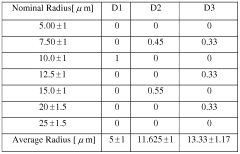
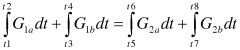How to Test Lewis Acid Strength Using NMR?
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid NMR Testing Background and Objectives
Lewis acids, defined as electron pair acceptors according to the Lewis theory of acids and bases, play a crucial role in numerous chemical reactions and catalytic processes. The ability to accurately measure and compare the strength of Lewis acids is fundamental to understanding their reactivity patterns and selecting appropriate catalysts for specific applications. Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as one of the most powerful and versatile analytical techniques for assessing Lewis acid strength due to its non-destructive nature and ability to provide detailed structural information at the molecular level.
The development of NMR-based methods for Lewis acid characterization dates back to the 1960s, with significant advancements occurring in the 1980s and 1990s as NMR technology improved. The evolution of this field has been driven by the increasing demand for more efficient catalysts in pharmaceutical manufacturing, materials science, and green chemistry applications, where precise understanding of Lewis acidity is essential for optimizing reaction conditions and outcomes.
Current technological trends in this domain include the development of more sensitive NMR probes specifically designed for Lewis acid characterization, integration of computational methods with experimental NMR data, and the establishment of standardized protocols for comparing acid strengths across different chemical families. The advancement of multinuclear NMR techniques has expanded the toolkit available to researchers, allowing for more comprehensive analysis of Lewis acid-base interactions.
The primary objective of Lewis acid strength testing via NMR is to establish quantitative scales that accurately reflect relative acidities, enabling rational catalyst design and selection. This involves correlating spectroscopic parameters with actual catalytic performance, developing universal reference systems that allow comparison between different classes of Lewis acids, and creating predictive models that can anticipate the behavior of novel acid systems.
Secondary goals include elucidating the relationship between electronic structure and Lewis acidity, understanding solvent effects on acid strength measurements, and developing high-throughput NMR methods for rapid screening of potential catalysts. These objectives align with broader industry trends toward more efficient and sustainable chemical processes, where precise control of Lewis acid catalysis can lead to significant improvements in reaction selectivity and yield.
The technological significance of this field extends beyond academic interest, as accurate Lewis acid characterization directly impacts industrial applications ranging from polymer synthesis to pharmaceutical manufacturing, where even small improvements in catalyst performance can translate to substantial economic and environmental benefits.
The development of NMR-based methods for Lewis acid characterization dates back to the 1960s, with significant advancements occurring in the 1980s and 1990s as NMR technology improved. The evolution of this field has been driven by the increasing demand for more efficient catalysts in pharmaceutical manufacturing, materials science, and green chemistry applications, where precise understanding of Lewis acidity is essential for optimizing reaction conditions and outcomes.
Current technological trends in this domain include the development of more sensitive NMR probes specifically designed for Lewis acid characterization, integration of computational methods with experimental NMR data, and the establishment of standardized protocols for comparing acid strengths across different chemical families. The advancement of multinuclear NMR techniques has expanded the toolkit available to researchers, allowing for more comprehensive analysis of Lewis acid-base interactions.
The primary objective of Lewis acid strength testing via NMR is to establish quantitative scales that accurately reflect relative acidities, enabling rational catalyst design and selection. This involves correlating spectroscopic parameters with actual catalytic performance, developing universal reference systems that allow comparison between different classes of Lewis acids, and creating predictive models that can anticipate the behavior of novel acid systems.
Secondary goals include elucidating the relationship between electronic structure and Lewis acidity, understanding solvent effects on acid strength measurements, and developing high-throughput NMR methods for rapid screening of potential catalysts. These objectives align with broader industry trends toward more efficient and sustainable chemical processes, where precise control of Lewis acid catalysis can lead to significant improvements in reaction selectivity and yield.
The technological significance of this field extends beyond academic interest, as accurate Lewis acid characterization directly impacts industrial applications ranging from polymer synthesis to pharmaceutical manufacturing, where even small improvements in catalyst performance can translate to substantial economic and environmental benefits.
Market Applications for Lewis Acid Strength Determination
The determination of Lewis acid strength using NMR spectroscopy has significant market applications across multiple industries, driven by the critical role Lewis acids play in various chemical processes. The pharmaceutical industry represents one of the largest markets, where precise Lewis acid strength measurements enable optimization of catalytic reactions for drug synthesis. Companies like Pfizer, Novartis, and Merck rely on NMR-based Lewis acid characterization to develop more efficient synthetic routes for active pharmaceutical ingredients, reducing production costs and environmental impact.
In the petrochemical sector, Lewis acid catalysts are fundamental to processes such as alkylation, isomerization, and cracking. Major corporations including ExxonMobil, Shell, and BASF utilize NMR techniques to evaluate and fine-tune catalyst performance. The ability to accurately measure Lewis acid strength translates directly to improved process efficiency and product selectivity, creating substantial economic value in large-scale operations.
The polymer industry represents another significant market application, where Lewis acids catalyze polymerization reactions. Companies producing specialty polymers can leverage NMR-based Lewis acid strength determination to develop materials with precisely controlled properties. This capability is particularly valuable in high-performance applications such as aerospace components, medical devices, and electronic materials.
The fine chemicals industry benefits from NMR-based Lewis acid characterization in developing selective synthetic methodologies for complex molecules. Specialty chemical manufacturers can optimize reaction conditions based on quantitative understanding of Lewis acidity, leading to higher yields and fewer side products. This application is especially relevant for companies producing flavors, fragrances, and agricultural chemicals.
Academic and industrial research laboratories constitute a smaller but growing market segment. The increasing focus on green chemistry and sustainable processes has heightened interest in Lewis acid catalysis as an alternative to traditional methods. Research institutions and R&D departments invest in NMR equipment and expertise specifically for catalyst characterization.
The analytical services sector represents an emerging market opportunity, with specialized laboratories offering Lewis acid strength determination as a service to companies lacking in-house capabilities. This business model allows smaller chemical companies to access sophisticated analytical techniques without major capital investment.
Market growth for Lewis acid strength determination technologies is being driven by increasing regulatory pressure for cleaner chemical processes, the push toward more efficient catalytic systems, and the trend toward computational modeling of chemical reactions that requires precise experimental data for validation.
In the petrochemical sector, Lewis acid catalysts are fundamental to processes such as alkylation, isomerization, and cracking. Major corporations including ExxonMobil, Shell, and BASF utilize NMR techniques to evaluate and fine-tune catalyst performance. The ability to accurately measure Lewis acid strength translates directly to improved process efficiency and product selectivity, creating substantial economic value in large-scale operations.
The polymer industry represents another significant market application, where Lewis acids catalyze polymerization reactions. Companies producing specialty polymers can leverage NMR-based Lewis acid strength determination to develop materials with precisely controlled properties. This capability is particularly valuable in high-performance applications such as aerospace components, medical devices, and electronic materials.
The fine chemicals industry benefits from NMR-based Lewis acid characterization in developing selective synthetic methodologies for complex molecules. Specialty chemical manufacturers can optimize reaction conditions based on quantitative understanding of Lewis acidity, leading to higher yields and fewer side products. This application is especially relevant for companies producing flavors, fragrances, and agricultural chemicals.
Academic and industrial research laboratories constitute a smaller but growing market segment. The increasing focus on green chemistry and sustainable processes has heightened interest in Lewis acid catalysis as an alternative to traditional methods. Research institutions and R&D departments invest in NMR equipment and expertise specifically for catalyst characterization.
The analytical services sector represents an emerging market opportunity, with specialized laboratories offering Lewis acid strength determination as a service to companies lacking in-house capabilities. This business model allows smaller chemical companies to access sophisticated analytical techniques without major capital investment.
Market growth for Lewis acid strength determination technologies is being driven by increasing regulatory pressure for cleaner chemical processes, the push toward more efficient catalytic systems, and the trend toward computational modeling of chemical reactions that requires precise experimental data for validation.
Current NMR Methods and Technical Limitations
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful analytical tool for determining Lewis acid strength due to its non-destructive nature and ability to provide detailed structural information at the molecular level. Current methodologies primarily focus on monitoring chemical shift changes when Lewis acids interact with probe molecules.
The most widely employed NMR method for Lewis acid strength determination is the Gutmann-Beckett method, which utilizes triethylphosphine oxide (Et3PO) as a probe molecule. This approach measures the 31P NMR chemical shift of the phosphine oxide when coordinated to Lewis acids. The magnitude of downfield shift correlates directly with Lewis acid strength, providing a quantitative acceptor number (AN) scale.
Another established technique is the Childs method, which employs crotonaldehyde as the probe molecule. This method monitors the 1H NMR chemical shift of the olefinic proton, which experiences deshielding proportional to the Lewis acid strength. The resulting chemical shift difference creates a reliable scale for comparing various Lewis acids.
Fluoride ion affinity (FIA) measurements using 19F NMR represent another valuable approach. By monitoring the 19F chemical shift of a fluoride-containing probe molecule upon interaction with Lewis acids, researchers can quantify binding strength and establish relative acidity scales.
Despite these advances, current NMR methods face several technical limitations. Signal overlap in complex systems often complicates interpretation, particularly when analyzing mixtures of Lewis acids or when working with compounds containing multiple NMR-active nuclei. This challenge becomes especially pronounced in heterogeneous catalytic systems.
Sensitivity constraints represent another significant limitation. Traditional NMR requires relatively high concentrations of both Lewis acids and probe molecules, which may not reflect actual catalytic conditions where Lewis acids operate at much lower concentrations. This concentration disparity can lead to misleading strength assessments.
Temperature dependence of chemical shifts introduces additional complications. NMR measurements at different temperatures may yield varying results, making standardization difficult across different research groups and experimental conditions. This variability hampers the establishment of universal Lewis acidity scales.
Furthermore, solvent effects significantly impact NMR measurements. Different solvents can compete with probe molecules for coordination sites on Lewis acids, altering the observed chemical shifts and potentially masking the true acidity. This solvent dependence necessitates careful standardization of measurement conditions.
Time-resolution limitations also restrict the application of NMR for studying dynamic Lewis acid-base interactions. Traditional NMR techniques struggle to capture rapid exchange processes that occur faster than the NMR timescale, potentially missing important mechanistic details in catalytic systems.
The most widely employed NMR method for Lewis acid strength determination is the Gutmann-Beckett method, which utilizes triethylphosphine oxide (Et3PO) as a probe molecule. This approach measures the 31P NMR chemical shift of the phosphine oxide when coordinated to Lewis acids. The magnitude of downfield shift correlates directly with Lewis acid strength, providing a quantitative acceptor number (AN) scale.
Another established technique is the Childs method, which employs crotonaldehyde as the probe molecule. This method monitors the 1H NMR chemical shift of the olefinic proton, which experiences deshielding proportional to the Lewis acid strength. The resulting chemical shift difference creates a reliable scale for comparing various Lewis acids.
Fluoride ion affinity (FIA) measurements using 19F NMR represent another valuable approach. By monitoring the 19F chemical shift of a fluoride-containing probe molecule upon interaction with Lewis acids, researchers can quantify binding strength and establish relative acidity scales.
Despite these advances, current NMR methods face several technical limitations. Signal overlap in complex systems often complicates interpretation, particularly when analyzing mixtures of Lewis acids or when working with compounds containing multiple NMR-active nuclei. This challenge becomes especially pronounced in heterogeneous catalytic systems.
Sensitivity constraints represent another significant limitation. Traditional NMR requires relatively high concentrations of both Lewis acids and probe molecules, which may not reflect actual catalytic conditions where Lewis acids operate at much lower concentrations. This concentration disparity can lead to misleading strength assessments.
Temperature dependence of chemical shifts introduces additional complications. NMR measurements at different temperatures may yield varying results, making standardization difficult across different research groups and experimental conditions. This variability hampers the establishment of universal Lewis acidity scales.
Furthermore, solvent effects significantly impact NMR measurements. Different solvents can compete with probe molecules for coordination sites on Lewis acids, altering the observed chemical shifts and potentially masking the true acidity. This solvent dependence necessitates careful standardization of measurement conditions.
Time-resolution limitations also restrict the application of NMR for studying dynamic Lewis acid-base interactions. Traditional NMR techniques struggle to capture rapid exchange processes that occur faster than the NMR timescale, potentially missing important mechanistic details in catalytic systems.
Established NMR Protocols for Lewis Acidity Measurement
01 Methods for measuring Lewis acid strength
Various analytical techniques are employed to measure and quantify Lewis acid strength in chemical compounds. These methods include spectroscopic techniques, calorimetric measurements, and computational approaches that evaluate electron-accepting capabilities. The strength of Lewis acids can be determined by measuring their interaction with standard Lewis bases, where stronger acids form more stable complexes. These testing methodologies help in characterizing catalysts and understanding reaction mechanisms.- Methods for testing Lewis acid strength: Various methods are employed to test and measure the strength of Lewis acids. These methods include spectroscopic techniques, calorimetric measurements, and reaction rate studies. The strength of Lewis acids can be determined by measuring their ability to accept electron pairs from Lewis bases, which is reflected in the formation constants of the resulting complexes. These testing methods help in characterizing and comparing different Lewis acids for various applications.
- Lewis acid catalysts in chemical reactions: Lewis acids serve as important catalysts in various chemical reactions, including polymerization, alkylation, and isomerization processes. The catalytic activity of these Lewis acids is directly related to their acid strength, with stronger Lewis acids generally showing higher catalytic efficiency. The selection of appropriate Lewis acid catalysts based on their strength is crucial for optimizing reaction conditions and achieving desired product yields and selectivity.
- Relationship between structure and Lewis acid strength: The strength of Lewis acids is significantly influenced by their molecular structure and composition. Factors such as the electronegativity of central atoms, steric hindrance, and the presence of electron-withdrawing groups affect the Lewis acidity. Understanding these structure-property relationships helps in designing Lewis acids with tailored strengths for specific applications. Computational methods are often used to predict Lewis acid strength based on structural parameters.
- Lewis acid strength in zeolites and solid catalysts: Zeolites and other solid materials containing Lewis acid sites are widely used as heterogeneous catalysts. The strength and distribution of Lewis acid sites in these materials significantly impact their catalytic performance. Various techniques, including temperature-programmed desorption and infrared spectroscopy with probe molecules, are used to characterize the Lewis acid strength in these solid catalysts. Modification methods can be employed to tune the Lewis acid strength for specific catalytic applications.
- Industrial applications of Lewis acids based on strength: The industrial application of Lewis acids is largely determined by their acid strength. Stronger Lewis acids are typically used in reactions requiring higher activation energy, while milder Lewis acids find applications in more selective transformations. Industries such as petrochemicals, pharmaceuticals, and polymer manufacturing utilize Lewis acids of varying strengths for different processes. The selection of appropriate Lewis acids based on their strength is crucial for process efficiency, product quality, and economic viability.
02 Lewis acid catalysts in chemical synthesis
Lewis acids serve as important catalysts in various chemical synthesis processes. Their electron-accepting properties enable them to activate substrates by coordinating with electron-rich sites, facilitating reactions like alkylation, acylation, and polymerization. The strength of these Lewis acid catalysts directly impacts reaction rates, selectivity, and yield. Different Lewis acids exhibit varying catalytic activities depending on their strength, which can be tailored for specific applications in pharmaceutical and industrial chemical synthesis.Expand Specific Solutions03 Relationship between structure and Lewis acidity
The structural characteristics of compounds significantly influence their Lewis acid strength. Factors such as the central atom's electronegativity, coordination number, and surrounding ligands affect electron-accepting capabilities. Metal-based Lewis acids with higher oxidation states typically exhibit stronger acidity. Steric hindrance around the acidic center can modulate accessibility to Lewis basic sites. Understanding these structure-activity relationships enables the rational design of Lewis acids with predictable strengths for specific applications.Expand Specific Solutions04 Lewis acid strength in catalytic processes
The strength of Lewis acids plays a crucial role in determining their effectiveness in catalytic processes. Stronger Lewis acids generally show higher catalytic activity but may lead to unwanted side reactions or product degradation. Moderate strength acids often provide better selectivity for certain transformations. The optimal Lewis acid strength depends on the specific reaction requirements, substrate sensitivity, and desired product distribution. Testing and comparing acid strengths helps in selecting the most appropriate catalyst for industrial applications.Expand Specific Solutions05 Novel Lewis acid systems and modifications
Research has led to the development of novel Lewis acid systems with enhanced properties and tunable strengths. These include supported Lewis acids, Lewis acid-surfactant combined catalysts, and heterogeneous systems that offer advantages like recyclability and easier separation. Modifications such as the addition of electron-withdrawing groups or changing counter ions can fine-tune Lewis acidity. Some innovations involve combining Lewis acids with other catalytic functionalities to create bifunctional catalysts with synergistic effects, expanding their application range.Expand Specific Solutions
Leading Research Groups and Industrial Players
Nuclear Magnetic Resonance (NMR) spectroscopy for Lewis acid strength testing represents a growing technical field currently in its early maturity phase. The market is expanding steadily with an estimated size of $300-400 million, driven by applications in catalysis research and materials science. Technologically, the field has reached moderate maturity with established methodologies but continues to evolve. Key players demonstrate varying specialization levels: Schlumberger and Baker Hughes leverage NMR for energy applications; JEOL and Bruker BioSpin lead in instrumentation development; while academic institutions like École Polytechnique Fédérale de Lausanne and University of Maryland contribute fundamental research. Pharmaceutical companies including F. Hoffmann-La Roche and Chugai Pharmaceutical utilize this technology for drug development applications, indicating cross-industry relevance.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has developed a sophisticated NMR-based methodology for quantifying Lewis acid strength in heterogeneous catalysts, particularly zeolites and metal-organic frameworks used in petrochemical processes. Their approach combines solid-state 1H, 13C, and 27Al MAS (Magic Angle Spinning) NMR with carefully selected probe molecules like pyridine, acetonitrile, and trimethylphosphine. The company's proprietary technique involves correlating 31P chemical shifts of adsorbed trimethylphosphine with catalytic activity measurements to establish quantitative Lewis acidity scales. IFP has further enhanced this methodology by implementing 2D correlation experiments that distinguish between Brønsted and Lewis acid sites in complex catalyst systems. Their research has established standardized protocols for sample preparation, including controlled dehydration procedures that preserve the integrity of Lewis acid sites during NMR analysis. Additionally, IFP has developed computational models that correlate NMR parameters with quantum chemical calculations to provide deeper insights into the electronic properties of Lewis acid centers.
Strengths: Specialized expertise in heterogeneous catalysts relevant to petrochemical industry; established correlation between NMR parameters and catalytic performance; comprehensive approach combining multiple techniques. Weaknesses: Methods optimized primarily for petroleum-related catalysts; requires specialized solid-state NMR equipment; time-intensive analysis procedures.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed a field-deployable NMR technology for Lewis acid characterization in drilling fluids and well cementing materials. Their approach utilizes low-field NMR relaxometry combined with specialized probe molecules to assess Lewis acidity in complex geological environments. The company's proprietary MagTraC™ system incorporates temperature-resistant NMR probes capable of operating under downhole conditions, allowing real-time monitoring of Lewis acid-base interactions that affect drilling fluid stability and cement setting kinetics. Baker Hughes has established correlations between 11B and 27Al NMR parameters and the performance of boron and aluminum-based Lewis acids used in oilfield chemicals. Their methodology includes standardized protocols for sample preparation under anaerobic conditions to prevent oxidation of air-sensitive Lewis acidic components. The company has further developed data processing algorithms that compensate for the heterogeneity of geological samples, enabling accurate Lewis acid strength determination even in complex mineral mixtures containing paramagnetic impurities.
Strengths: Robust technology designed for harsh field conditions; specialized expertise in oilfield-relevant Lewis acid systems; integration with other analytical techniques for comprehensive characterization. Weaknesses: Lower resolution compared to high-field laboratory NMR systems; optimized primarily for petroleum industry applications; limited sensitivity for weak Lewis acid sites.
Key Spectroscopic Principles in Lewis Acid Testing
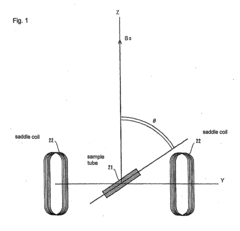
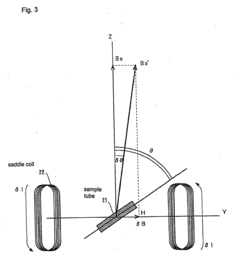
Method and apparatus for accurately adjusting magic angle in NMR
PatentActiveEP2306215A1
Innovation
- The method involves using a uniform magnetic field coil assembly to vectorially combine the external magnetic field with a uniform magnetic field generated by electric currents flowing through shim coils, allowing for precise adjustment of the magic angle without mechanical operations, suitable for high-resolution solid-state NMR systems including those with vacuum and cryo-coil configurations.
Magnetic resonance method for analyzing PORE size distribution
PatentWO2013021390A1
Innovation
- A method involving a series of magnetic resonance experiments using bipolar gradient pulse subsequences characterized by specific wavevectors and angles, followed by a three-dimensional analysis to extract pore size distribution, which includes solving linearized equations and calculating expected signals for different pore sizes.
Standardization and Reproducibility Challenges
One of the most significant challenges in using NMR to test Lewis acid strength is the lack of standardized protocols across the scientific community. Different research groups often employ varying experimental conditions, including solvent selection, temperature control, and sample preparation methods, which can lead to inconsistent results when comparing Lewis acid strengths. For instance, the choice between deuterated solvents such as CDCl3, CD2Cl2, or CD3CN can significantly impact the observed chemical shifts due to different solvent-acid interactions, making direct comparisons between studies problematic.
Temperature control represents another critical variable affecting reproducibility. NMR measurements of Lewis acid-base interactions are temperature-dependent, with chemical shifts and coupling constants varying significantly even with small temperature fluctuations. Despite this sensitivity, many published studies fail to report precise temperature conditions or utilize inadequate temperature calibration methods, further complicating cross-study comparisons.
Sample concentration issues also contribute to reproducibility challenges. The concentration of both Lewis acids and probe molecules can dramatically influence observed chemical shifts, particularly when self-association or aggregation phenomena occur. Without standardized concentration ranges, researchers may unknowingly operate in different interaction regimes, leading to contradictory strength assessments.
Reference standards present another obstacle to consistent measurements. While tetramethylsilane (TMS) serves as a universal reference for 1H and 13C NMR, the field lacks consensus on appropriate reference compounds for evaluating Lewis acid strength. This absence of agreed-upon standards makes it difficult to establish absolute strength scales that remain consistent across different laboratories and instruments.
Instrument variability further compounds these challenges. Different NMR spectrometer field strengths, probe designs, and acquisition parameters can yield varying results for identical samples. Modern high-field instruments may resolve interactions that remain undetectable on older or lower-field systems, creating a technological bias in the literature.
Data processing and analysis methodologies also lack standardization. Researchers employ diverse approaches to peak fitting, integration, and interpretation of complex spectra, particularly when dealing with dynamic exchange processes common in Lewis acid-base interactions. Without established data analysis protocols, subjective interpretations can lead to divergent conclusions from essentially identical raw data.
Temperature control represents another critical variable affecting reproducibility. NMR measurements of Lewis acid-base interactions are temperature-dependent, with chemical shifts and coupling constants varying significantly even with small temperature fluctuations. Despite this sensitivity, many published studies fail to report precise temperature conditions or utilize inadequate temperature calibration methods, further complicating cross-study comparisons.
Sample concentration issues also contribute to reproducibility challenges. The concentration of both Lewis acids and probe molecules can dramatically influence observed chemical shifts, particularly when self-association or aggregation phenomena occur. Without standardized concentration ranges, researchers may unknowingly operate in different interaction regimes, leading to contradictory strength assessments.
Reference standards present another obstacle to consistent measurements. While tetramethylsilane (TMS) serves as a universal reference for 1H and 13C NMR, the field lacks consensus on appropriate reference compounds for evaluating Lewis acid strength. This absence of agreed-upon standards makes it difficult to establish absolute strength scales that remain consistent across different laboratories and instruments.
Instrument variability further compounds these challenges. Different NMR spectrometer field strengths, probe designs, and acquisition parameters can yield varying results for identical samples. Modern high-field instruments may resolve interactions that remain undetectable on older or lower-field systems, creating a technological bias in the literature.
Data processing and analysis methodologies also lack standardization. Researchers employ diverse approaches to peak fitting, integration, and interpretation of complex spectra, particularly when dealing with dynamic exchange processes common in Lewis acid-base interactions. Without established data analysis protocols, subjective interpretations can lead to divergent conclusions from essentially identical raw data.
Computational Methods Supporting NMR Analysis
Computational methods have become indispensable tools in modern NMR analysis of Lewis acid strength, offering predictive capabilities that complement experimental measurements. Density Functional Theory (DFT) calculations stand at the forefront, enabling researchers to predict chemical shifts, coupling constants, and other NMR parameters with remarkable accuracy. These calculations can simulate the interaction between Lewis acids and probe molecules, providing theoretical chemical shift values that correlate with acid strength before experimental verification.
Quantum mechanical calculations particularly excel at modeling the electronic environment around nuclei affected by Lewis acid coordination. By calculating the electron density distribution and resulting magnetic shielding effects, these methods can predict the magnitude of chemical shift changes upon acid-base interactions. This approach proves especially valuable when dealing with highly reactive or unstable Lewis acid species that present experimental challenges.
Machine learning algorithms have recently emerged as powerful tools for analyzing complex NMR datasets related to Lewis acidity. These computational approaches can identify patterns and correlations between spectral features and acid strength that might not be immediately apparent through conventional analysis. Neural networks trained on extensive NMR datasets can predict Lewis acid strength based on spectral fingerprints with increasing accuracy.
Molecular dynamics simulations complement static computational models by accounting for dynamic effects in solution. These simulations can model solvent effects, molecular motion, and temperature dependencies that influence NMR measurements of Lewis acid strength. By incorporating these dynamic factors, researchers obtain more realistic predictions that better match experimental conditions.
Computational chemistry software packages like Gaussian, ORCA, and ADF now include specialized modules for NMR parameter calculation. These tools have democratized computational NMR analysis, allowing researchers without extensive computational expertise to predict and interpret NMR data for Lewis acid characterization. Modern graphical interfaces and automated workflows have significantly reduced the technical barriers to implementing these methods.
Correlation analysis between computational and experimental NMR data has established valuable scaling factors and correction parameters that improve prediction accuracy. These empirical corrections account for systematic errors in computational methods and have enhanced the reliability of computational approaches for quantifying Lewis acid strength through NMR parameters.
Quantum mechanical calculations particularly excel at modeling the electronic environment around nuclei affected by Lewis acid coordination. By calculating the electron density distribution and resulting magnetic shielding effects, these methods can predict the magnitude of chemical shift changes upon acid-base interactions. This approach proves especially valuable when dealing with highly reactive or unstable Lewis acid species that present experimental challenges.
Machine learning algorithms have recently emerged as powerful tools for analyzing complex NMR datasets related to Lewis acidity. These computational approaches can identify patterns and correlations between spectral features and acid strength that might not be immediately apparent through conventional analysis. Neural networks trained on extensive NMR datasets can predict Lewis acid strength based on spectral fingerprints with increasing accuracy.
Molecular dynamics simulations complement static computational models by accounting for dynamic effects in solution. These simulations can model solvent effects, molecular motion, and temperature dependencies that influence NMR measurements of Lewis acid strength. By incorporating these dynamic factors, researchers obtain more realistic predictions that better match experimental conditions.
Computational chemistry software packages like Gaussian, ORCA, and ADF now include specialized modules for NMR parameter calculation. These tools have democratized computational NMR analysis, allowing researchers without extensive computational expertise to predict and interpret NMR data for Lewis acid characterization. Modern graphical interfaces and automated workflows have significantly reduced the technical barriers to implementing these methods.
Correlation analysis between computational and experimental NMR data has established valuable scaling factors and correction parameters that improve prediction accuracy. These empirical corrections account for systematic errors in computational methods and have enhanced the reliability of computational approaches for quantifying Lewis acid strength through NMR parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!