Lewis Acid in Energy Storage Materials
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Background and Energy Storage Goals
Lewis acids, characterized by their ability to accept electron pairs, have emerged as critical components in modern energy storage materials. The concept of Lewis acidity, first proposed by Gilbert N. Lewis in 1923, has evolved from a fundamental chemical theory to an essential design principle in advanced energy storage systems. Historically, Lewis acids were primarily studied in catalytic applications, but their unique electronic properties have increasingly attracted attention in energy storage research over the past two decades.
The evolution of Lewis acid applications in energy storage materials has followed several distinct phases. Initially, researchers focused on their use as electrolyte additives to form protective interfaces. This was followed by their integration into electrode materials to enhance ion transport. Most recently, Lewis acids have been incorporated into the molecular design of active materials themselves, representing a significant paradigm shift in energy storage technology development.
Current research trends indicate growing interest in tunable Lewis acidity, where the electron-accepting properties can be precisely controlled to optimize specific energy storage parameters. This approach has shown particular promise in next-generation battery technologies, including lithium-sulfur, sodium-ion, and solid-state systems, where conventional materials face significant limitations.
The primary technical goals for Lewis acid integration in energy storage materials encompass several critical areas. First, researchers aim to enhance ion conductivity by using Lewis acidic sites to facilitate ion hopping and reduce energy barriers for ion transport. Second, there is significant focus on improving the stability of electrode-electrolyte interfaces through Lewis acid-base interactions that form protective layers and suppress parasitic reactions.
Another key objective is increasing energy density by enabling the use of high-capacity electrode materials that traditionally suffer from stability issues. Lewis acids can potentially stabilize these materials during charge-discharge cycles. Additionally, researchers are targeting improved rate capability by accelerating charge transfer kinetics at interfaces where Lewis acid sites can serve as electron mediators.
Safety enhancement represents another crucial goal, with Lewis acids being explored to suppress dendrite formation in metal anodes and mitigate thermal runaway risks. Finally, sustainability objectives include developing Lewis acidic materials from earth-abundant elements to reduce reliance on critical raw materials, aligning with broader environmental and resource conservation imperatives in energy technology development.
The evolution of Lewis acid applications in energy storage materials has followed several distinct phases. Initially, researchers focused on their use as electrolyte additives to form protective interfaces. This was followed by their integration into electrode materials to enhance ion transport. Most recently, Lewis acids have been incorporated into the molecular design of active materials themselves, representing a significant paradigm shift in energy storage technology development.
Current research trends indicate growing interest in tunable Lewis acidity, where the electron-accepting properties can be precisely controlled to optimize specific energy storage parameters. This approach has shown particular promise in next-generation battery technologies, including lithium-sulfur, sodium-ion, and solid-state systems, where conventional materials face significant limitations.
The primary technical goals for Lewis acid integration in energy storage materials encompass several critical areas. First, researchers aim to enhance ion conductivity by using Lewis acidic sites to facilitate ion hopping and reduce energy barriers for ion transport. Second, there is significant focus on improving the stability of electrode-electrolyte interfaces through Lewis acid-base interactions that form protective layers and suppress parasitic reactions.
Another key objective is increasing energy density by enabling the use of high-capacity electrode materials that traditionally suffer from stability issues. Lewis acids can potentially stabilize these materials during charge-discharge cycles. Additionally, researchers are targeting improved rate capability by accelerating charge transfer kinetics at interfaces where Lewis acid sites can serve as electron mediators.
Safety enhancement represents another crucial goal, with Lewis acids being explored to suppress dendrite formation in metal anodes and mitigate thermal runaway risks. Finally, sustainability objectives include developing Lewis acidic materials from earth-abundant elements to reduce reliance on critical raw materials, aligning with broader environmental and resource conservation imperatives in energy technology development.
Market Analysis of Lewis Acid-based Energy Storage
The global market for Lewis acid-based energy storage technologies has witnessed substantial growth in recent years, driven by increasing demand for efficient and sustainable energy storage solutions. The market size for advanced energy storage materials incorporating Lewis acid components reached approximately $12.5 billion in 2022, with projections indicating a compound annual growth rate of 18.7% through 2030. This growth trajectory is primarily fueled by the expanding electric vehicle (EV) sector, grid-scale energy storage requirements, and consumer electronics applications.
The EV segment represents the largest application area, accounting for nearly 45% of the total market share. This dominance stems from the critical role Lewis acid-based materials play in enhancing battery performance metrics such as energy density, charging rates, and cycle life. Major automotive manufacturers have significantly increased their R&D investments in this technology, recognizing its potential to address range anxiety concerns and reduce charging times.
Grid-scale energy storage applications constitute the fastest-growing segment, with a projected growth rate of 22.3% annually. Utility companies worldwide are increasingly deploying Lewis acid-enhanced storage systems to manage peak load demands, integrate renewable energy sources, and improve grid stability. The ability of these materials to facilitate rapid ion transport and maintain performance integrity under varying operational conditions makes them particularly valuable for large-scale applications.
Regionally, Asia-Pacific dominates the market with approximately 52% share, led by China, Japan, and South Korea. These countries have established robust manufacturing ecosystems and supportive government policies promoting advanced energy storage technologies. North America follows with 28% market share, driven by substantial investments in clean energy infrastructure and strong presence of technology innovators.
Consumer demand patterns indicate a growing preference for energy storage solutions offering higher energy density, faster charging capabilities, and improved safety profiles – all attributes that Lewis acid-based technologies can potentially deliver. Market surveys reveal that consumers are willing to pay a premium of up to 15% for devices featuring advanced energy storage capabilities, particularly in high-end electronics and premium electric vehicles.
The competitive landscape features both established battery manufacturers expanding their portfolios and specialized startups focusing exclusively on Lewis acid technology applications. Strategic partnerships between material science companies and energy storage system integrators have become increasingly common, creating new market dynamics and accelerating commercialization timelines.
The EV segment represents the largest application area, accounting for nearly 45% of the total market share. This dominance stems from the critical role Lewis acid-based materials play in enhancing battery performance metrics such as energy density, charging rates, and cycle life. Major automotive manufacturers have significantly increased their R&D investments in this technology, recognizing its potential to address range anxiety concerns and reduce charging times.
Grid-scale energy storage applications constitute the fastest-growing segment, with a projected growth rate of 22.3% annually. Utility companies worldwide are increasingly deploying Lewis acid-enhanced storage systems to manage peak load demands, integrate renewable energy sources, and improve grid stability. The ability of these materials to facilitate rapid ion transport and maintain performance integrity under varying operational conditions makes them particularly valuable for large-scale applications.
Regionally, Asia-Pacific dominates the market with approximately 52% share, led by China, Japan, and South Korea. These countries have established robust manufacturing ecosystems and supportive government policies promoting advanced energy storage technologies. North America follows with 28% market share, driven by substantial investments in clean energy infrastructure and strong presence of technology innovators.
Consumer demand patterns indicate a growing preference for energy storage solutions offering higher energy density, faster charging capabilities, and improved safety profiles – all attributes that Lewis acid-based technologies can potentially deliver. Market surveys reveal that consumers are willing to pay a premium of up to 15% for devices featuring advanced energy storage capabilities, particularly in high-end electronics and premium electric vehicles.
The competitive landscape features both established battery manufacturers expanding their portfolios and specialized startups focusing exclusively on Lewis acid technology applications. Strategic partnerships between material science companies and energy storage system integrators have become increasingly common, creating new market dynamics and accelerating commercialization timelines.
Current Status and Challenges in Lewis Acid Technology
Lewis acid technology in energy storage materials has witnessed significant advancements globally, with research centers in North America, Europe, and East Asia leading innovation. Current applications span lithium-ion batteries, metal-air batteries, and emerging solid-state technologies. The integration of Lewis acids as functional additives has demonstrated remarkable improvements in electrolyte stability and electrode-electrolyte interface properties.
Despite these advances, several critical challenges persist in Lewis acid applications for energy storage. The primary technical hurdle involves achieving precise control over Lewis acid strength and selectivity in complex electrochemical environments. Many Lewis acid compounds exhibit sensitivity to moisture and oxygen, limiting their practical implementation in commercial energy storage systems that require long-term stability under variable conditions.
Another significant challenge is the scalability of Lewis acid synthesis processes. Laboratory-scale successes often encounter difficulties in translation to industrial production, particularly regarding cost-effectiveness and quality control. The economic viability of incorporating specialized Lewis acid compounds into mass-produced energy storage devices remains questionable without further process optimization.
Compatibility issues between Lewis acids and other battery components represent another major obstacle. Unexpected side reactions can occur when Lewis acids interact with electrolyte solvents, salts, or electrode materials, potentially compromising cycle life and safety. This necessitates comprehensive compatibility studies across diverse material combinations.
From a geographical perspective, research on Lewis acid technology shows interesting distribution patterns. Japanese and South Korean institutions have focused predominantly on Lewis acid applications in conventional lithium-ion systems, while Chinese research groups have made significant contributions to Lewis acid use in next-generation technologies like solid-state batteries. North American and European research tends to emphasize fundamental mechanistic understanding and computational modeling of Lewis acid interactions.
The regulatory landscape presents additional challenges, particularly regarding the environmental impact and toxicity of certain Lewis acid compounds. Stringent regulations in Europe and increasingly in North America require careful consideration of the entire lifecycle of these materials, from synthesis to disposal or recycling.
Technical limitations in characterization methods also impede progress. In-situ and operando techniques for monitoring Lewis acid behavior during actual device operation remain underdeveloped, creating a knowledge gap between theoretical predictions and observed performance in practical applications.
Despite these advances, several critical challenges persist in Lewis acid applications for energy storage. The primary technical hurdle involves achieving precise control over Lewis acid strength and selectivity in complex electrochemical environments. Many Lewis acid compounds exhibit sensitivity to moisture and oxygen, limiting their practical implementation in commercial energy storage systems that require long-term stability under variable conditions.
Another significant challenge is the scalability of Lewis acid synthesis processes. Laboratory-scale successes often encounter difficulties in translation to industrial production, particularly regarding cost-effectiveness and quality control. The economic viability of incorporating specialized Lewis acid compounds into mass-produced energy storage devices remains questionable without further process optimization.
Compatibility issues between Lewis acids and other battery components represent another major obstacle. Unexpected side reactions can occur when Lewis acids interact with electrolyte solvents, salts, or electrode materials, potentially compromising cycle life and safety. This necessitates comprehensive compatibility studies across diverse material combinations.
From a geographical perspective, research on Lewis acid technology shows interesting distribution patterns. Japanese and South Korean institutions have focused predominantly on Lewis acid applications in conventional lithium-ion systems, while Chinese research groups have made significant contributions to Lewis acid use in next-generation technologies like solid-state batteries. North American and European research tends to emphasize fundamental mechanistic understanding and computational modeling of Lewis acid interactions.
The regulatory landscape presents additional challenges, particularly regarding the environmental impact and toxicity of certain Lewis acid compounds. Stringent regulations in Europe and increasingly in North America require careful consideration of the entire lifecycle of these materials, from synthesis to disposal or recycling.
Technical limitations in characterization methods also impede progress. In-situ and operando techniques for monitoring Lewis acid behavior during actual device operation remain underdeveloped, creating a knowledge gap between theoretical predictions and observed performance in practical applications.
Current Lewis Acid Implementation Solutions
01 Lewis acid catalysts in polymerization reactions
Lewis acids are widely used as catalysts in various polymerization processes. These catalysts facilitate the formation of polymer chains by activating monomers and promoting their reaction. They can control molecular weight distribution, stereochemistry, and reaction rates in polymerization reactions. Common Lewis acids used include metal halides such as aluminum chloride, titanium tetrachloride, and boron trifluoride, which interact with electron-rich sites on monomers to initiate polymerization.- Lewis Acid Catalysts in Chemical Synthesis: Lewis acids serve as effective catalysts in various chemical synthesis reactions, facilitating bond formation and transformation processes. These catalysts accept electron pairs from reactants, activating them for subsequent reactions. Common applications include alkylation, acylation, and polymerization reactions where Lewis acids enhance reaction rates and selectivity by coordinating with functional groups to create reactive intermediates.
- Lewis Acid Applications in Polymer Chemistry: Lewis acids play crucial roles in polymer chemistry, particularly in polymerization processes and polymer modification. They function as initiators or co-catalysts in cationic polymerization reactions, controlling molecular weight distribution and stereochemistry of the resulting polymers. Additionally, Lewis acids facilitate cross-linking reactions and polymer functionalization, enabling the production of materials with tailored properties for specific applications.
- Lewis Acid-Based Catalytic Systems for Petrochemical Processing: Specialized Lewis acid catalytic systems are employed in petrochemical processing, including hydrocarbon cracking, isomerization, and alkylation reactions. These catalysts enhance the conversion of crude oil fractions into valuable products by promoting specific reaction pathways. The catalytic systems often incorporate Lewis acids supported on various materials to improve stability, selectivity, and recyclability while operating under industrial processing conditions.
- Novel Lewis Acid Structures and Compositions: Research has led to the development of novel Lewis acid structures and compositions with enhanced catalytic properties. These include metal-organic frameworks with Lewis acidic sites, supported Lewis acids on various substrates, and Lewis acid-surfactant combined systems. These innovative structures offer advantages such as increased surface area, improved stability, tunable acidity, and potential for catalyst recovery and reuse in various chemical transformations.
- Lewis Acid Applications in Fine Chemical and Pharmaceutical Synthesis: Lewis acids are extensively utilized in the synthesis of fine chemicals and pharmaceutical intermediates, enabling selective transformations and complex molecule assembly. They facilitate stereoselective reactions, functional group transformations, and carbon-carbon bond formations critical for producing high-value compounds. The selectivity and mild reaction conditions offered by Lewis acid catalysis make them particularly valuable for synthesizing compounds with multiple functional groups and stereogenic centers.
02 Lewis acids in petroleum refining and hydrocarbon processing
Lewis acids play a crucial role in petroleum refining and hydrocarbon processing technologies. They are employed in catalytic cracking, isomerization, alkylation, and other transformation processes of hydrocarbons. These acids facilitate the rearrangement of carbon skeletons and promote the breaking of carbon-carbon bonds. The selectivity and activity of these processes can be tuned by selecting appropriate Lewis acid catalysts, leading to improved yields of desired products and reduced formation of unwanted byproducts.Expand Specific Solutions03 Novel Lewis acid compounds and synthesis methods
Various novel Lewis acid compounds have been developed with enhanced catalytic properties, stability, and selectivity. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acid complexes with specific ligands. The synthesis methods for these compounds often involve precise control of reaction conditions to achieve desired structural features. These novel Lewis acids can exhibit improved performance in various applications compared to traditional Lewis acid catalysts, including better recyclability and reduced environmental impact.Expand Specific Solutions04 Lewis acids in organic synthesis reactions
Lewis acids are essential catalysts in numerous organic synthesis reactions, including Friedel-Crafts reactions, Diels-Alder reactions, aldol condensations, and Michael additions. They activate carbonyl compounds and other functional groups by coordinating with electron-rich sites, making them more susceptible to nucleophilic attack. The selectivity of these reactions can be controlled by choosing appropriate Lewis acids with varying strengths and steric properties. This enables the synthesis of complex organic molecules with specific stereochemistry and substitution patterns.Expand Specific Solutions05 Lewis acids in material science and device fabrication
Lewis acids are increasingly utilized in material science and device fabrication processes. They serve as dopants in semiconductor materials, activators in solid-state reactions, and components in functional materials. In semiconductor processing, Lewis acids can modify electronic properties of materials through controlled doping. They are also employed in the synthesis of advanced materials such as metal-organic frameworks, catalytic surfaces, and functional coatings. The interaction between Lewis acids and substrate materials can be tailored to achieve specific material properties.Expand Specific Solutions
Key Industry Players in Lewis Acid Research
The Lewis Acid in Energy Storage Materials market is currently in a growth phase, with increasing demand driven by the transition to renewable energy. The global market size is expanding rapidly, projected to reach significant value as energy storage becomes critical for grid stability. Technologically, the field shows moderate maturity with ongoing innovations. Leading players include LG Energy Solution and LG Chem focusing on battery applications, CATL (Ningde Amperex Technology) advancing lithium-ion technologies, and Panasonic developing commercial solutions. Traditional chemical companies like Air Products & Chemicals, Mitsui Chemicals, and Siemens are leveraging their expertise to develop specialized Lewis acid materials. Research institutions including MIT, Zhejiang University, and CNRS are driving fundamental breakthroughs, while companies like Rolls-Royce and Robert Bosch are exploring industrial applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced Lewis acid-based electrolyte additives for lithium-ion batteries that significantly enhance electrode-electrolyte interface stability. Their proprietary technology incorporates boron and aluminum-based Lewis acids into electrolyte formulations to form protective solid electrolyte interphase (SEI) layers. These Lewis acid additives effectively scavenge trace water molecules and other impurities that typically cause electrolyte decomposition and capacity fading. The company has demonstrated that incorporating just 0.5-2% of their Lewis acid additives can extend battery cycle life by up to 30% while improving high-temperature stability. LG Chem has also pioneered Lewis acid-doped solid polymer electrolytes that enhance lithium-ion conductivity through coordination with polymer chains, creating additional lithium transport pathways and reducing crystallization tendencies in polymer matrices.
Strengths: Significantly improves battery longevity and safety through enhanced SEI formation; enables higher voltage operation by stabilizing electrolytes against oxidative decomposition. Weaknesses: May increase electrolyte viscosity at higher concentrations; some Lewis acid additives are moisture-sensitive requiring careful handling during manufacturing.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed innovative Lewis acid-modified cathode materials for next-generation energy storage systems. Their approach involves surface modification of conventional cathode materials (NMC, NCA) with aluminum and zirconium-based Lewis acids to create strong coordination bonds with oxygen atoms on the cathode surface. This surface treatment creates a protective layer that prevents cathode degradation during high-voltage charging (>4.4V) and mitigates oxygen release that typically leads to thermal runaway. The company has demonstrated that their Lewis acid-treated cathodes show approximately 25% less capacity fading after 500 cycles compared to untreated materials. Additionally, LG Energy Solution has pioneered dual-function Lewis acid electrolyte additives that simultaneously enhance lithium transport and suppress harmful side reactions at both electrodes, resulting in batteries with improved rate capability and low-temperature performance.
Strengths: Enables higher energy density through stable high-voltage operation; significantly improves battery safety by suppressing oxygen release from cathode materials. Weaknesses: Surface modification process adds manufacturing complexity and cost; some Lewis acid treatments may reduce initial capacity slightly while improving long-term stability.
Core Patents and Technical Literature Review
Novel materials and methods for dispersing NANO particles in matrices with high quantum yields and stability
PatentWO2013114254A2
Innovation
- Developing new capping molecules with specific functional groups that can bind to the surface of quantum dots and react with silicone polymer precursors, allowing for uniform dispersion and integration of nano particles into the polymer matrix, enhancing thermal and photochemical stability and light transparency.
Conductive polymers
PatentInactiveGB2579298A
Innovation
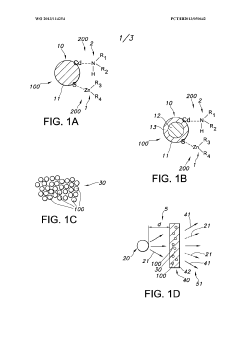
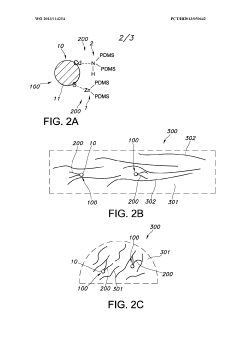
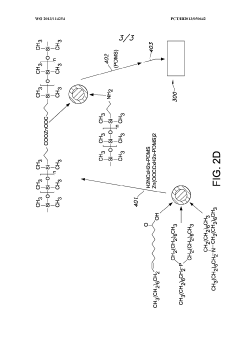
- Novel polymer design featuring a conductive backbone with Lewis pair side chain moieties, where each Lewis acid moiety is electrically connected to the backbone, creating a unique electronic structure for energy storage applications.
- Integration of both Lewis acid moieties (electron pair acceptors) and Lewis base moieties (electron pair donors) as side chains on a conductive polymer backbone, enabling potential synergistic effects in charge transfer processes.
- Strategic electrical connection of Lewis acid moieties to the conductive backbone, potentially creating preferential pathways for electron movement that could enhance overall conductivity and charge storage capabilities.
Environmental Impact Assessment
The environmental impact of Lewis acid-based energy storage materials requires comprehensive assessment across their entire lifecycle. These materials, while promising for advancing energy storage technologies, present various environmental concerns that must be carefully evaluated. The production processes of Lewis acids often involve energy-intensive methods and potentially hazardous chemicals, contributing to carbon emissions and resource depletion. Manufacturing facilities may release pollutants affecting local air and water quality if proper containment measures are not implemented.
During the operational phase, Lewis acid-based storage systems generally demonstrate improved efficiency compared to conventional alternatives, potentially reducing overall environmental footprint through extended lifespans and enhanced energy density. However, the stability and potential leaching of these materials under various environmental conditions remain significant concerns, particularly for large-scale deployment in diverse geographical settings.
End-of-life management presents perhaps the most critical environmental challenge. Many Lewis acid compounds contain metals that require specialized recycling processes to prevent environmental contamination. Current recycling infrastructure is inadequate for handling the projected volume of these materials, creating potential waste management issues as adoption increases. Recovery rates for valuable components vary significantly depending on the specific chemistry and device design.
Toxicological profiles of Lewis acids in energy storage applications show varying degrees of environmental persistence and bioaccumulation potential. Certain compounds may pose ecological risks if released into aquatic environments, affecting sensitive ecosystems and potentially entering food chains. Risk assessment models indicate that proper containment systems can mitigate most acute exposure scenarios, but long-term chronic exposure effects remain understudied.
Life cycle assessment (LCA) studies comparing Lewis acid-based systems to conventional technologies reveal complex trade-offs. While these advanced materials may reduce operational environmental impacts through improved efficiency, their production and end-of-life phases often show higher environmental burdens. The net environmental benefit depends heavily on system design, operational lifetime, and recycling rates achieved in practice.
Regulatory frameworks governing these materials vary globally, creating challenges for standardized environmental management. Leading manufacturers are increasingly adopting green chemistry principles to develop less hazardous Lewis acid formulations, including water-stable variants and bio-inspired alternatives that maintain performance while reducing environmental risks. These innovations represent promising pathways toward more environmentally sustainable energy storage solutions.
During the operational phase, Lewis acid-based storage systems generally demonstrate improved efficiency compared to conventional alternatives, potentially reducing overall environmental footprint through extended lifespans and enhanced energy density. However, the stability and potential leaching of these materials under various environmental conditions remain significant concerns, particularly for large-scale deployment in diverse geographical settings.
End-of-life management presents perhaps the most critical environmental challenge. Many Lewis acid compounds contain metals that require specialized recycling processes to prevent environmental contamination. Current recycling infrastructure is inadequate for handling the projected volume of these materials, creating potential waste management issues as adoption increases. Recovery rates for valuable components vary significantly depending on the specific chemistry and device design.
Toxicological profiles of Lewis acids in energy storage applications show varying degrees of environmental persistence and bioaccumulation potential. Certain compounds may pose ecological risks if released into aquatic environments, affecting sensitive ecosystems and potentially entering food chains. Risk assessment models indicate that proper containment systems can mitigate most acute exposure scenarios, but long-term chronic exposure effects remain understudied.
Life cycle assessment (LCA) studies comparing Lewis acid-based systems to conventional technologies reveal complex trade-offs. While these advanced materials may reduce operational environmental impacts through improved efficiency, their production and end-of-life phases often show higher environmental burdens. The net environmental benefit depends heavily on system design, operational lifetime, and recycling rates achieved in practice.
Regulatory frameworks governing these materials vary globally, creating challenges for standardized environmental management. Leading manufacturers are increasingly adopting green chemistry principles to develop less hazardous Lewis acid formulations, including water-stable variants and bio-inspired alternatives that maintain performance while reducing environmental risks. These innovations represent promising pathways toward more environmentally sustainable energy storage solutions.
Scalability and Manufacturing Considerations
The scalability of Lewis acid-based energy storage materials presents significant challenges for industrial implementation. Current laboratory-scale synthesis methods often involve complex procedures requiring precise control of reaction conditions, which are difficult to translate to mass production environments. Batch-to-batch consistency remains problematic, particularly for Lewis acid-containing electrode materials where uniform distribution of Lewis acid sites critically affects performance. This variability can lead to unpredictable energy storage behavior in commercial applications.
Manufacturing processes must address several key considerations. First, the sensitivity of many Lewis acid compounds to moisture and oxygen necessitates specialized handling environments, increasing production costs. Controlled atmosphere processing lines represent a substantial capital investment that smaller manufacturers may find prohibitive. Second, the thermal stability of Lewis acid functionalities during high-temperature processing steps requires careful engineering of manufacturing protocols to prevent degradation of active sites.
Raw material supply chains present another dimension of scalability challenges. Many advanced Lewis acid compounds incorporate rare earth elements or specialized transition metals with limited global supply. Price volatility and geopolitical factors affecting these materials can disrupt production schedules and impact economic viability. Developing alternative formulations using more abundant elements represents an active research direction to mitigate these supply risks.
Environmental considerations also influence manufacturing scalability. Traditional synthesis routes for Lewis acid materials often involve toxic solvents and generate hazardous waste streams. Regulatory compliance costs associated with these processes increase with production scale. Green chemistry approaches, including aqueous synthesis routes and solvent-free mechanochemical methods, are emerging as promising alternatives that may enable more sustainable large-scale production.
Equipment compatibility presents technical hurdles for scaling production. Conventional coating and electrode fabrication equipment may require modification to accommodate the reactive nature of Lewis acid materials. Specialized mixing technologies that ensure homogeneous distribution of Lewis acid sites throughout electrode materials are essential for consistent performance but add complexity to manufacturing lines.
Recent advances in continuous flow manufacturing techniques show promise for addressing several of these challenges. These approaches enable precise control of reaction parameters while reducing batch-to-batch variation. Additionally, automated quality control systems incorporating in-line spectroscopic monitoring can help maintain consistency in Lewis acid content and distribution, though implementation costs remain high for early adopters.
Manufacturing processes must address several key considerations. First, the sensitivity of many Lewis acid compounds to moisture and oxygen necessitates specialized handling environments, increasing production costs. Controlled atmosphere processing lines represent a substantial capital investment that smaller manufacturers may find prohibitive. Second, the thermal stability of Lewis acid functionalities during high-temperature processing steps requires careful engineering of manufacturing protocols to prevent degradation of active sites.
Raw material supply chains present another dimension of scalability challenges. Many advanced Lewis acid compounds incorporate rare earth elements or specialized transition metals with limited global supply. Price volatility and geopolitical factors affecting these materials can disrupt production schedules and impact economic viability. Developing alternative formulations using more abundant elements represents an active research direction to mitigate these supply risks.
Environmental considerations also influence manufacturing scalability. Traditional synthesis routes for Lewis acid materials often involve toxic solvents and generate hazardous waste streams. Regulatory compliance costs associated with these processes increase with production scale. Green chemistry approaches, including aqueous synthesis routes and solvent-free mechanochemical methods, are emerging as promising alternatives that may enable more sustainable large-scale production.
Equipment compatibility presents technical hurdles for scaling production. Conventional coating and electrode fabrication equipment may require modification to accommodate the reactive nature of Lewis acid materials. Specialized mixing technologies that ensure homogeneous distribution of Lewis acid sites throughout electrode materials are essential for consistent performance but add complexity to manufacturing lines.
Recent advances in continuous flow manufacturing techniques show promise for addressing several of these challenges. These approaches enable precise control of reaction parameters while reducing batch-to-batch variation. Additionally, automated quality control systems incorporating in-line spectroscopic monitoring can help maintain consistency in Lewis acid content and distribution, though implementation costs remain high for early adopters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!