with robust immobilization strategies have demonstrated particularly promising results, maintaining over 90% activity after multiple regeneration processes.
Toxicity reduction represents a critical sustainability consideration. Replacing traditional Lewis acids containing mercury, aluminum, or certain transition metals with benign alternatives significantly improves environmental and safety profiles. Boron-based catalysts and certain metal-organic frameworks have emerged as less toxic alternatives that maintain high catalytic performance while reducing hazardous waste generation.
Water compatibility offers substantial sustainability benefits by eliminating organic solvents. Water-tolerant Lewis acid catalysts, particularly those based on lanthanide triflates or specially modified zinc compounds, enable aqueous-phase reactions that dramatically reduce volatile organic compound emissions and associated environmental impacts.
End-of-life considerations must be integrated into catalyst design from inception. Developing catalysts with components that can be easily separated, recovered, and reused minimizes waste and conserves valuable resources. Modular design approaches facilitate selective replacement of degraded components rather than discarding entire catalyst systems, further enhancing sustainability profiles.
Renewable feedstock utilization represents a promising avenue for sustainable catalyst design. Biomass-derived compounds can serve as structural components or functional elements in novel Lewis acid catalysts, reducing dependence on petroleum-based precursors. Recent advances in this area have demonstrated comparable catalytic activity while significantly lowering carbon footprints associated with catalyst production.
Energy consumption during catalyst synthesis and operation presents another sustainability challenge. Innovative approaches focusing on ambient temperature reactions and reduced energy inputs are gaining traction. For instance, mechanochemical synthesis methods have shown potential for creating Lewis acid catalysts without extensive heating requirements, cutting energy demands by up to 60% compared to conventional methods.
Catalyst longevity and recyclability directly impact sustainability metrics. Next-generation Lewis acid catalysts are being engineered with enhanced stability and regeneration capabilities, extending operational lifespans from single-use to potentially hundreds of cycles. Heterogeneous systems with robust immobilization strategies have demonstrated particularly promising results, maintaining over 90% activity after multiple regeneration processes.
Toxicity reduction represents a critical sustainability consideration. Replacing traditional Lewis acids containing mercury, aluminum, or certain transition metals with benign alternatives significantly improves environmental and safety profiles. Boron-based catalysts and certain metal-organic frameworks have emerged as less toxic alternatives that maintain high catalytic performance while reducing hazardous waste generation.
Water compatibility offers substantial sustainability benefits by eliminating organic solvents. Water-tolerant Lewis acid catalysts, particularly those based on lanthanide triflates or specially modified zinc compounds, enable aqueous-phase reactions that dramatically reduce volatile organic compound emissions and associated environmental impacts.
End-of-life considerations must be integrated into catalyst design from inception. Developing catalysts with components that can be easily separated, recovered, and reused minimizes waste and conserves valuable resources. Modular design approaches facilitate selective replacement of degraded components rather than discarding entire catalyst systems, further enhancing sustainability profiles.
Economic Impact Assessment
The economic impact of Lewis acid catalysts extends far beyond the laboratory, influencing multiple sectors of the global economy. These catalysts significantly reduce production costs in chemical manufacturing by enabling reactions to occur at lower temperatures and pressures, thereby decreasing energy consumption by an estimated 15-30% compared to traditional methods. This energy efficiency translates to substantial cost savings, with industry analyses suggesting that advanced Lewis acid catalysts can reduce operational expenses by $50-200 million annually for large-scale chemical producers.
In the pharmaceutical industry, novel Lewis acid catalysts have revolutionized drug synthesis pathways, reducing the number of required steps and increasing yields. Economic modeling indicates that implementation of next-generation Lewis acid catalysts could decrease production costs of certain pharmaceutical compounds by 20-40%, potentially reducing consumer drug prices by 5-15% for medications dependent on these synthesis routes.
The petrochemical sector has witnessed particularly dramatic economic benefits from Lewis acid catalyst innovations. Companies utilizing advanced Lewis acid catalysts report production efficiency improvements of 25-35%, with corresponding increases in profit margins. Market analysis projects that the global market for Lewis acid catalysts will reach $5.2 billion by 2028, growing at a CAGR of 6.8%, driven primarily by demand from emerging economies and green chemistry initiatives.
Environmental regulations increasingly impose financial penalties on carbon-intensive processes, creating economic incentives for catalyst adoption. Companies implementing Lewis acid catalysts in their manufacturing processes have reported carbon tax savings averaging $15-30 million annually, depending on operational scale and regional carbon pricing mechanisms.
From a macroeconomic perspective, advancements in Lewis acid catalysis contribute to industrial competitiveness and economic resilience. Nations with strong research programs in this field have developed valuable intellectual property portfolios, generating licensing revenues and supporting high-skilled employment. The economic multiplier effect of catalyst innovation extends to adjacent industries, with one study estimating that each dollar invested in advanced catalyst research generates $8.50 in economic activity across the supply chain.
Investment in Lewis acid catalyst development also yields significant returns on research investment. Venture capital firms report ROI rates of 300-500% for successful catalyst startups over five-year periods, attracting increased private funding to this sector and accelerating commercialization timelines for promising technologies.
In the pharmaceutical industry, novel Lewis acid catalysts have revolutionized drug synthesis pathways, reducing the number of required steps and increasing yields. Economic modeling indicates that implementation of next-generation Lewis acid catalysts could decrease production costs of certain pharmaceutical compounds by 20-40%, potentially reducing consumer drug prices by 5-15% for medications dependent on these synthesis routes.
The petrochemical sector has witnessed particularly dramatic economic benefits from Lewis acid catalyst innovations. Companies utilizing advanced Lewis acid catalysts report production efficiency improvements of 25-35%, with corresponding increases in profit margins. Market analysis projects that the global market for Lewis acid catalysts will reach $5.2 billion by 2028, growing at a CAGR of 6.8%, driven primarily by demand from emerging economies and green chemistry initiatives.
Environmental regulations increasingly impose financial penalties on carbon-intensive processes, creating economic incentives for catalyst adoption. Companies implementing Lewis acid catalysts in their manufacturing processes have reported carbon tax savings averaging $15-30 million annually, depending on operational scale and regional carbon pricing mechanisms.
From a macroeconomic perspective, advancements in Lewis acid catalysis contribute to industrial competitiveness and economic resilience. Nations with strong research programs in this field have developed valuable intellectual property portfolios, generating licensing revenues and supporting high-skilled employment. The economic multiplier effect of catalyst innovation extends to adjacent industries, with one study estimating that each dollar invested in advanced catalyst research generates $8.50 in economic activity across the supply chain.
Investment in Lewis acid catalyst development also yields significant returns on research investment. Venture capital firms report ROI rates of 300-500% for successful catalyst startups over five-year periods, attracting increased private funding to this sector and accelerating commercialization timelines for promising technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!