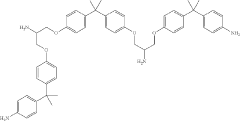
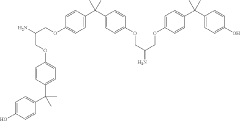
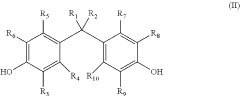
How to Correlate Lewis Acid and Reaction Selectivity?
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Chemistry Background and Objectives
Lewis acids have been fundamental components in organic chemistry since the early 20th century, following Gilbert N. Lewis's groundbreaking definition in 1923. These electron pair acceptors have evolved from simple catalysts to sophisticated reagents capable of directing complex chemical transformations with remarkable precision. The historical trajectory of Lewis acid chemistry reveals a progressive refinement in understanding structure-activity relationships, moving from empirical observations to mechanistic insights based on quantum chemical principles.
The field has witnessed significant milestones, including the development of Friedel-Crafts reactions using aluminum chloride, the emergence of organoboron chemistry pioneered by H.C. Brown, and the revolutionary asymmetric catalysis work by Sharpless and Noyori. Each advancement has expanded the application scope while simultaneously deepening theoretical understanding of Lewis acid behavior in reaction environments.
Current technological trends in Lewis acid chemistry focus on designing highly selective catalysts that can discriminate between similar functional groups or create specific stereochemical outcomes. The integration of computational modeling with experimental validation has accelerated this development, allowing for rational design approaches rather than traditional trial-and-error methodologies. Machine learning algorithms are increasingly being employed to predict Lewis acid performance across diverse reaction conditions.
The correlation between Lewis acid properties and reaction selectivity represents a critical frontier in synthetic chemistry. Understanding how subtle electronic and steric variations in Lewis acid structure translate to predictable reaction outcomes would enable unprecedented control over chemical transformations. This correlation encompasses multiple dimensions: chemoselectivity (preferential reaction at one functional group over others), regioselectivity (reaction at a specific position), and stereoselectivity (formation of specific stereoisomers).
The primary objective of this technical research is to establish quantifiable relationships between Lewis acid characteristics (such as LUMO energy, coordination geometry, steric parameters, and hardness/softness profiles) and observed reaction selectivity patterns. Secondary goals include developing predictive models that can guide Lewis acid selection for specific transformations, identifying novel Lewis acid scaffolds with enhanced selectivity profiles, and creating a standardized framework for evaluating and comparing Lewis acid performance across reaction classes.
This research aims to bridge the gap between theoretical understanding and practical application, ultimately enabling chemists to rationally select or design Lewis acids for specific synthetic challenges rather than relying on empirical precedent or extensive screening. Success in this domain would significantly impact pharmaceutical development, materials science, and fine chemical production by reducing development timelines and expanding the synthetic toolbox available to researchers.
The field has witnessed significant milestones, including the development of Friedel-Crafts reactions using aluminum chloride, the emergence of organoboron chemistry pioneered by H.C. Brown, and the revolutionary asymmetric catalysis work by Sharpless and Noyori. Each advancement has expanded the application scope while simultaneously deepening theoretical understanding of Lewis acid behavior in reaction environments.
Current technological trends in Lewis acid chemistry focus on designing highly selective catalysts that can discriminate between similar functional groups or create specific stereochemical outcomes. The integration of computational modeling with experimental validation has accelerated this development, allowing for rational design approaches rather than traditional trial-and-error methodologies. Machine learning algorithms are increasingly being employed to predict Lewis acid performance across diverse reaction conditions.
The correlation between Lewis acid properties and reaction selectivity represents a critical frontier in synthetic chemistry. Understanding how subtle electronic and steric variations in Lewis acid structure translate to predictable reaction outcomes would enable unprecedented control over chemical transformations. This correlation encompasses multiple dimensions: chemoselectivity (preferential reaction at one functional group over others), regioselectivity (reaction at a specific position), and stereoselectivity (formation of specific stereoisomers).
The primary objective of this technical research is to establish quantifiable relationships between Lewis acid characteristics (such as LUMO energy, coordination geometry, steric parameters, and hardness/softness profiles) and observed reaction selectivity patterns. Secondary goals include developing predictive models that can guide Lewis acid selection for specific transformations, identifying novel Lewis acid scaffolds with enhanced selectivity profiles, and creating a standardized framework for evaluating and comparing Lewis acid performance across reaction classes.
This research aims to bridge the gap between theoretical understanding and practical application, ultimately enabling chemists to rationally select or design Lewis acids for specific synthetic challenges rather than relying on empirical precedent or extensive screening. Success in this domain would significantly impact pharmaceutical development, materials science, and fine chemical production by reducing development timelines and expanding the synthetic toolbox available to researchers.
Market Applications of Lewis Acid Catalysis
Lewis acid catalysis has emerged as a cornerstone technology across multiple industrial sectors, with applications spanning from pharmaceuticals to materials science. The global market for Lewis acid catalysts was valued at approximately $2.3 billion in 2022 and is projected to grow at a compound annual growth rate of 6.7% through 2030, driven by increasing demand for efficient and selective chemical transformations.
In the pharmaceutical industry, Lewis acid catalysis enables the synthesis of complex drug molecules with precise stereochemistry. Companies like Pfizer, Novartis, and Merck have incorporated Lewis acid-mediated reactions into their manufacturing processes for various blockbuster drugs. The ability to control reaction selectivity using tailored Lewis acids has revolutionized the production of chiral pharmaceuticals, reducing waste and improving product purity.
The fine chemicals sector represents another significant market for Lewis acid catalysis. Manufacturers of fragrances, flavors, and specialty chemicals rely on Lewis acid-catalyzed reactions to produce high-value compounds with specific structural features. The market for these specialty chemicals exceeds $30 billion globally, with Lewis acid catalysis playing a crucial role in production efficiency.
Polymer chemistry has also benefited substantially from advances in Lewis acid catalysis. Metallocene catalysts, a specific class of Lewis acids, have transformed polyolefin production by enabling precise control over polymer architecture. This technology has created a multi-billion dollar market for specialty polymers with tailored properties for applications ranging from medical devices to automotive components.
The agrochemical industry utilizes Lewis acid catalysis in the synthesis of pesticides and herbicides, where reaction selectivity is paramount for producing effective and environmentally acceptable products. Companies like BASF, Bayer, and Syngenta have invested heavily in developing selective catalytic processes based on Lewis acid chemistry.
Emerging applications include green chemistry initiatives, where Lewis acids are being employed to valorize biomass and convert renewable feedstocks into platform chemicals. This sector is growing rapidly as sustainability concerns drive industrial innovation, with projected market growth exceeding 10% annually.
The electronics industry has also found valuable applications for Lewis acid catalysis in the production of semiconductor materials and specialty coatings. As devices continue to miniaturize, the demand for precisely engineered materials with specific electronic properties continues to grow, creating new opportunities for selective Lewis acid-catalyzed processes.
In the pharmaceutical industry, Lewis acid catalysis enables the synthesis of complex drug molecules with precise stereochemistry. Companies like Pfizer, Novartis, and Merck have incorporated Lewis acid-mediated reactions into their manufacturing processes for various blockbuster drugs. The ability to control reaction selectivity using tailored Lewis acids has revolutionized the production of chiral pharmaceuticals, reducing waste and improving product purity.
The fine chemicals sector represents another significant market for Lewis acid catalysis. Manufacturers of fragrances, flavors, and specialty chemicals rely on Lewis acid-catalyzed reactions to produce high-value compounds with specific structural features. The market for these specialty chemicals exceeds $30 billion globally, with Lewis acid catalysis playing a crucial role in production efficiency.
Polymer chemistry has also benefited substantially from advances in Lewis acid catalysis. Metallocene catalysts, a specific class of Lewis acids, have transformed polyolefin production by enabling precise control over polymer architecture. This technology has created a multi-billion dollar market for specialty polymers with tailored properties for applications ranging from medical devices to automotive components.
The agrochemical industry utilizes Lewis acid catalysis in the synthesis of pesticides and herbicides, where reaction selectivity is paramount for producing effective and environmentally acceptable products. Companies like BASF, Bayer, and Syngenta have invested heavily in developing selective catalytic processes based on Lewis acid chemistry.
Emerging applications include green chemistry initiatives, where Lewis acids are being employed to valorize biomass and convert renewable feedstocks into platform chemicals. This sector is growing rapidly as sustainability concerns drive industrial innovation, with projected market growth exceeding 10% annually.
The electronics industry has also found valuable applications for Lewis acid catalysis in the production of semiconductor materials and specialty coatings. As devices continue to miniaturize, the demand for precisely engineered materials with specific electronic properties continues to grow, creating new opportunities for selective Lewis acid-catalyzed processes.
Current Challenges in Lewis Acid Selectivity
Despite significant advancements in Lewis acid catalysis, several fundamental challenges persist in establishing reliable correlations between Lewis acid properties and reaction selectivity outcomes. The multifaceted nature of Lewis acid-substrate interactions creates complex reaction environments that are difficult to predict and control with precision. This complexity stems from the interplay of electronic, steric, and coordination effects that simultaneously influence reaction pathways.
One primary challenge is the quantification of Lewis acidity itself. While methods such as Gutmann-Beckett measurements, fluoride ion affinity, and computational approaches provide valuable insights, they often fail to capture the dynamic behavior of Lewis acids under actual reaction conditions. The Lewis acidity measured in isolation frequently differs from its effective strength when interacting with specific substrates in various solvents.
The solvent effect presents another significant obstacle. Solvation can dramatically alter Lewis acid strength and coordination geometry, leading to unpredictable changes in selectivity patterns. Polar solvents may coordinate to Lewis acids, effectively reducing their acidity and changing their selectivity profile. This solvent-dependent behavior makes it difficult to establish universal correlations applicable across different reaction media.
Counter-ion effects further complicate selectivity predictions. In many Lewis acid systems, especially those derived from metal salts, the counter-ion can participate in the reaction mechanism, either by stabilizing transition states or by altering the coordination sphere of the Lewis acidic center. These effects are often substrate-specific and difficult to predict a priori.
The dynamic nature of Lewis acid-substrate complexes poses additional challenges. Many reactions proceed through multiple equilibria involving different coordination modes, each potentially leading to different product distributions. Capturing these dynamic processes experimentally remains difficult, limiting our understanding of the actual reaction mechanisms.
Temperature dependence of selectivity represents another layer of complexity. Different activation barriers for competing pathways can lead to dramatically different selectivity profiles at varying temperatures. This temperature-dependent behavior often follows non-linear patterns that defy simple predictive models.
Perhaps most challenging is the substrate-specific nature of Lewis acid interactions. The same Lewis acid can exhibit completely different selectivity patterns with structurally similar substrates due to subtle electronic or steric differences. This specificity makes it difficult to develop general predictive frameworks that span diverse substrate classes.
Advanced computational methods offer promising approaches to address these challenges, but they still struggle with accurately modeling the full complexity of reaction environments, particularly in solution phase with explicit solvent molecules and counter-ions.
One primary challenge is the quantification of Lewis acidity itself. While methods such as Gutmann-Beckett measurements, fluoride ion affinity, and computational approaches provide valuable insights, they often fail to capture the dynamic behavior of Lewis acids under actual reaction conditions. The Lewis acidity measured in isolation frequently differs from its effective strength when interacting with specific substrates in various solvents.
The solvent effect presents another significant obstacle. Solvation can dramatically alter Lewis acid strength and coordination geometry, leading to unpredictable changes in selectivity patterns. Polar solvents may coordinate to Lewis acids, effectively reducing their acidity and changing their selectivity profile. This solvent-dependent behavior makes it difficult to establish universal correlations applicable across different reaction media.
Counter-ion effects further complicate selectivity predictions. In many Lewis acid systems, especially those derived from metal salts, the counter-ion can participate in the reaction mechanism, either by stabilizing transition states or by altering the coordination sphere of the Lewis acidic center. These effects are often substrate-specific and difficult to predict a priori.
The dynamic nature of Lewis acid-substrate complexes poses additional challenges. Many reactions proceed through multiple equilibria involving different coordination modes, each potentially leading to different product distributions. Capturing these dynamic processes experimentally remains difficult, limiting our understanding of the actual reaction mechanisms.
Temperature dependence of selectivity represents another layer of complexity. Different activation barriers for competing pathways can lead to dramatically different selectivity profiles at varying temperatures. This temperature-dependent behavior often follows non-linear patterns that defy simple predictive models.
Perhaps most challenging is the substrate-specific nature of Lewis acid interactions. The same Lewis acid can exhibit completely different selectivity patterns with structurally similar substrates due to subtle electronic or steric differences. This specificity makes it difficult to develop general predictive frameworks that span diverse substrate classes.
Advanced computational methods offer promising approaches to address these challenges, but they still struggle with accurately modeling the full complexity of reaction environments, particularly in solution phase with explicit solvent molecules and counter-ions.
Established Methods for Selectivity Control
01 Lewis acid catalysts for regioselective reactions
Lewis acid catalysts can be used to control the regioselectivity of chemical reactions by coordinating with specific functional groups and directing the reaction pathway. These catalysts can influence the electronic distribution in substrates, leading to preferential attack at specific sites. The selectivity can be tuned by varying the strength and steric properties of the Lewis acid, allowing for precise control over reaction outcomes in complex molecular transformations.- Lewis acid catalysts for regioselective reactions: Lewis acid catalysts can be used to control the regioselectivity of chemical reactions by coordinating with specific functional groups and directing the reaction pathway. These catalysts interact with electron-rich sites in substrates, influencing the position where reactions occur. The selectivity can be tuned by modifying the steric and electronic properties of the Lewis acid, allowing for precise control over reaction outcomes in complex organic transformations.
- Stereoselective synthesis using Lewis acids: Lewis acids play a crucial role in stereoselective synthesis by controlling the facial approach of reactants. By coordinating with carbonyl groups or other functional moieties, these catalysts create chiral environments that favor the formation of specific stereoisomers. The stereoselectivity can be enhanced by using chiral Lewis acids or by combining Lewis acids with chiral ligands, enabling the synthesis of enantiomerically pure compounds for pharmaceutical and fine chemical applications.
- Lewis acid-mediated C-C bond formation: Lewis acids are effective catalysts for carbon-carbon bond formation reactions, including Friedel-Crafts alkylations, acylations, and various coupling reactions. These catalysts activate electrophiles by increasing their reactivity toward nucleophilic attack. The selectivity of these reactions can be controlled by adjusting the Lewis acid strength, reaction temperature, and solvent conditions. This approach enables the construction of complex molecular frameworks with high efficiency and selectivity.
- Heterogeneous Lewis acid catalysts for selective transformations: Heterogeneous Lewis acid catalysts offer advantages in terms of recyclability, ease of separation, and enhanced selectivity for various chemical transformations. These catalysts can be supported on solid materials such as silica, alumina, or zeolites, providing unique reaction environments that influence selectivity. The surface properties and pore structures of these supports can be tailored to enhance the selectivity of Lewis acid-catalyzed reactions, making them valuable for industrial applications requiring high efficiency and minimal waste generation.
- Lewis acid strength and selectivity relationships: The strength of Lewis acids significantly impacts reaction selectivity, with stronger Lewis acids generally promoting faster reactions but potentially lower selectivity. By carefully tuning the Lewis acidity through ligand modification or metal center selection, chemists can optimize the balance between reactivity and selectivity. Temperature control, solvent effects, and additives can further modulate the effective Lewis acidity, providing additional tools for achieving desired selectivity in complex transformations. Understanding these relationships enables rational catalyst design for specific applications.
02 Stereoselective synthesis using Lewis acids
Lewis acids play a crucial role in stereoselective synthesis by controlling the facial approach of reactants. By forming coordination complexes with substrates, Lewis acids can create chiral environments that favor the formation of specific stereoisomers. This approach is particularly valuable in the synthesis of pharmaceuticals and natural products where stereochemistry is critical. The stereoselectivity can be enhanced by using chiral Lewis acids or by combining Lewis acids with chiral ligands.Expand Specific Solutions03 Lewis acid-mediated C-C bond formation
Lewis acids are effective catalysts for carbon-carbon bond formation reactions, including Friedel-Crafts alkylation, acylation, and various coupling reactions. The selectivity of these reactions can be controlled by the choice of Lewis acid, reaction conditions, and substrate structure. Lewis acids activate electrophiles by increasing their electrophilicity, thereby facilitating nucleophilic attack at specific positions. This approach enables the construction of complex molecular frameworks with high regioselectivity.Expand Specific Solutions04 Tuning Lewis acid strength for reaction control
The strength of Lewis acids can be systematically tuned to achieve optimal reaction selectivity. Stronger Lewis acids generally provide higher reactivity but may lead to decreased selectivity, while weaker Lewis acids often offer better selectivity at the cost of reduced reaction rates. By carefully selecting the metal center, ligands, and counterions, the Lewis acidity can be precisely adjusted to match the requirements of specific transformations. This approach allows for fine control over chemoselectivity, regioselectivity, and stereoselectivity in complex reactions.Expand Specific Solutions05 Lewis acid-substrate complexation for selective transformations
The formation of specific Lewis acid-substrate complexes is key to achieving selective transformations. By understanding the coordination preferences of different Lewis acids with various functional groups, chemists can design reactions where the Lewis acid selectively activates one functional group over others. This approach is particularly valuable in multifunctional molecules where selective activation of a specific site is required. The selectivity can be further enhanced by controlling reaction parameters such as temperature, solvent, and concentration.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Lewis acid-reaction selectivity correlation field is in a growth phase, with increasing market interest driven by pharmaceutical and chemical industries seeking more efficient catalytic processes. The technology is moderately mature, with academic institutions like California Institute of Technology, Zhejiang University of Technology, and Texas A&M University leading fundamental research, while companies including ExxonMobil Chemical Patents, Sinopec, and Firmenich SA focus on industrial applications. Pharmaceutical companies such as Gilead Sciences, Chugai Pharmaceutical, and Kyowa Kirin are leveraging this technology for drug development. The competitive landscape shows a balanced distribution between academic research pushing theoretical boundaries and industrial players implementing practical applications, with growing cross-sector collaborations accelerating innovation in selective chemical transformations.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has made groundbreaking contributions to understanding Lewis acid-controlled selectivity through innovative catalyst design and mechanistic investigations. Their researchers have developed bifunctional Lewis acid catalysts featuring precisely positioned secondary interaction sites that enforce substrate orientation, leading to exceptional levels of stereocontrol. Caltech's work on conformationally rigid metallocene-based Lewis acids has revealed how catalyst symmetry directly influences reaction stereoselectivity, with C2-symmetric complexes often providing superior enantioselectivity compared to C1 or C3 alternatives. Their studies have established quantitative correlations between Lewis acid metal-ligand bond distances and resulting selectivity patterns in various transformations. Caltech researchers pioneered the development of chiral scandium triflate complexes that achieve remarkable levels of enantioselectivity (often >98% ee) in challenging carbon-carbon bond-forming reactions through well-defined transition state organization.
Strengths: Exceptional ability to design highly selective catalysts based on fundamental understanding of transition state organization and weak secondary interactions. Their catalysts often achieve high selectivity at very low catalyst loadings (0.5-2 mol%). Weaknesses: Some of their most selective catalyst systems require air and moisture-sensitive handling techniques, limiting practical applications outside specialized laboratory settings.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed industrial-scale applications of Lewis acid catalysis for selective hydrocarbon transformations. Their research has focused on heterogeneous Lewis acid catalysts, particularly modified zeolites and metal-organic frameworks (MOFs), that can selectively activate specific C-H bonds in complex hydrocarbon mixtures. Sinopec has pioneered the development of hierarchical zeolite catalysts with precisely tuned Lewis acidity that achieve remarkable shape-selective transformations in petroleum refining processes. Their catalysts incorporate carefully positioned metal centers (particularly Al, Ga, and Zn) within zeolite frameworks to control reaction pathways. Sinopec researchers have established correlations between Lewis acid site density, acid strength distribution, and resulting product selectivity in processes like alkylation, isomerization, and cracking reactions. Their recent work has focused on developing regenerable Lewis acid catalysts that maintain selectivity over multiple reaction cycles in industrial settings, addressing a key challenge in large-scale applications.
Strengths: Exceptional expertise in developing industrially viable, heterogeneous Lewis acid catalysts that can operate at scale under demanding conditions. Their catalysts often demonstrate excellent stability and regenerability in continuous flow processes. Weaknesses: Their catalyst systems typically require higher temperatures (150-500°C) than homogeneous alternatives, potentially limiting applications with thermally sensitive substrates.
Key Mechanistic Studies and Computational Models
A cross-linkable ethylene polymer composition comprising epoxy-groups and a cross-linking agent
PatentActiveUS20210221989A1
Innovation
- An ethylene polymer composition incorporating epoxy groups and a cross-linking agent comprising organo-metallic Lewis acids, amino compounds, and hydroxyl compounds, allowing for effective cross-linking at moderate temperatures and short times, thereby reducing volatile by-products and eliminating the need for high peroxide amounts.
Methods of preparing quinolone analogs
PatentActiveEP1928887A1
Innovation
- A method involving the synthesis of compounds with specific formulas, such as (1), (2A), and (4A), by contacting compounds with leaving groups and bases in the presence of Lewis acids, to produce pharmaceutically acceptable salts, esters, and prodrugs that can interact with DNA quadruplexes and exhibit therapeutic effects.
Green Chemistry Implications
The integration of Lewis acid catalysis with green chemistry principles represents a significant frontier in sustainable reaction development. Lewis acids play a crucial role in enhancing reaction selectivity while potentially reducing energy requirements and waste generation. When properly selected and applied, these catalysts can operate under milder conditions than traditional methods, thereby decreasing the overall environmental footprint of chemical processes.
The environmental benefits of Lewis acid catalysis extend beyond energy efficiency. Many modern Lewis acid catalysts can be immobilized on solid supports, facilitating their recovery and reuse across multiple reaction cycles. This recyclability directly addresses one of green chemistry's core principles by minimizing waste generation and resource consumption. Furthermore, certain Lewis acids enable reactions in aqueous media or solvent-free conditions, eliminating the need for hazardous organic solvents.
Recent advances have demonstrated that tuning Lewis acid properties can dramatically improve atom economy in chemical transformations. By precisely controlling the coordination environment and electronic properties of Lewis acidic centers, chemists can design highly selective catalysts that minimize side reactions and unwanted byproducts. This selectivity enhancement translates directly to greener processes with higher yields and reduced purification requirements.
The development of heterogeneous Lewis acid catalysts represents another significant contribution to green chemistry. These materials combine the selectivity advantages of Lewis acids with practical benefits of heterogeneous catalysis, including simplified product separation and catalyst recovery. Innovations in this area include Lewis acidic metal-organic frameworks (MOFs), functionalized silicas, and supported metal catalysts that maintain high activity while offering environmental advantages.
Life cycle assessment studies have confirmed that properly designed Lewis acid-catalyzed processes can significantly reduce the environmental impact compared to traditional synthetic routes. Key metrics showing improvement include reduced carbon footprint, decreased E-factor (waste-to-product ratio), and lower process mass intensity. These quantifiable benefits strengthen the case for incorporating Lewis acid catalysis into industrial green chemistry initiatives.
Looking forward, the correlation between Lewis acid properties and reaction selectivity offers promising pathways for developing next-generation sustainable processes. By understanding the fundamental interactions between Lewis acids and substrates, researchers can design increasingly efficient catalytic systems that operate under ambient conditions with minimal environmental impact. This approach aligns perfectly with the principles of green chemistry while maintaining the high selectivity and efficiency demanded by modern chemical manufacturing.
The environmental benefits of Lewis acid catalysis extend beyond energy efficiency. Many modern Lewis acid catalysts can be immobilized on solid supports, facilitating their recovery and reuse across multiple reaction cycles. This recyclability directly addresses one of green chemistry's core principles by minimizing waste generation and resource consumption. Furthermore, certain Lewis acids enable reactions in aqueous media or solvent-free conditions, eliminating the need for hazardous organic solvents.
Recent advances have demonstrated that tuning Lewis acid properties can dramatically improve atom economy in chemical transformations. By precisely controlling the coordination environment and electronic properties of Lewis acidic centers, chemists can design highly selective catalysts that minimize side reactions and unwanted byproducts. This selectivity enhancement translates directly to greener processes with higher yields and reduced purification requirements.
The development of heterogeneous Lewis acid catalysts represents another significant contribution to green chemistry. These materials combine the selectivity advantages of Lewis acids with practical benefits of heterogeneous catalysis, including simplified product separation and catalyst recovery. Innovations in this area include Lewis acidic metal-organic frameworks (MOFs), functionalized silicas, and supported metal catalysts that maintain high activity while offering environmental advantages.
Life cycle assessment studies have confirmed that properly designed Lewis acid-catalyzed processes can significantly reduce the environmental impact compared to traditional synthetic routes. Key metrics showing improvement include reduced carbon footprint, decreased E-factor (waste-to-product ratio), and lower process mass intensity. These quantifiable benefits strengthen the case for incorporating Lewis acid catalysis into industrial green chemistry initiatives.
Looking forward, the correlation between Lewis acid properties and reaction selectivity offers promising pathways for developing next-generation sustainable processes. By understanding the fundamental interactions between Lewis acids and substrates, researchers can design increasingly efficient catalytic systems that operate under ambient conditions with minimal environmental impact. This approach aligns perfectly with the principles of green chemistry while maintaining the high selectivity and efficiency demanded by modern chemical manufacturing.
Scale-up Considerations for Industrial Applications
When scaling up Lewis acid-catalyzed reactions from laboratory to industrial scale, several critical factors must be addressed to maintain selectivity while achieving economic viability. Heat transfer becomes particularly challenging as reaction volumes increase, potentially leading to temperature gradients that can significantly alter selectivity profiles. Industrial equipment typically has lower surface-to-volume ratios, necessitating modified cooling strategies to maintain precise temperature control essential for Lewis acid coordination and subsequent reaction pathways.
Mass transfer limitations represent another significant challenge in scale-up operations. The interaction between Lewis acids and substrates depends heavily on efficient mixing, which becomes more difficult to achieve in larger reactors. Implementing advanced mixing technologies, such as static mixers or specialized impeller designs, can help maintain homogeneity and ensure consistent Lewis acid distribution throughout the reaction medium.
Catalyst recovery and recycling systems must be integrated into industrial processes to address economic and environmental concerns. While laboratory-scale reactions might tolerate single-use Lewis acid catalysts, industrial applications require efficient recovery methods such as immobilization on solid supports, membrane filtration systems, or continuous extraction processes. These recovery systems must be designed to preserve catalyst activity and selectivity characteristics across multiple cycles.
Material compatibility presents additional challenges at industrial scale. Lewis acids often exhibit corrosive properties that may be manageable in laboratory glassware but become problematic with industrial metal reactors. Selection of appropriate construction materials, protective linings, or specialized alloys resistant to Lewis acid degradation is essential for maintaining process integrity and preventing contamination that could affect reaction selectivity.
Continuous flow processing offers promising alternatives to batch operations for Lewis acid-catalyzed reactions. Flow chemistry can provide more precise control over reaction parameters, allowing for consistent Lewis acid-substrate interactions and improved selectivity. Microreactor and flow technologies enable better heat management, controlled mixing, and potentially reduced catalyst loading, though they require significant engineering expertise to implement successfully at industrial scale.
Safety considerations become paramount during scale-up, particularly with moisture-sensitive Lewis acids that can generate hazardous conditions upon exposure to air or water. Implementing robust containment systems, inert gas handling protocols, and automated safety controls is essential. These safety measures must be designed to protect both personnel and maintain reaction selectivity by preventing unintended Lewis acid decomposition or side reactions.
Mass transfer limitations represent another significant challenge in scale-up operations. The interaction between Lewis acids and substrates depends heavily on efficient mixing, which becomes more difficult to achieve in larger reactors. Implementing advanced mixing technologies, such as static mixers or specialized impeller designs, can help maintain homogeneity and ensure consistent Lewis acid distribution throughout the reaction medium.
Catalyst recovery and recycling systems must be integrated into industrial processes to address economic and environmental concerns. While laboratory-scale reactions might tolerate single-use Lewis acid catalysts, industrial applications require efficient recovery methods such as immobilization on solid supports, membrane filtration systems, or continuous extraction processes. These recovery systems must be designed to preserve catalyst activity and selectivity characteristics across multiple cycles.
Material compatibility presents additional challenges at industrial scale. Lewis acids often exhibit corrosive properties that may be manageable in laboratory glassware but become problematic with industrial metal reactors. Selection of appropriate construction materials, protective linings, or specialized alloys resistant to Lewis acid degradation is essential for maintaining process integrity and preventing contamination that could affect reaction selectivity.
Continuous flow processing offers promising alternatives to batch operations for Lewis acid-catalyzed reactions. Flow chemistry can provide more precise control over reaction parameters, allowing for consistent Lewis acid-substrate interactions and improved selectivity. Microreactor and flow technologies enable better heat management, controlled mixing, and potentially reduced catalyst loading, though they require significant engineering expertise to implement successfully at industrial scale.
Safety considerations become paramount during scale-up, particularly with moisture-sensitive Lewis acids that can generate hazardous conditions upon exposure to air or water. Implementing robust containment systems, inert gas handling protocols, and automated safety controls is essential. These safety measures must be designed to protect both personnel and maintain reaction selectivity by preventing unintended Lewis acid decomposition or side reactions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!