Lewis Acid Deployment in Advanced Chemical Sensing
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Sensing Background and Objectives
Lewis acids have been fundamental components in chemical research since their conceptualization by Gilbert N. Lewis in 1923. These electron pair acceptors have evolved from simple theoretical constructs to sophisticated tools in modern analytical chemistry. The trajectory of Lewis acid application in sensing technologies has accelerated dramatically over the past two decades, driven by increasing demands for highly selective, sensitive, and rapid detection methods across multiple industries.
The evolution of Lewis acid-based sensing began with rudimentary colorimetric indicators and has progressed through several technological generations. Early applications primarily focused on pH detection and simple metal ion sensing. The 1980s and 1990s witnessed significant breakthroughs in molecular design principles that allowed for more specific Lewis acid-base interactions to be harnessed for sensing applications.
Recent advancements in materials science, particularly in the fields of nanomaterials and supramolecular chemistry, have revolutionized Lewis acid deployment in sensing platforms. The integration of Lewis acidic centers into polymers, metal-organic frameworks (MOFs), and quantum dots has expanded the toolkit available for chemical detection. These developments have enabled unprecedented sensitivity levels, often reaching parts-per-billion detection thresholds for various analytes.
The primary objective of current Lewis acid sensing research is to develop next-generation chemical sensors that combine high selectivity with real-time detection capabilities. This includes creating systems capable of distinguishing between structurally similar compounds in complex matrices—a persistent challenge in environmental monitoring, medical diagnostics, and industrial quality control. Additionally, researchers aim to design sensors that maintain performance under variable conditions such as fluctuating temperatures, humidity levels, and in the presence of interfering substances.
Another critical goal is miniaturization and integration with existing technologies. The push toward portable, field-deployable devices necessitates Lewis acid-based sensing elements that are stable, reproducible, and compatible with microfluidic platforms and electronic readout systems. This convergence of chemical sensing with digital technology represents a frontier in the field.
Sustainability considerations have also emerged as important objectives in Lewis acid sensing development. Current research emphasizes environmentally benign materials, reduced energy consumption during operation, and minimized waste generation throughout the sensor lifecycle. These green chemistry principles are increasingly guiding the design of new Lewis acid-based sensing technologies.
The ultimate aim of this technological trajectory is to establish versatile sensing platforms that can be rapidly adapted to emerging detection needs across sectors ranging from healthcare and environmental protection to national security and space exploration.
The evolution of Lewis acid-based sensing began with rudimentary colorimetric indicators and has progressed through several technological generations. Early applications primarily focused on pH detection and simple metal ion sensing. The 1980s and 1990s witnessed significant breakthroughs in molecular design principles that allowed for more specific Lewis acid-base interactions to be harnessed for sensing applications.
Recent advancements in materials science, particularly in the fields of nanomaterials and supramolecular chemistry, have revolutionized Lewis acid deployment in sensing platforms. The integration of Lewis acidic centers into polymers, metal-organic frameworks (MOFs), and quantum dots has expanded the toolkit available for chemical detection. These developments have enabled unprecedented sensitivity levels, often reaching parts-per-billion detection thresholds for various analytes.
The primary objective of current Lewis acid sensing research is to develop next-generation chemical sensors that combine high selectivity with real-time detection capabilities. This includes creating systems capable of distinguishing between structurally similar compounds in complex matrices—a persistent challenge in environmental monitoring, medical diagnostics, and industrial quality control. Additionally, researchers aim to design sensors that maintain performance under variable conditions such as fluctuating temperatures, humidity levels, and in the presence of interfering substances.
Another critical goal is miniaturization and integration with existing technologies. The push toward portable, field-deployable devices necessitates Lewis acid-based sensing elements that are stable, reproducible, and compatible with microfluidic platforms and electronic readout systems. This convergence of chemical sensing with digital technology represents a frontier in the field.
Sustainability considerations have also emerged as important objectives in Lewis acid sensing development. Current research emphasizes environmentally benign materials, reduced energy consumption during operation, and minimized waste generation throughout the sensor lifecycle. These green chemistry principles are increasingly guiding the design of new Lewis acid-based sensing technologies.
The ultimate aim of this technological trajectory is to establish versatile sensing platforms that can be rapidly adapted to emerging detection needs across sectors ranging from healthcare and environmental protection to national security and space exploration.
Market Analysis for Chemical Sensing Applications
The chemical sensing market is experiencing robust growth, driven by increasing demand across multiple sectors including environmental monitoring, healthcare diagnostics, industrial safety, and homeland security. The global chemical sensors market was valued at approximately 22 billion USD in 2021 and is projected to reach 33 billion USD by 2027, growing at a CAGR of around 7% during the forecast period. This growth trajectory is particularly significant for Lewis acid-based sensing technologies, which are gaining prominence due to their high selectivity and sensitivity.
Environmental monitoring represents one of the largest application segments, accounting for nearly 25% of the market share. The increasing focus on air and water quality monitoring, coupled with stringent environmental regulations worldwide, has created substantial demand for advanced chemical sensing solutions. Lewis acid-based sensors are particularly valuable in this sector due to their ability to detect trace amounts of pollutants and toxic compounds.
The healthcare and biomedical diagnostics sector is another rapidly expanding market for chemical sensing technologies. The growing prevalence of chronic diseases and the shift towards personalized medicine have accelerated the adoption of point-of-care testing devices, many of which incorporate chemical sensing elements. Lewis acid-based sensors show promising applications in detecting biomarkers, monitoring glucose levels, and analyzing breath compounds for disease diagnosis.
Industrial safety applications constitute a significant market segment, with chemical sensors being deployed for leak detection, process monitoring, and worker safety. The oil and gas, chemical manufacturing, and mining industries are major consumers of these technologies. Lewis acid-based sensors offer advantages in harsh industrial environments due to their stability and resistance to interference.
The Asia-Pacific region is emerging as the fastest-growing market for chemical sensing technologies, with China, Japan, and South Korea leading the development and adoption of advanced sensing solutions. North America and Europe remain significant markets, primarily driven by stringent regulatory frameworks and substantial R&D investments.
Key market trends include miniaturization of sensing devices, integration with IoT platforms, and the development of multi-parameter sensing arrays. The shift towards wearable and portable sensing devices is creating new opportunities for Lewis acid-based sensing technologies, particularly in personal health monitoring and environmental surveillance applications.
Customer requirements are increasingly focused on real-time monitoring capabilities, low power consumption, extended sensor lifetime, and seamless data integration. These demands are shaping the development trajectory of Lewis acid-based chemical sensing technologies, pushing researchers and manufacturers to develop more efficient, durable, and user-friendly solutions.
Environmental monitoring represents one of the largest application segments, accounting for nearly 25% of the market share. The increasing focus on air and water quality monitoring, coupled with stringent environmental regulations worldwide, has created substantial demand for advanced chemical sensing solutions. Lewis acid-based sensors are particularly valuable in this sector due to their ability to detect trace amounts of pollutants and toxic compounds.
The healthcare and biomedical diagnostics sector is another rapidly expanding market for chemical sensing technologies. The growing prevalence of chronic diseases and the shift towards personalized medicine have accelerated the adoption of point-of-care testing devices, many of which incorporate chemical sensing elements. Lewis acid-based sensors show promising applications in detecting biomarkers, monitoring glucose levels, and analyzing breath compounds for disease diagnosis.
Industrial safety applications constitute a significant market segment, with chemical sensors being deployed for leak detection, process monitoring, and worker safety. The oil and gas, chemical manufacturing, and mining industries are major consumers of these technologies. Lewis acid-based sensors offer advantages in harsh industrial environments due to their stability and resistance to interference.
The Asia-Pacific region is emerging as the fastest-growing market for chemical sensing technologies, with China, Japan, and South Korea leading the development and adoption of advanced sensing solutions. North America and Europe remain significant markets, primarily driven by stringent regulatory frameworks and substantial R&D investments.
Key market trends include miniaturization of sensing devices, integration with IoT platforms, and the development of multi-parameter sensing arrays. The shift towards wearable and portable sensing devices is creating new opportunities for Lewis acid-based sensing technologies, particularly in personal health monitoring and environmental surveillance applications.
Customer requirements are increasingly focused on real-time monitoring capabilities, low power consumption, extended sensor lifetime, and seamless data integration. These demands are shaping the development trajectory of Lewis acid-based chemical sensing technologies, pushing researchers and manufacturers to develop more efficient, durable, and user-friendly solutions.
Current Lewis Acid Technology Landscape
Lewis acids have emerged as critical components in modern chemical sensing technologies, with applications spanning environmental monitoring, medical diagnostics, industrial quality control, and security screening. The current landscape of Lewis acid technology demonstrates significant advancements in both fundamental understanding and practical applications, particularly in sensing platforms.
Traditional Lewis acid-based sensors have primarily relied on simple coordination chemistry, where the Lewis acid acts as an electron pair acceptor to form adducts with analyte molecules containing electron-donating groups. This interaction typically produces detectable changes in optical, electrical, or mass-sensitive properties. However, recent developments have shifted toward more sophisticated and selective sensing mechanisms.
Metal-organic frameworks (MOFs) incorporating Lewis acidic metal centers represent one of the most promising current technologies. These materials offer exceptional surface areas and tunable pore structures, enabling highly selective detection of target molecules even in complex mixtures. Companies like BASF and MOF Technologies have commercialized several Lewis acid MOF platforms specifically designed for sensing applications, with detection limits reaching parts-per-billion levels for certain volatile organic compounds.
Polymer-supported Lewis acids constitute another significant segment of the current technology landscape. These materials combine the advantages of homogeneous Lewis acid catalysis with the practical benefits of heterogeneous systems. Notable examples include perfluorinated polymers functionalized with boron-based Lewis acids, which demonstrate remarkable stability in harsh environments while maintaining high sensitivity to nucleophilic analytes.
Nanostructured Lewis acid sensors represent the cutting edge of current technology. Quantum dots, nanoparticles, and two-dimensional materials (like MXenes) functionalized with Lewis acidic groups have demonstrated unprecedented sensitivity and response times. These nanomaterials often exploit quantum confinement effects to amplify the sensing signal, enabling detection at the single-molecule level in some cases.
Microfluidic and lab-on-chip devices incorporating Lewis acid sensing elements have gained significant traction in point-of-care diagnostics. These integrated systems combine sample preparation, sensing, and signal processing in compact formats. Companies like Illumina and Thermo Fisher Scientific have invested heavily in developing such platforms for rapid medical diagnostics and environmental monitoring.
Computational design has revolutionized Lewis acid sensor development, with machine learning algorithms now capable of predicting Lewis acid-analyte interactions with remarkable accuracy. This approach has accelerated the discovery of novel Lewis acidic materials with tailored selectivity profiles, reducing development time from years to months in some cases.
Despite these advances, challenges remain in achieving long-term stability, reducing cross-sensitivity, and lowering production costs. The field continues to evolve rapidly, with increasing focus on sustainable materials and miniaturization for portable applications.
Traditional Lewis acid-based sensors have primarily relied on simple coordination chemistry, where the Lewis acid acts as an electron pair acceptor to form adducts with analyte molecules containing electron-donating groups. This interaction typically produces detectable changes in optical, electrical, or mass-sensitive properties. However, recent developments have shifted toward more sophisticated and selective sensing mechanisms.
Metal-organic frameworks (MOFs) incorporating Lewis acidic metal centers represent one of the most promising current technologies. These materials offer exceptional surface areas and tunable pore structures, enabling highly selective detection of target molecules even in complex mixtures. Companies like BASF and MOF Technologies have commercialized several Lewis acid MOF platforms specifically designed for sensing applications, with detection limits reaching parts-per-billion levels for certain volatile organic compounds.
Polymer-supported Lewis acids constitute another significant segment of the current technology landscape. These materials combine the advantages of homogeneous Lewis acid catalysis with the practical benefits of heterogeneous systems. Notable examples include perfluorinated polymers functionalized with boron-based Lewis acids, which demonstrate remarkable stability in harsh environments while maintaining high sensitivity to nucleophilic analytes.
Nanostructured Lewis acid sensors represent the cutting edge of current technology. Quantum dots, nanoparticles, and two-dimensional materials (like MXenes) functionalized with Lewis acidic groups have demonstrated unprecedented sensitivity and response times. These nanomaterials often exploit quantum confinement effects to amplify the sensing signal, enabling detection at the single-molecule level in some cases.
Microfluidic and lab-on-chip devices incorporating Lewis acid sensing elements have gained significant traction in point-of-care diagnostics. These integrated systems combine sample preparation, sensing, and signal processing in compact formats. Companies like Illumina and Thermo Fisher Scientific have invested heavily in developing such platforms for rapid medical diagnostics and environmental monitoring.
Computational design has revolutionized Lewis acid sensor development, with machine learning algorithms now capable of predicting Lewis acid-analyte interactions with remarkable accuracy. This approach has accelerated the discovery of novel Lewis acidic materials with tailored selectivity profiles, reducing development time from years to months in some cases.
Despite these advances, challenges remain in achieving long-term stability, reducing cross-sensitivity, and lowering production costs. The field continues to evolve rapidly, with increasing focus on sustainable materials and miniaturization for portable applications.
Current Lewis Acid Deployment Methodologies
01 Lewis acid-based chemical sensors for gas detection
Lewis acids can be utilized in chemical sensors for detecting various gases and volatile compounds. These sensors work by forming complexes with target analytes that have Lewis base properties, resulting in measurable changes in electrical, optical, or other physical properties. The sensitivity and selectivity of these sensors can be tuned by modifying the Lewis acid structure or incorporating them into various substrates or matrices.- Lewis acid-based chemical sensors for gas detection: Lewis acids can be utilized in chemical sensors for detecting various gases. These sensors work by forming complexes with target gas molecules, which changes the electronic properties of the sensing material. This change can be measured through various methods such as electrical conductivity, optical properties, or electrochemical responses. The sensitivity and selectivity of these sensors can be tuned by modifying the Lewis acid structure or incorporating them into composite materials.
- Fluorescent and luminescent Lewis acid sensors: Lewis acids can be incorporated into fluorescent or luminescent compounds to create chemical sensors. When these compounds interact with analytes, changes in their fluorescence or luminescence properties occur, allowing for detection and quantification. These optical changes may include shifts in emission wavelength, intensity changes, or lifetime alterations. Such sensors are particularly useful for detecting analytes in biological systems, environmental monitoring, and industrial applications.
- Metal-organic frameworks with Lewis acid sites for sensing: Metal-organic frameworks (MOFs) containing Lewis acid sites can function as effective chemical sensors. The porous structure of MOFs provides high surface area and tunable pore sizes, while the Lewis acid sites offer selective binding to target molecules. These materials can detect various analytes through changes in their optical, electrical, or structural properties upon analyte binding. The modular nature of MOFs allows for customization of sensing properties through selection of metal centers and organic linkers.
- Electrochemical sensors based on Lewis acid-base interactions: Electrochemical sensors utilizing Lewis acid-base interactions can detect various analytes through changes in electrical properties. These sensors typically incorporate Lewis acid moieties on electrode surfaces or within conductive polymers. When target molecules interact with the Lewis acid sites, changes in electron transfer kinetics, impedance, or redox potentials occur, generating measurable electrical signals. This approach enables the development of sensitive and selective sensors for environmental pollutants, biological molecules, and industrial chemicals.
- Lewis acid catalysts with sensing capabilities: Lewis acid catalysts can be designed with dual functionality as both catalysts and sensors. These materials can catalyze specific reactions while simultaneously providing information about reaction progress or the presence of certain compounds through detectable changes in their properties. The sensing mechanism may involve changes in color, fluorescence, or electrical properties when the Lewis acid interacts with reactants, products, or other species in the reaction mixture. This approach enables real-time monitoring of chemical processes.
02 Fluorescent and luminescent Lewis acid sensors
Lewis acids can be incorporated into fluorescent or luminescent compounds to create highly sensitive chemical sensors. When these Lewis acid-containing molecules interact with target analytes, they exhibit changes in their emission properties such as wavelength shifts, intensity changes, or lifetime alterations. These optical changes provide a convenient and sensitive method for detecting and quantifying various chemical species in solution or gas phase.Expand Specific Solutions03 Metal-organic frameworks with Lewis acid sites for sensing
Metal-organic frameworks (MOFs) containing Lewis acid metal centers can function as effective chemical sensors. These porous materials combine high surface area with tunable Lewis acidity, allowing for selective interaction with target molecules. The sensing mechanism typically involves changes in the framework's optical properties, conductivity, or mass upon analyte binding to the Lewis acid sites, enabling detection of various compounds including gases, volatile organics, and ions.Expand Specific Solutions04 Electrochemical sensors based on Lewis acid-base interactions
Electrochemical sensors utilizing Lewis acid-base interactions can detect various analytes through changes in electrical properties. These sensors typically incorporate Lewis acidic components on electrode surfaces or within conductive polymers. When target molecules interact with these Lewis acid sites, the resulting complexation alters electron transfer processes, impedance, or redox potentials, generating measurable electrical signals proportional to analyte concentration.Expand Specific Solutions05 Lewis acid-functionalized nanomaterials for sensing applications
Nanomaterials functionalized with Lewis acidic groups offer enhanced sensing capabilities due to their high surface area and unique physical properties. These materials include modified nanoparticles, quantum dots, carbon nanotubes, and graphene derivatives. The incorporation of Lewis acid moieties enables selective binding of target analytes, while the nanomaterial platform provides signal transduction mechanisms through changes in optical, electrical, or magnetic properties, resulting in highly sensitive and selective chemical sensors.Expand Specific Solutions
Leading Companies in Lewis Acid Sensing
The Lewis acid sensing technology market is currently in a growth phase, characterized by increasing applications in chemical detection and monitoring systems. The market size is expanding steadily, driven by demand in pharmaceutical research, environmental monitoring, and industrial process control. Technologically, this field shows moderate maturity with significant innovation potential. Leading players demonstrate varying levels of advancement: Novartis AG and California Institute of Technology are pioneering research applications in pharmaceutical sensing, while Japan Science & Technology Agency and Centre National de la Recherche Scientifique lead in fundamental research. Companies like BASF Corp. and Mitsubishi Gas Chemical are developing industrial applications, with Micron Technology exploring semiconductor-related implementations. University collaborations with Idaho Research Foundation and Massachusetts Institute of Technology are accelerating innovation through cross-disciplinary approaches.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has pioneered innovative Lewis acid-based chemical sensing technologies through their development of lanthanide-based coordination polymers with tunable Lewis acidity. Their approach utilizes europium and terbium complexes incorporated into porous frameworks that exhibit dramatic luminescence changes upon interaction with target analytes. The sensing mechanism relies on the displacement of coordinated water molecules by analytes at Lewis acidic metal centers, resulting in characteristic changes in emission spectra that can be correlated to specific compounds and concentrations. CNRS researchers have optimized these materials for detecting oxygen-containing volatile organic compounds with exceptional selectivity, even in complex mixtures. Their recent advancements include the development of 3D-printable sensor composites that maintain the sensing capabilities of the active materials while enabling custom-designed sensor geometries for specific applications. The technology has been successfully demonstrated for indoor air quality monitoring and early detection of food spoilage through the sensing of aldehyde and ketone compounds.
Strengths: Exceptional selectivity for oxygen-containing compounds; visual readout capability without complex instrumentation; long shelf-life and stability. Weaknesses: Limited response to non-polar analytes; potential for signal quenching in certain environments; slower response time compared to electronic sensors.
California Institute of Technology
Technical Solution: California Institute of Technology has developed innovative Lewis acid-based chemical sensing platforms utilizing metal-organic frameworks (MOFs) with tunable Lewis acidic sites. Their approach incorporates lanthanide and transition metal centers into MOF structures to create highly selective sensors for volatile organic compounds and environmental pollutants. The technology employs luminescence-based detection mechanisms where the interaction between Lewis acidic sites and target analytes produces measurable spectral shifts and intensity changes. Caltech researchers have demonstrated detection limits in the parts-per-billion range for formaldehyde and other carbonyl compounds through their proprietary signal amplification techniques. Their recent advancements include the development of portable, real-time monitoring devices that integrate these Lewis acid sensors with wireless communication capabilities for environmental and industrial applications.
Strengths: Exceptional sensitivity and selectivity for specific analytes; highly customizable platform through metal center substitution; non-destructive detection method. Weaknesses: Potential interference from humidity in real-world environments; higher manufacturing costs compared to conventional sensors; requires periodic recalibration for optimal performance.
Key Patents in Lewis Acid Sensing Technology
Dissociating agents, formulations and methods providing enhanced solubility of fluorides
PatentWO2008105916A2
Innovation
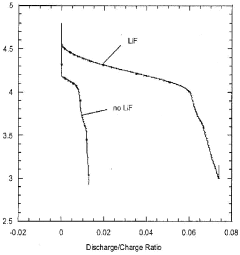
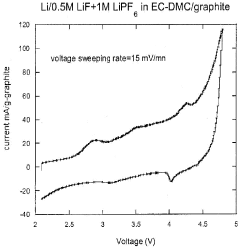
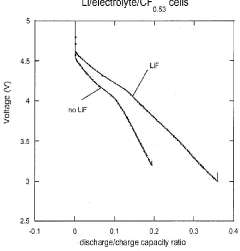
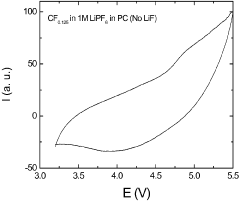
- Incorporation of dissociating agents such as Lewis acids, Lewis bases, anion receptors, and cation receptors into lithium battery electrolytes to enhance the solubility and stability of lithium salts, particularly lithium fluoride, in non-aqueous organic solvents, facilitating higher ionic conductivity and improved electrochemical performance.
Dissociating agents, formulations and methods providing enhanced solubility of fluorides
PatentActiveUS20080171268A1
Innovation
- Incorporation of dissociating agents such as Lewis acids, Lewis bases, anion receptors, and cation receptors into the electrolyte formulations to enhance the dissolution and solubility of lithium salts, particularly lithium fluoride, in nonaqueous organic solvents, thereby increasing ionic conductivity and stability.
Environmental Impact Assessment
The deployment of Lewis acids in advanced chemical sensing technologies presents significant environmental considerations that must be thoroughly evaluated. These compounds, while valuable for their sensing capabilities, can potentially impact various environmental compartments including air, water, soil, and biological systems. The environmental fate of Lewis acids depends largely on their specific chemical composition, with some compounds demonstrating persistence in environmental matrices while others undergo rapid degradation or transformation.
Water systems are particularly vulnerable to impacts from Lewis acid-based sensors. When improperly disposed of or in the event of leakage, these compounds can alter aquatic pH levels and potentially form complexes with naturally occurring organic matter. Studies have shown that certain metal-based Lewis acids can bioaccumulate in aquatic organisms, potentially disrupting ecosystem functions at multiple trophic levels.
Atmospheric emissions during the manufacturing, operation, or disposal of Lewis acid-based sensing devices represent another environmental concern. Volatile Lewis acid compounds may contribute to air quality degradation, particularly in indoor environments where chemical sensing applications are increasingly common. The potential for these compounds to participate in atmospheric chemistry reactions, possibly forming secondary pollutants, requires careful assessment.
Soil contamination presents a third pathway of environmental impact, particularly for in-situ sensing applications deployed in terrestrial environments. The mobility and persistence of Lewis acids in soil matrices vary significantly based on soil properties including pH, organic content, and mineral composition. Long-term monitoring studies have indicated potential for certain Lewis acid compounds to alter soil microbial community structures.
Life cycle assessment (LCA) methodologies reveal that environmental impacts extend beyond the operational phase of Lewis acid-based sensors. Raw material extraction, particularly for rare earth elements often used in advanced sensing technologies, can result in significant environmental disruption including habitat destruction and resource depletion. Manufacturing processes typically involve energy-intensive steps and the use of additional hazardous substances.
Mitigation strategies are emerging to address these environmental concerns. Green chemistry approaches are being applied to develop Lewis acid sensors with reduced environmental footprints, including the use of biodegradable substrates and less toxic Lewis acid variants. Closed-loop recycling systems for recovering valuable components from end-of-life sensors are showing promise for reducing waste streams and conserving resources.
Regulatory frameworks governing the environmental aspects of Lewis acid deployment vary considerably across jurisdictions, creating challenges for global implementation of these technologies. Harmonization of standards and adoption of precautionary principles in emerging markets represent important steps toward ensuring environmentally responsible deployment of these advanced chemical sensing technologies.
Water systems are particularly vulnerable to impacts from Lewis acid-based sensors. When improperly disposed of or in the event of leakage, these compounds can alter aquatic pH levels and potentially form complexes with naturally occurring organic matter. Studies have shown that certain metal-based Lewis acids can bioaccumulate in aquatic organisms, potentially disrupting ecosystem functions at multiple trophic levels.
Atmospheric emissions during the manufacturing, operation, or disposal of Lewis acid-based sensing devices represent another environmental concern. Volatile Lewis acid compounds may contribute to air quality degradation, particularly in indoor environments where chemical sensing applications are increasingly common. The potential for these compounds to participate in atmospheric chemistry reactions, possibly forming secondary pollutants, requires careful assessment.
Soil contamination presents a third pathway of environmental impact, particularly for in-situ sensing applications deployed in terrestrial environments. The mobility and persistence of Lewis acids in soil matrices vary significantly based on soil properties including pH, organic content, and mineral composition. Long-term monitoring studies have indicated potential for certain Lewis acid compounds to alter soil microbial community structures.
Life cycle assessment (LCA) methodologies reveal that environmental impacts extend beyond the operational phase of Lewis acid-based sensors. Raw material extraction, particularly for rare earth elements often used in advanced sensing technologies, can result in significant environmental disruption including habitat destruction and resource depletion. Manufacturing processes typically involve energy-intensive steps and the use of additional hazardous substances.
Mitigation strategies are emerging to address these environmental concerns. Green chemistry approaches are being applied to develop Lewis acid sensors with reduced environmental footprints, including the use of biodegradable substrates and less toxic Lewis acid variants. Closed-loop recycling systems for recovering valuable components from end-of-life sensors are showing promise for reducing waste streams and conserving resources.
Regulatory frameworks governing the environmental aspects of Lewis acid deployment vary considerably across jurisdictions, creating challenges for global implementation of these technologies. Harmonization of standards and adoption of precautionary principles in emerging markets represent important steps toward ensuring environmentally responsible deployment of these advanced chemical sensing technologies.
Sensor Miniaturization Challenges
The miniaturization of chemical sensors incorporating Lewis acid technology presents significant engineering challenges that must be addressed to enable widespread deployment in portable and embedded applications. Current sensor platforms utilizing Lewis acid-based detection mechanisms typically require substantial space for sample handling, reagent storage, and detection components, limiting their integration into compact devices.
One primary challenge involves the reduction of reaction chambers while maintaining sensitivity and selectivity. Traditional Lewis acid sensors often require relatively large volumes of analyte and reagent to achieve reliable detection thresholds. Miniaturizing these chambers introduces surface-to-volume ratio effects that can dramatically alter reaction kinetics and sensor performance characteristics.
Material compatibility issues become increasingly critical at smaller scales. The highly reactive nature of many Lewis acids necessitates specialized containment materials that maintain their structural and chemical integrity over time. Finding materials that are simultaneously compatible with Lewis acids, amenable to microfabrication techniques, and cost-effective remains problematic for widespread commercial implementation.
Power requirements represent another significant hurdle in sensor miniaturization. Many Lewis acid-based detection schemes require temperature control or other energy-intensive processes to maintain optimal reaction conditions. Developing low-power alternatives while preserving detection capabilities is essential for battery-operated portable devices and remote sensing applications.
Signal transduction mechanisms must also evolve to accommodate smaller form factors. Traditional optical detection methods often rely on specific path lengths and detection volumes that become challenging to maintain in miniaturized platforms. Alternative transduction approaches, such as impedance-based or field-effect sensing, show promise but require careful engineering to achieve comparable performance metrics.
Integration with microfluidic systems presents both opportunities and challenges. While microfluidics enables precise handling of small sample volumes, the integration of Lewis acid chemistry with these platforms requires addressing issues of channel fouling, reagent depletion, and long-term stability. Novel approaches combining digital microfluidics with solid-phase Lewis acid materials show potential for overcoming some of these limitations.
Manufacturing scalability represents a final critical challenge. Transitioning from laboratory prototypes to mass-produced miniaturized sensors requires production processes compatible with existing semiconductor or microelectromechanical systems (MEMS) fabrication techniques. Developing standardized approaches for incorporating Lewis acid chemistry into these established manufacturing paradigms remains an active area of research and development.
One primary challenge involves the reduction of reaction chambers while maintaining sensitivity and selectivity. Traditional Lewis acid sensors often require relatively large volumes of analyte and reagent to achieve reliable detection thresholds. Miniaturizing these chambers introduces surface-to-volume ratio effects that can dramatically alter reaction kinetics and sensor performance characteristics.
Material compatibility issues become increasingly critical at smaller scales. The highly reactive nature of many Lewis acids necessitates specialized containment materials that maintain their structural and chemical integrity over time. Finding materials that are simultaneously compatible with Lewis acids, amenable to microfabrication techniques, and cost-effective remains problematic for widespread commercial implementation.
Power requirements represent another significant hurdle in sensor miniaturization. Many Lewis acid-based detection schemes require temperature control or other energy-intensive processes to maintain optimal reaction conditions. Developing low-power alternatives while preserving detection capabilities is essential for battery-operated portable devices and remote sensing applications.
Signal transduction mechanisms must also evolve to accommodate smaller form factors. Traditional optical detection methods often rely on specific path lengths and detection volumes that become challenging to maintain in miniaturized platforms. Alternative transduction approaches, such as impedance-based or field-effect sensing, show promise but require careful engineering to achieve comparable performance metrics.
Integration with microfluidic systems presents both opportunities and challenges. While microfluidics enables precise handling of small sample volumes, the integration of Lewis acid chemistry with these platforms requires addressing issues of channel fouling, reagent depletion, and long-term stability. Novel approaches combining digital microfluidics with solid-phase Lewis acid materials show potential for overcoming some of these limitations.
Manufacturing scalability represents a final critical challenge. Transitioning from laboratory prototypes to mass-produced miniaturized sensors requires production processes compatible with existing semiconductor or microelectromechanical systems (MEMS) fabrication techniques. Developing standardized approaches for incorporating Lewis acid chemistry into these established manufacturing paradigms remains an active area of research and development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!