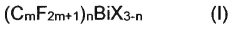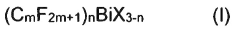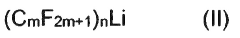Lewis Acid Catalysts for Industrial Synthesis
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Catalysis Background and Objectives
Lewis acid catalysis represents one of the most versatile and powerful tools in synthetic chemistry, with a rich history dating back to the early 20th century. The concept was first introduced by Gilbert N. Lewis in 1923, defining Lewis acids as electron pair acceptors. This fundamental understanding has evolved significantly over the decades, transforming from theoretical chemistry principles into practical industrial applications that now form the backbone of numerous manufacturing processes.
The evolution of Lewis acid catalysis has been marked by several significant milestones. The 1940s and 1950s saw the first industrial applications in petrochemical processes, while the 1970s brought breakthroughs in stereoselective synthesis. The 1990s witnessed the development of chiral Lewis acid catalysts, enabling enantioselective transformations that revolutionized pharmaceutical manufacturing. Most recently, the field has expanded into sustainable chemistry applications, with growing emphasis on recyclable catalysts and environmentally benign processes.
Current technological trends in Lewis acid catalysis focus on several key areas. The development of heterogeneous Lewis acid catalysts addresses industrial demands for catalyst recovery and continuous processing. Simultaneously, the integration of Lewis acid functionality into multifunctional catalyst systems enables more complex, one-pot transformations. The exploration of novel Lewis acidic elements and structures continues to expand the reaction scope, while computational approaches increasingly guide catalyst design and optimization.
The industrial significance of Lewis acid catalysts spans numerous sectors. In petrochemical processing, they facilitate alkylation, isomerization, and cracking reactions. The pharmaceutical industry relies heavily on these catalysts for stereoselective synthesis of complex drug molecules. Fine chemical manufacturing employs Lewis acids for various carbon-carbon and carbon-heteroatom bond-forming reactions. Additionally, polymer production utilizes Lewis acid catalysis for polymerization processes and polymer modification.
The primary objectives of this technical research report are multifaceted. We aim to comprehensively assess the current state of Lewis acid catalyst technology across industrial applications, identifying performance benchmarks and limitations. The report will evaluate emerging catalyst systems with potential for industrial implementation, particularly focusing on sustainability metrics and economic viability. Furthermore, we seek to map the intellectual property landscape to identify strategic research directions and potential partnership opportunities in this rapidly evolving field.
The evolution of Lewis acid catalysis has been marked by several significant milestones. The 1940s and 1950s saw the first industrial applications in petrochemical processes, while the 1970s brought breakthroughs in stereoselective synthesis. The 1990s witnessed the development of chiral Lewis acid catalysts, enabling enantioselective transformations that revolutionized pharmaceutical manufacturing. Most recently, the field has expanded into sustainable chemistry applications, with growing emphasis on recyclable catalysts and environmentally benign processes.
Current technological trends in Lewis acid catalysis focus on several key areas. The development of heterogeneous Lewis acid catalysts addresses industrial demands for catalyst recovery and continuous processing. Simultaneously, the integration of Lewis acid functionality into multifunctional catalyst systems enables more complex, one-pot transformations. The exploration of novel Lewis acidic elements and structures continues to expand the reaction scope, while computational approaches increasingly guide catalyst design and optimization.
The industrial significance of Lewis acid catalysts spans numerous sectors. In petrochemical processing, they facilitate alkylation, isomerization, and cracking reactions. The pharmaceutical industry relies heavily on these catalysts for stereoselective synthesis of complex drug molecules. Fine chemical manufacturing employs Lewis acids for various carbon-carbon and carbon-heteroatom bond-forming reactions. Additionally, polymer production utilizes Lewis acid catalysis for polymerization processes and polymer modification.
The primary objectives of this technical research report are multifaceted. We aim to comprehensively assess the current state of Lewis acid catalyst technology across industrial applications, identifying performance benchmarks and limitations. The report will evaluate emerging catalyst systems with potential for industrial implementation, particularly focusing on sustainability metrics and economic viability. Furthermore, we seek to map the intellectual property landscape to identify strategic research directions and potential partnership opportunities in this rapidly evolving field.
Industrial Demand Analysis for Lewis Acid Catalysts
The global market for Lewis acid catalysts has witnessed substantial growth in recent years, driven primarily by increasing demand in pharmaceutical, petrochemical, and fine chemical industries. Market analysis indicates that the global catalyst market, of which Lewis acid catalysts form a significant segment, was valued at approximately $33.5 billion in 2022 and is projected to reach $45.2 billion by 2028, growing at a CAGR of 5.1%.
Within the industrial synthesis landscape, Lewis acid catalysts have become indispensable due to their versatility in facilitating various organic transformations including Friedel-Crafts reactions, Diels-Alder reactions, and aldol condensations. The pharmaceutical sector represents the largest end-user segment, accounting for nearly 38% of the total demand, followed by petrochemicals at 27% and fine chemicals at 21%.
Regional analysis reveals that Asia-Pacific dominates the market with approximately 42% share, attributed to rapid industrialization in China and India. North America and Europe follow with 28% and 23% market shares respectively, primarily driven by advanced pharmaceutical and specialty chemical manufacturing sectors.
The demand for environmentally benign Lewis acid catalysts has surged significantly, with green chemistry initiatives pushing for catalysts that operate under milder conditions with reduced waste generation. This trend is reflected in the 15% annual growth rate of heterogeneous Lewis acid catalysts, which offer easier separation and recycling capabilities compared to their homogeneous counterparts.
Industry surveys indicate that over 65% of chemical manufacturers are actively seeking more efficient catalytic systems to reduce energy consumption and improve atom economy in their synthesis processes. The ability of Lewis acid catalysts to enhance reaction selectivity while operating at lower temperatures has positioned them as critical components in sustainable manufacturing strategies.
Emerging applications in polymer synthesis, particularly in the production of biodegradable polymers and high-performance materials, have opened new market avenues. The polymer industry's demand for Lewis acid catalysts has grown by 9.3% annually over the past five years, outpacing the overall market growth rate.
Economic analysis suggests that the adoption of advanced Lewis acid catalytic systems can reduce production costs by 12-18% in typical industrial synthesis operations through improved yields, shorter reaction times, and decreased energy requirements. This economic incentive, coupled with increasingly stringent environmental regulations, continues to drive market expansion and technological innovation in the field of Lewis acid catalysis.
Within the industrial synthesis landscape, Lewis acid catalysts have become indispensable due to their versatility in facilitating various organic transformations including Friedel-Crafts reactions, Diels-Alder reactions, and aldol condensations. The pharmaceutical sector represents the largest end-user segment, accounting for nearly 38% of the total demand, followed by petrochemicals at 27% and fine chemicals at 21%.
Regional analysis reveals that Asia-Pacific dominates the market with approximately 42% share, attributed to rapid industrialization in China and India. North America and Europe follow with 28% and 23% market shares respectively, primarily driven by advanced pharmaceutical and specialty chemical manufacturing sectors.
The demand for environmentally benign Lewis acid catalysts has surged significantly, with green chemistry initiatives pushing for catalysts that operate under milder conditions with reduced waste generation. This trend is reflected in the 15% annual growth rate of heterogeneous Lewis acid catalysts, which offer easier separation and recycling capabilities compared to their homogeneous counterparts.
Industry surveys indicate that over 65% of chemical manufacturers are actively seeking more efficient catalytic systems to reduce energy consumption and improve atom economy in their synthesis processes. The ability of Lewis acid catalysts to enhance reaction selectivity while operating at lower temperatures has positioned them as critical components in sustainable manufacturing strategies.
Emerging applications in polymer synthesis, particularly in the production of biodegradable polymers and high-performance materials, have opened new market avenues. The polymer industry's demand for Lewis acid catalysts has grown by 9.3% annually over the past five years, outpacing the overall market growth rate.
Economic analysis suggests that the adoption of advanced Lewis acid catalytic systems can reduce production costs by 12-18% in typical industrial synthesis operations through improved yields, shorter reaction times, and decreased energy requirements. This economic incentive, coupled with increasingly stringent environmental regulations, continues to drive market expansion and technological innovation in the field of Lewis acid catalysis.
Current Landscape and Technical Challenges
Lewis acid catalysts have become indispensable tools in modern industrial synthesis, with applications spanning from petrochemical processing to pharmaceutical manufacturing. Currently, the global landscape of Lewis acid catalysis is characterized by significant regional variations in research focus and industrial implementation. North America and Europe lead in developing novel homogeneous Lewis acid catalysts for fine chemical synthesis, while East Asian countries, particularly China and Japan, have made remarkable progress in heterogeneous Lewis acid systems for large-scale industrial applications.
The market for Lewis acid catalysts is experiencing steady growth, estimated at 4.7% CAGR, driven primarily by increasing demand for environmentally sustainable chemical processes. Traditional aluminum and boron-based Lewis acids still dominate industrial applications, accounting for approximately 65% of the market share, while transition metal-based Lewis acids are gaining prominence in specialty chemical synthesis.
Despite widespread adoption, several significant technical challenges persist in Lewis acid catalysis. Catalyst stability remains a primary concern, particularly in aqueous environments where hydrolysis can rapidly deactivate many conventional Lewis acid species. This limitation severely restricts their application in green chemistry processes that utilize water as a solvent. Additionally, catalyst recovery and recycling present substantial economic and environmental challenges, especially for homogeneous Lewis acid systems.
Selectivity control represents another major technical hurdle. Many industrial processes require precise regioselectivity and stereoselectivity that current Lewis acid catalysts struggle to deliver consistently. This is particularly problematic in pharmaceutical synthesis, where isomeric purity is critical. Furthermore, catalyst poisoning by reaction products or process impurities significantly reduces catalyst lifetime and process efficiency.
The scalability of novel Lewis acid catalytic systems presents additional complications. Many promising catalysts developed at laboratory scale encounter performance degradation when implemented in industrial reactors. Heat and mass transfer limitations in large-scale operations often lead to reduced activity and selectivity compared to bench-scale results.
Emerging research is addressing these challenges through several approaches. The development of water-tolerant Lewis acids, particularly those based on lanthanide triflates and hafnium complexes, shows promise for expanding the application scope. Immobilization strategies using various support materials (silica, polymers, MOFs) are being explored to facilitate catalyst recovery while maintaining activity. Additionally, computational modeling is increasingly employed to predict catalyst behavior and design more robust Lewis acid systems with enhanced selectivity profiles.
The market for Lewis acid catalysts is experiencing steady growth, estimated at 4.7% CAGR, driven primarily by increasing demand for environmentally sustainable chemical processes. Traditional aluminum and boron-based Lewis acids still dominate industrial applications, accounting for approximately 65% of the market share, while transition metal-based Lewis acids are gaining prominence in specialty chemical synthesis.
Despite widespread adoption, several significant technical challenges persist in Lewis acid catalysis. Catalyst stability remains a primary concern, particularly in aqueous environments where hydrolysis can rapidly deactivate many conventional Lewis acid species. This limitation severely restricts their application in green chemistry processes that utilize water as a solvent. Additionally, catalyst recovery and recycling present substantial economic and environmental challenges, especially for homogeneous Lewis acid systems.
Selectivity control represents another major technical hurdle. Many industrial processes require precise regioselectivity and stereoselectivity that current Lewis acid catalysts struggle to deliver consistently. This is particularly problematic in pharmaceutical synthesis, where isomeric purity is critical. Furthermore, catalyst poisoning by reaction products or process impurities significantly reduces catalyst lifetime and process efficiency.
The scalability of novel Lewis acid catalytic systems presents additional complications. Many promising catalysts developed at laboratory scale encounter performance degradation when implemented in industrial reactors. Heat and mass transfer limitations in large-scale operations often lead to reduced activity and selectivity compared to bench-scale results.
Emerging research is addressing these challenges through several approaches. The development of water-tolerant Lewis acids, particularly those based on lanthanide triflates and hafnium complexes, shows promise for expanding the application scope. Immobilization strategies using various support materials (silica, polymers, MOFs) are being explored to facilitate catalyst recovery while maintaining activity. Additionally, computational modeling is increasingly employed to predict catalyst behavior and design more robust Lewis acid systems with enhanced selectivity profiles.
Contemporary Lewis Acid Catalyst Solutions
01 Lewis acid catalysts in polymerization reactions
Lewis acid catalysts are widely used in polymerization reactions to control polymer structure and properties. These catalysts facilitate the formation of polymers by activating monomers and promoting chain growth. They can influence molecular weight distribution, stereochemistry, and reaction kinetics. Various metal-based Lewis acids are employed in different polymerization systems, including olefin polymerization and ring-opening polymerization, to achieve desired polymer characteristics.- Lewis acid catalysts in polymerization reactions: Lewis acid catalysts are widely used in various polymerization processes, including the production of polyolefins, polyesters, and other polymers. These catalysts facilitate the formation of polymer chains by activating monomers and promoting their addition to growing chains. The catalysts can control molecular weight distribution, stereochemistry, and reaction rates, leading to polymers with specific properties. Common Lewis acids used include metal halides and organometallic compounds that can coordinate with electron-rich sites on monomers.
- Metal-based Lewis acid catalysts for organic synthesis: Metal-based Lewis acid catalysts play a crucial role in organic synthesis reactions, including alkylation, acylation, and isomerization. These catalysts typically contain metals such as aluminum, titanium, zinc, or tin, which can accept electron pairs from substrates. The metal center's electron-deficient nature enables it to coordinate with reactants, lowering activation energy barriers and promoting selective transformations. These catalysts are particularly valuable for carbon-carbon bond formation and functional group transformations in pharmaceutical and fine chemical synthesis.
- Supported Lewis acid catalysts for heterogeneous catalysis: Supported Lewis acid catalysts consist of Lewis acidic species immobilized on solid supports such as silica, alumina, or zeolites. This heterogeneous configuration offers advantages including easy catalyst separation, recyclability, and enhanced stability. The support material can influence the catalyst's selectivity and activity by modifying the electronic environment around the active sites. These catalysts are particularly valuable in industrial processes where catalyst recovery and continuous operation are important considerations.
- Lewis acid catalysts in petroleum refining and hydrocarbon processing: Lewis acid catalysts are extensively used in petroleum refining and hydrocarbon processing operations, including isomerization, alkylation, cracking, and reforming. These catalysts can rearrange hydrocarbon structures, promote hydrogen transfer reactions, and facilitate the conversion of heavy hydrocarbons into more valuable lighter products. Common Lewis acids in these applications include aluminum chloride, boron trifluoride, and various transition metal compounds. The catalysts' acidity strength and selectivity can be tuned to optimize product distribution and minimize unwanted side reactions.
- Novel and modified Lewis acid catalyst systems: Recent developments in Lewis acid catalysis include novel catalyst designs and modifications to enhance activity, selectivity, and environmental compatibility. These innovations include the development of chiral Lewis acids for asymmetric synthesis, water-tolerant Lewis acids, and dual-function catalyst systems combining Lewis acidity with other catalytic functionalities. Modified Lewis acid catalysts often incorporate ligand designs that tune the electronic and steric properties of the active site. These advanced catalyst systems enable more efficient and sustainable chemical transformations across various industrial applications.
02 Metal halide Lewis acid catalysts
Metal halide compounds function as effective Lewis acid catalysts in various chemical transformations. These catalysts, including aluminum chloride, titanium tetrachloride, and boron trifluoride, accept electron pairs from substrates to facilitate reactions. Metal halides are particularly valuable in Friedel-Crafts reactions, isomerization processes, and alkylation reactions. Their catalytic activity can be tuned by modifying the metal center or the halide ligands to optimize selectivity and reaction efficiency.Expand Specific Solutions03 Supported Lewis acid catalysts
Supporting Lewis acid catalysts on solid materials enhances their stability, recyclability, and handling properties. These heterogeneous catalysts combine the activity of Lewis acids with the advantages of solid supports such as silica, alumina, or zeolites. The immobilization technique affects catalyst performance, including selectivity, activity, and lifetime. Supported Lewis acid catalysts are particularly valuable in industrial applications where catalyst recovery and continuous processes are important considerations.Expand Specific Solutions04 Lewis acid catalysts in organic synthesis
Lewis acid catalysts enable various organic transformations including carbon-carbon bond formation, rearrangements, and functional group modifications. These catalysts activate carbonyl compounds, alkenes, and other functional groups by coordinating with electron-rich sites. They can promote reactions such as Diels-Alder cycloadditions, aldol condensations, and Michael additions with high efficiency and selectivity. The choice of Lewis acid catalyst significantly influences reaction pathways and product distributions in complex organic syntheses.Expand Specific Solutions05 Novel Lewis acid catalyst structures and compositions
Research on novel Lewis acid catalyst structures focuses on developing catalysts with enhanced activity, selectivity, and stability. These innovations include bimetallic systems, organometallic complexes, and nanostructured materials with Lewis acidic properties. Modified Lewis acids with tailored electronic and steric properties can catalyze challenging transformations under milder conditions. Advanced characterization techniques help understand structure-activity relationships, enabling rational design of next-generation Lewis acid catalysts for specific applications.Expand Specific Solutions
Leading Companies and Research Institutions
The Lewis Acid Catalysts for Industrial Synthesis market is currently in a growth phase, with increasing demand driven by sustainable chemistry initiatives and process efficiency requirements. The global market size is estimated at $3-4 billion, expected to grow at 5-7% annually through 2028. Technologically, the field shows varying maturity levels, with companies like BASF, Dow Global Technologies, and ExxonMobil Chemical Patents leading commercial applications through extensive patent portfolios. Japanese firms including Tosoh Corp., AGC, and Asahi Kasei have established strong positions in specialty applications, while academic-industrial partnerships from institutions like Zhejiang University and North Carolina State University are advancing next-generation catalysts. Chinese players such as China Petroleum & Chemical Corp. are rapidly expanding their technological capabilities, particularly in petrochemical applications.
BASF Corp.
Technical Solution: BASF has developed a comprehensive portfolio of Lewis acid catalysts, particularly focusing on metal-organic frameworks (MOFs) with tunable Lewis acidity. Their CathoFin™ technology incorporates aluminum and iron-based Lewis acid sites into heterogeneous catalysts for industrial applications. BASF's catalysts feature precisely engineered pore structures that enhance selectivity in complex organic transformations. Their proprietary immobilization techniques allow for the creation of supported Lewis acid catalysts with exceptional stability under industrial conditions, enabling continuous flow processes with catalyst lifetimes exceeding 5,000 hours in some applications. BASF has also pioneered water-tolerant Lewis acid systems based on perfluorinated metal complexes that maintain activity in the presence of moisture, addressing a traditional limitation of Lewis acid catalysis.
Strengths: Exceptional catalyst stability and reusability in industrial settings; advanced manufacturing capabilities allowing precise control of Lewis acid site density and strength. Weaknesses: Higher production costs compared to traditional homogeneous Lewis acids; some systems require specialized handling due to air/moisture sensitivity.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary solid Lewis acid catalysts based on modified zeolites and mesoporous materials for petroleum refining and petrochemical synthesis. Their technology incorporates rare earth metals and transition metal species into hierarchical porous frameworks, creating multifunctional catalytic systems. Sinopec's catalysts feature optimized acid strength distribution, with controlled ratios of Lewis to Brønsted acid sites tailored for specific reactions like alkylation, isomerization, and oligomerization. Their ZRP-1 catalyst series demonstrates exceptional activity in the conversion of methanol to propylene with selectivity exceeding 70%, significantly higher than conventional catalysts. Sinopec has also pioneered regeneration protocols that restore catalyst activity after deactivation, extending operational lifetimes in industrial settings by up to 300% compared to previous generation catalysts.
Strengths: Extensive industrial implementation experience; cost-effective manufacturing processes; catalysts designed specifically for large-scale petrochemical applications. Weaknesses: Less versatile for fine chemical synthesis; intellectual property primarily focused on petroleum-related applications rather than broader chemical synthesis.
Key Patents and Scientific Breakthroughs
Lewis acid catalyst composition
PatentInactiveUS7084088B2
Innovation
- A Lewis acid catalyst composition using a mixed medium of fluorinated and non-fluorinated compounds, where the catalyst is a compound with specific perfluorinated and partially substituted hydrocarbon groups, enhancing solubility and allowing for rapid phase separation of the reaction mixture, facilitating easy recovery and continuous reaction processes.
Bismuth compounds containing perfluoroalkyl groups as Lewis acid catalysts
PatentActiveJP2018535817A
Innovation
- Development of bismuth compounds containing perfluoroalkyl groups, specifically Bi(CmF2m+1)X, where m represents 2, 3, or 4, and X is F, Cl, Br, or OSO2CF3, which are catalytically active and stable, allowing reactions to be performed in good yields with reduced catalyst amounts.
Environmental Impact and Green Chemistry Considerations
The environmental impact of Lewis acid catalysts in industrial synthesis represents a critical consideration in modern chemical manufacturing. Traditional Lewis acid catalysts such as aluminum chloride (AlCl₃), boron trifluoride (BF₃), and titanium tetrachloride (TiCl₄) have historically posed significant environmental challenges. These conventional catalysts often require stoichiometric quantities, generate substantial waste streams, and involve hazardous handling procedures that increase environmental risk profiles across manufacturing operations.
Recent advancements in green chemistry have driven the development of environmentally benign Lewis acid catalysts. Solid supported Lewis acids, including zeolites, clays, and metal-organic frameworks (MOFs), offer substantial environmental benefits through their recyclability and reduced waste generation. These heterogeneous systems facilitate easier separation from reaction mixtures, dramatically decreasing the environmental footprint associated with catalyst recovery and disposal processes.
Water-compatible Lewis acids represent another significant environmental innovation. Catalysts such as scandium triflate [Sc(OTf)₃] and lanthanide triflates can operate effectively in aqueous media, eliminating the need for environmentally harmful organic solvents. This advancement aligns with green chemistry principles by reducing volatile organic compound (VOC) emissions and minimizing the environmental impact of solvent disposal.
Life cycle assessment (LCA) studies of Lewis acid catalyzed processes reveal substantial environmental improvements when implementing newer catalytic systems. Quantitative analyses demonstrate reductions in carbon footprint by 30-60% compared to traditional methods, with corresponding decreases in ecotoxicity indicators and resource depletion metrics. These environmental benefits often translate to economic advantages through reduced waste treatment costs and improved regulatory compliance.
Regulatory frameworks increasingly influence catalyst selection in industrial synthesis. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar regulations worldwide have accelerated the transition toward greener Lewis acid catalysts. Industries must now consider end-of-life management strategies for catalytic materials, including recycling protocols and safe disposal methodologies that prevent environmental contamination.
Emerging research focuses on developing bio-based Lewis acid catalysts derived from renewable resources. Metal complexes incorporating ligands from biomass sources show promising catalytic activity while reducing dependence on petrochemical feedstocks. Additionally, the integration of continuous flow technologies with environmentally benign Lewis acids enables process intensification, reducing energy requirements and enhancing atom economy in industrial synthesis operations.
Recent advancements in green chemistry have driven the development of environmentally benign Lewis acid catalysts. Solid supported Lewis acids, including zeolites, clays, and metal-organic frameworks (MOFs), offer substantial environmental benefits through their recyclability and reduced waste generation. These heterogeneous systems facilitate easier separation from reaction mixtures, dramatically decreasing the environmental footprint associated with catalyst recovery and disposal processes.
Water-compatible Lewis acids represent another significant environmental innovation. Catalysts such as scandium triflate [Sc(OTf)₃] and lanthanide triflates can operate effectively in aqueous media, eliminating the need for environmentally harmful organic solvents. This advancement aligns with green chemistry principles by reducing volatile organic compound (VOC) emissions and minimizing the environmental impact of solvent disposal.
Life cycle assessment (LCA) studies of Lewis acid catalyzed processes reveal substantial environmental improvements when implementing newer catalytic systems. Quantitative analyses demonstrate reductions in carbon footprint by 30-60% compared to traditional methods, with corresponding decreases in ecotoxicity indicators and resource depletion metrics. These environmental benefits often translate to economic advantages through reduced waste treatment costs and improved regulatory compliance.
Regulatory frameworks increasingly influence catalyst selection in industrial synthesis. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe and similar regulations worldwide have accelerated the transition toward greener Lewis acid catalysts. Industries must now consider end-of-life management strategies for catalytic materials, including recycling protocols and safe disposal methodologies that prevent environmental contamination.
Emerging research focuses on developing bio-based Lewis acid catalysts derived from renewable resources. Metal complexes incorporating ligands from biomass sources show promising catalytic activity while reducing dependence on petrochemical feedstocks. Additionally, the integration of continuous flow technologies with environmentally benign Lewis acids enables process intensification, reducing energy requirements and enhancing atom economy in industrial synthesis operations.
Scalability and Economic Feasibility Assessment
The scalability of Lewis acid catalysts from laboratory to industrial scale represents a critical factor in their commercial viability. Current industrial implementations demonstrate varying degrees of success, with aluminum chloride (AlCl3) and boron trifluoride (BF3) showing excellent scalability in petrochemical processes despite handling challenges. These traditional catalysts benefit from established engineering solutions and decades of process optimization, allowing for cost-effective large-scale operations.
Economic analysis reveals that catalyst cost typically constitutes 2-8% of total production expenses in fine chemical synthesis, though this percentage can rise significantly for precious metal-based Lewis acids. The total cost consideration must include not only the initial catalyst price but also regeneration costs, equipment corrosion expenses, and waste treatment requirements. Notably, heterogeneous Lewis acid catalysts often present superior economic profiles due to their recyclability, despite higher initial investment costs.
Infrastructure requirements vary substantially across different Lewis acid systems. Homogeneous catalysts generally demand less specialized equipment but incur higher downstream separation costs. Conversely, heterogeneous systems require more sophisticated reactor designs but offer simplified product isolation. Recent advances in continuous flow processing with supported Lewis acids have demonstrated promising economic returns by reducing reactor volumes by up to 80% while maintaining equivalent throughput.
Risk assessment indicates that volatile Lewis acids like aluminum chloride present significant safety concerns that translate to higher operational costs through specialized containment systems and rigorous safety protocols. The economic impact of these safety measures can add 15-25% to overall production costs compared to more stable catalyst systems. Additionally, regulatory compliance costs for hazardous Lewis acids continue to increase, particularly in Europe and North America.
Emerging supported Lewis acid technologies show promising economic profiles through extended catalyst lifetimes and reduced waste generation. Recent industrial case studies demonstrate that immobilized scandium triflate catalysts, despite higher initial costs, deliver superior return on investment through 20-30 reaction cycles without significant activity loss. Similarly, metal-organic framework (MOF) supported Lewis acids are approaching economic viability with current production costs decreasing by approximately 40% over the past five years.
The economic feasibility of Lewis acid catalysts ultimately depends on the specific application, with bulk chemical processes demanding extremely cost-effective solutions while fine chemical and pharmaceutical applications can tolerate higher catalyst costs when offset by improved selectivity and reduced purification requirements. Future economic viability will be significantly influenced by continued innovations in catalyst recovery technologies and the development of more stable, longer-lasting Lewis acid systems.
Economic analysis reveals that catalyst cost typically constitutes 2-8% of total production expenses in fine chemical synthesis, though this percentage can rise significantly for precious metal-based Lewis acids. The total cost consideration must include not only the initial catalyst price but also regeneration costs, equipment corrosion expenses, and waste treatment requirements. Notably, heterogeneous Lewis acid catalysts often present superior economic profiles due to their recyclability, despite higher initial investment costs.
Infrastructure requirements vary substantially across different Lewis acid systems. Homogeneous catalysts generally demand less specialized equipment but incur higher downstream separation costs. Conversely, heterogeneous systems require more sophisticated reactor designs but offer simplified product isolation. Recent advances in continuous flow processing with supported Lewis acids have demonstrated promising economic returns by reducing reactor volumes by up to 80% while maintaining equivalent throughput.
Risk assessment indicates that volatile Lewis acids like aluminum chloride present significant safety concerns that translate to higher operational costs through specialized containment systems and rigorous safety protocols. The economic impact of these safety measures can add 15-25% to overall production costs compared to more stable catalyst systems. Additionally, regulatory compliance costs for hazardous Lewis acids continue to increase, particularly in Europe and North America.
Emerging supported Lewis acid technologies show promising economic profiles through extended catalyst lifetimes and reduced waste generation. Recent industrial case studies demonstrate that immobilized scandium triflate catalysts, despite higher initial costs, deliver superior return on investment through 20-30 reaction cycles without significant activity loss. Similarly, metal-organic framework (MOF) supported Lewis acids are approaching economic viability with current production costs decreasing by approximately 40% over the past five years.
The economic feasibility of Lewis acid catalysts ultimately depends on the specific application, with bulk chemical processes demanding extremely cost-effective solutions while fine chemical and pharmaceutical applications can tolerate higher catalyst costs when offset by improved selectivity and reduced purification requirements. Future economic viability will be significantly influenced by continued innovations in catalyst recovery technologies and the development of more stable, longer-lasting Lewis acid systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!