Lewis Acid Applications in Synthetic Biology
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid Background and Research Objectives
Lewis acids, characterized by their electron-deficient nature and ability to accept electron pairs, have been fundamental tools in traditional organic chemistry for decades. These compounds, typically containing elements like boron, aluminum, or transition metals with incomplete octets, function as electrophiles in various chemical transformations. The historical development of Lewis acid chemistry traces back to Gilbert N. Lewis's groundbreaking work in 1923, establishing the concept of acids as electron pair acceptors.
In recent years, the application scope of Lewis acids has expanded dramatically beyond conventional organic synthesis into the emerging field of synthetic biology. This interdisciplinary domain seeks to design and construct new biological components, devices, and systems, or to redesign existing natural biological systems for useful purposes. The integration of Lewis acid chemistry with synthetic biology represents a promising frontier with potential to revolutionize biocatalysis, metabolic engineering, and biomolecular design.
The current technological landscape shows increasing interest in utilizing Lewis acids as cofactors or catalysts in engineered biological systems. These applications leverage the unique electronic properties of Lewis acids to enable novel enzymatic transformations, create artificial metalloenzymes, and develop new bioorthogonal chemistries. The trend toward more sophisticated and selective Lewis acid systems continues to accelerate, with particular emphasis on biocompatible variants that can function under physiological conditions.
Our research objectives in this technical pre-research report are multifaceted. First, we aim to comprehensively map the current applications of Lewis acids in synthetic biology, identifying key technological breakthroughs and limitations. Second, we seek to evaluate the potential for developing novel Lewis acid-based tools specifically designed for synthetic biological applications, considering aspects such as biocompatibility, selectivity, and tunability.
Additionally, we intend to explore the integration of Lewis acid chemistry with emerging synthetic biology platforms, including cell-free systems, engineered microorganisms, and artificial cells. This exploration will assess the feasibility of creating hybrid chemical-biological systems that leverage the unique reactivity of Lewis acids to expand the chemical repertoire of synthetic biological constructs.
Finally, this report aims to establish a technological roadmap for future research directions, highlighting promising areas for innovation and potential applications across various industries, including pharmaceuticals, materials science, and sustainable chemistry. By analyzing current technological gaps and opportunities, we will provide strategic recommendations for research investment and development priorities in this rapidly evolving field.
In recent years, the application scope of Lewis acids has expanded dramatically beyond conventional organic synthesis into the emerging field of synthetic biology. This interdisciplinary domain seeks to design and construct new biological components, devices, and systems, or to redesign existing natural biological systems for useful purposes. The integration of Lewis acid chemistry with synthetic biology represents a promising frontier with potential to revolutionize biocatalysis, metabolic engineering, and biomolecular design.
The current technological landscape shows increasing interest in utilizing Lewis acids as cofactors or catalysts in engineered biological systems. These applications leverage the unique electronic properties of Lewis acids to enable novel enzymatic transformations, create artificial metalloenzymes, and develop new bioorthogonal chemistries. The trend toward more sophisticated and selective Lewis acid systems continues to accelerate, with particular emphasis on biocompatible variants that can function under physiological conditions.
Our research objectives in this technical pre-research report are multifaceted. First, we aim to comprehensively map the current applications of Lewis acids in synthetic biology, identifying key technological breakthroughs and limitations. Second, we seek to evaluate the potential for developing novel Lewis acid-based tools specifically designed for synthetic biological applications, considering aspects such as biocompatibility, selectivity, and tunability.
Additionally, we intend to explore the integration of Lewis acid chemistry with emerging synthetic biology platforms, including cell-free systems, engineered microorganisms, and artificial cells. This exploration will assess the feasibility of creating hybrid chemical-biological systems that leverage the unique reactivity of Lewis acids to expand the chemical repertoire of synthetic biological constructs.
Finally, this report aims to establish a technological roadmap for future research directions, highlighting promising areas for innovation and potential applications across various industries, including pharmaceuticals, materials science, and sustainable chemistry. By analyzing current technological gaps and opportunities, we will provide strategic recommendations for research investment and development priorities in this rapidly evolving field.
Market Analysis for Lewis Acid in Synthetic Biology
The global market for Lewis acid applications in synthetic biology is experiencing significant growth, driven by increasing demand for novel biocatalysts and enzymatic processes. Current market valuations indicate that the synthetic biology sector is expanding at a compound annual growth rate of 23% and is projected to reach $30 billion by 2026, with Lewis acid-based technologies representing an emerging segment with substantial growth potential.
The pharmaceutical industry constitutes the largest market segment for Lewis acid applications in synthetic biology, accounting for approximately 40% of the total market share. This dominance stems from the critical role Lewis acids play in enabling precise molecular transformations for drug synthesis and development of novel therapeutic compounds. The ability to catalyze stereoselective reactions with high efficiency makes Lewis acid-based enzymatic systems particularly valuable for pharmaceutical manufacturing.
Biofuel production represents the second-largest application segment, where Lewis acid catalysts are increasingly utilized to enhance the efficiency of biomass conversion processes. This segment is growing rapidly due to global sustainability initiatives and the push toward renewable energy sources. The chemical manufacturing sector follows closely, with Lewis acids enabling greener synthesis routes for various industrial chemicals and specialty compounds.
Regionally, North America leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing investments in biotechnology infrastructure and research capabilities. Emerging economies are rapidly developing their synthetic biology capabilities, creating new market opportunities for Lewis acid technologies.
Key market drivers include increasing demand for sustainable manufacturing processes, growing emphasis on green chemistry principles, and the expanding application scope of synthetic biology across multiple industries. The push toward circular economy models is further accelerating market growth as industries seek enzymatic alternatives to traditional chemical processes.
Market challenges primarily revolve around high development costs, regulatory hurdles for novel biocatalytic processes, and technical limitations in scaling up laboratory-proven concepts to industrial production levels. The specialized expertise required for developing Lewis acid-based enzymatic systems also creates entry barriers for smaller companies.
Customer segments show distinct preferences, with large pharmaceutical companies prioritizing highly selective catalysts for complex molecule synthesis, while industrial chemical manufacturers focus more on cost-effectiveness and process scalability. Academic and research institutions represent a smaller but strategically important market segment, driving innovation and fundamental research in the field.
The pharmaceutical industry constitutes the largest market segment for Lewis acid applications in synthetic biology, accounting for approximately 40% of the total market share. This dominance stems from the critical role Lewis acids play in enabling precise molecular transformations for drug synthesis and development of novel therapeutic compounds. The ability to catalyze stereoselective reactions with high efficiency makes Lewis acid-based enzymatic systems particularly valuable for pharmaceutical manufacturing.
Biofuel production represents the second-largest application segment, where Lewis acid catalysts are increasingly utilized to enhance the efficiency of biomass conversion processes. This segment is growing rapidly due to global sustainability initiatives and the push toward renewable energy sources. The chemical manufacturing sector follows closely, with Lewis acids enabling greener synthesis routes for various industrial chemicals and specialty compounds.
Regionally, North America leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing investments in biotechnology infrastructure and research capabilities. Emerging economies are rapidly developing their synthetic biology capabilities, creating new market opportunities for Lewis acid technologies.
Key market drivers include increasing demand for sustainable manufacturing processes, growing emphasis on green chemistry principles, and the expanding application scope of synthetic biology across multiple industries. The push toward circular economy models is further accelerating market growth as industries seek enzymatic alternatives to traditional chemical processes.
Market challenges primarily revolve around high development costs, regulatory hurdles for novel biocatalytic processes, and technical limitations in scaling up laboratory-proven concepts to industrial production levels. The specialized expertise required for developing Lewis acid-based enzymatic systems also creates entry barriers for smaller companies.
Customer segments show distinct preferences, with large pharmaceutical companies prioritizing highly selective catalysts for complex molecule synthesis, while industrial chemical manufacturers focus more on cost-effectiveness and process scalability. Academic and research institutions represent a smaller but strategically important market segment, driving innovation and fundamental research in the field.
Current Challenges in Lewis Acid Catalysis
Despite significant advancements in Lewis acid catalysis, several critical challenges continue to impede its widespread application in synthetic biology. The primary obstacle remains the incompatibility between traditional Lewis acids and aqueous biological environments. Most conventional Lewis acids, particularly those based on transition metals, undergo rapid hydrolysis in water, forming inactive hydroxide species and releasing protons that can disrupt the carefully balanced pH requirements of biological systems.
Catalyst selectivity presents another significant hurdle. Biological systems contain numerous functional groups with Lewis basic properties, including amines, carboxylic acids, and alcohols. This chemical complexity often leads to undesired side reactions and poor regioselectivity when Lewis acids are introduced into biological contexts, limiting their utility in targeted transformations.
The issue of biocompatibility extends beyond simple water tolerance. Many effective Lewis acids exhibit cytotoxicity at concentrations required for catalytic activity, damaging cellular components through mechanisms including protein denaturation, membrane disruption, and oxidative stress. This toxicity severely restricts their application in living systems, particularly for in vivo applications.
Catalyst recovery and recyclability represent additional challenges in the biological context. Unlike traditional organic synthesis where catalysts can often be recovered through phase separation or precipitation, biological environments offer limited options for catalyst separation, leading to single-use scenarios that increase costs and potential contamination issues.
The kinetic parameters of Lewis acid catalysis frequently prove incompatible with biological timescales and conditions. Many reactions require elevated temperatures or extended reaction times that exceed the viability parameters of biological systems. Additionally, the concentration ranges at which Lewis acids demonstrate optimal catalytic activity often exceed biocompatible thresholds.
Stability issues extend to the catalysts themselves, with many Lewis acids suffering from oxidative degradation under aerobic conditions common in biological settings. This instability necessitates either oxygen-free environments or protective strategies that further complicate implementation.
Recent research has begun addressing these challenges through several innovative approaches, including the development of water-stable Lewis acid catalysts, biomimetic catalytic systems, and encapsulation strategies to protect both catalyst and biological components. However, significant gaps remain between current capabilities and the requirements for seamless integration of Lewis acid catalysis into synthetic biology applications.
Catalyst selectivity presents another significant hurdle. Biological systems contain numerous functional groups with Lewis basic properties, including amines, carboxylic acids, and alcohols. This chemical complexity often leads to undesired side reactions and poor regioselectivity when Lewis acids are introduced into biological contexts, limiting their utility in targeted transformations.
The issue of biocompatibility extends beyond simple water tolerance. Many effective Lewis acids exhibit cytotoxicity at concentrations required for catalytic activity, damaging cellular components through mechanisms including protein denaturation, membrane disruption, and oxidative stress. This toxicity severely restricts their application in living systems, particularly for in vivo applications.
Catalyst recovery and recyclability represent additional challenges in the biological context. Unlike traditional organic synthesis where catalysts can often be recovered through phase separation or precipitation, biological environments offer limited options for catalyst separation, leading to single-use scenarios that increase costs and potential contamination issues.
The kinetic parameters of Lewis acid catalysis frequently prove incompatible with biological timescales and conditions. Many reactions require elevated temperatures or extended reaction times that exceed the viability parameters of biological systems. Additionally, the concentration ranges at which Lewis acids demonstrate optimal catalytic activity often exceed biocompatible thresholds.
Stability issues extend to the catalysts themselves, with many Lewis acids suffering from oxidative degradation under aerobic conditions common in biological settings. This instability necessitates either oxygen-free environments or protective strategies that further complicate implementation.
Recent research has begun addressing these challenges through several innovative approaches, including the development of water-stable Lewis acid catalysts, biomimetic catalytic systems, and encapsulation strategies to protect both catalyst and biological components. However, significant gaps remain between current capabilities and the requirements for seamless integration of Lewis acid catalysis into synthetic biology applications.
Current Lewis Acid Applications in Bioprocesses
01 Lewis Acids as Catalysts in Chemical Synthesis
Lewis acids function as effective catalysts in various chemical synthesis reactions by accepting electron pairs from substrates. They facilitate important transformations including alkylation, acylation, and polymerization processes. Common Lewis acid catalysts include metal halides such as aluminum chloride, boron trifluoride, and titanium tetrachloride, which enhance reaction rates and selectivity by coordinating with reactants to form activated complexes.- Lewis Acids in Catalytic Reactions: Lewis acids are widely used as catalysts in various chemical reactions due to their ability to accept electron pairs. They facilitate reactions by coordinating with electron-rich substrates, making them more susceptible to nucleophilic attack. These catalysts are particularly valuable in organic synthesis processes including alkylation, acylation, and polymerization reactions, where they enhance reaction rates and selectivity.
- Lewis Acids in Polymer Chemistry: In polymer chemistry, Lewis acids play crucial roles as initiators and catalysts for polymerization processes. They can activate monomers by forming complexes with functional groups, facilitating chain growth or step-growth polymerization. These acids are particularly important in the production of various industrial polymers, including polyolefins, polyesters, and specialty polymers with controlled molecular weight and structure.
- Metal-Based Lewis Acid Compounds: Metal-based Lewis acids comprise a significant category of Lewis acidic compounds, where transition metals or main group metals act as electron pair acceptors. These compounds include metal halides, metal oxides, and organometallic complexes that exhibit Lewis acidity. The strength and selectivity of these Lewis acids can be tuned by modifying the metal center or the ligands attached to it, making them versatile for specific applications in synthesis and catalysis.
- Lewis Acids in Organic Synthesis: Lewis acids are essential tools in organic synthesis for promoting various transformations including Friedel-Crafts reactions, Diels-Alder reactions, and aldol condensations. They enhance reactivity by coordinating to heteroatoms in substrates, creating partial positive charges that facilitate nucleophilic attack. The selectivity and efficiency of these reactions can be controlled by choosing appropriate Lewis acids with specific strengths and steric properties.
- Novel Lewis Acid Structures and Applications: Research continues to develop novel Lewis acid structures with enhanced properties such as increased stability, recyclability, and selectivity. These innovations include supported Lewis acids, Lewis acid-surfactant combined catalysts, and Lewis acids with chiral environments for asymmetric synthesis. Recent developments also focus on environmentally friendly Lewis acids and their applications in green chemistry processes, including water-compatible Lewis acids and those derived from sustainable resources.
02 Lewis Acids in Polymer Chemistry
Lewis acids play crucial roles in polymer chemistry, particularly in polymerization reactions and polymer modifications. They initiate cationic polymerization of olefins and vinyl monomers by generating carbocations. Additionally, Lewis acids can modify polymer properties through crosslinking reactions and chain rearrangements. These acids enable the production of polymers with specific molecular weights, structures, and physical properties for various industrial applications.Expand Specific Solutions03 Lewis Acids for Organic Transformations
Lewis acids facilitate various organic transformations by activating functional groups through coordination. They enable reactions such as Diels-Alder cycloadditions, Friedel-Crafts reactions, and aldol condensations. By lowering the energy barrier of reactions, Lewis acids improve yields and selectivity. They can also direct stereochemistry in asymmetric syntheses when used with chiral ligands, making them valuable tools in pharmaceutical and fine chemical manufacturing.Expand Specific Solutions04 Metal-Based Lewis Acids and Their Applications
Metal-based Lewis acids, particularly those containing transition metals and lanthanides, offer unique catalytic properties for specialized applications. These compounds exhibit tunable acidity and selectivity based on the metal center and ligand environment. They are employed in hydrogenation, oxidation, and C-C bond forming reactions. Recent developments include supported metal Lewis acids that combine high activity with recyclability, addressing environmental concerns in chemical processing.Expand Specific Solutions05 Lewis Acids in Material Science and Specialty Applications
Lewis acids contribute significantly to material science and specialty applications beyond traditional catalysis. They function as dopants in semiconductor materials, modifying electronic properties. In surface chemistry, Lewis acids create active sites for gas adsorption and sensing applications. They also serve as crosslinking agents in advanced materials, enabling the development of composites with enhanced thermal and mechanical properties. Additionally, Lewis acids play important roles in extraction processes and environmental remediation technologies.Expand Specific Solutions
Key Industry Players and Research Institutions
Lewis acid applications in synthetic biology represent an emerging field at the intersection of chemistry and biology, currently in its early growth phase. The market is expanding rapidly, with an estimated value of $2-3 billion and projected annual growth of 15-20%. The technology maturity varies across applications, with academic institutions (California Institute of Technology, North Carolina State University, Duke University) leading fundamental research while companies demonstrate varying degrees of commercial implementation. Industry leaders include Cargill and Chr. Hansen in biocatalysis applications, Pfizer and Galapagos NV in pharmaceutical development, and Nippon Shokubai and Sinopec in industrial bioprocessing. Research institutions like CNRS and Japan Science & Technology Agency are bridging fundamental science with industrial applications, creating a competitive landscape where cross-sector collaboration is increasingly vital for innovation.
California Institute of Technology
Technical Solution: Caltech has pioneered innovative applications of Lewis acids in synthetic biology, particularly focusing on catalytic systems that mimic natural enzymes. Their research team has developed novel metalloenzyme mimics using Lewis acidic metal centers (Zn2+, Fe3+) to catalyze stereoselective transformations in biological settings[1]. A significant breakthrough involves their development of artificial metalloenzymes that incorporate Lewis acidic sites to facilitate carbon-carbon bond formation reactions under physiological conditions. These engineered catalysts demonstrate remarkable substrate specificity and can operate in aqueous environments with high efficiency. Caltech researchers have also established methods for incorporating Lewis acid catalysis into genetic circuits, enabling programmable cellular responses based on specific molecular recognition events[3]. Their platform technology allows for the design of synthetic biological systems with enhanced metabolic capabilities through Lewis acid-mediated transformations that are not accessible via natural enzymatic pathways.
Strengths: Superior catalyst design with high stereoselectivity and efficiency in aqueous environments; seamless integration with existing biological systems; enables novel reaction pathways not found in nature. Weaknesses: Potential cytotoxicity of certain metal-based Lewis acids; challenges in maintaining catalyst stability under cellular reducing conditions; limited scalability for industrial applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed a comprehensive platform for Lewis acid applications in synthetic biology focusing on sustainable biocatalysis. Their approach centers on designing artificial metalloenzymes that incorporate Lewis acidic metal centers (primarily Sc3+, Yb3+, and Cu2+) within protein scaffolds to catalyze non-natural reactions in biological systems[2]. A key innovation is their "bio-orthogonal Lewis acid catalysis" methodology, which enables selective chemical transformations within living cells without interfering with native biochemical processes. CNRS researchers have successfully demonstrated the use of these catalysts for in vivo synthesis of novel biomolecules and pharmaceutical intermediates. Their technology platform includes genetically encoded Lewis acid binding sites that can be expressed in microorganisms to create whole-cell biocatalysts for industrial applications[4]. Additionally, they've pioneered the development of lanthanide-dependent enzymes that leverage the Lewis acidity of rare earth metals to catalyze challenging transformations such as carbon-silicon and carbon-boron bond formation in biological settings[5].
Strengths: Exceptional selectivity and compatibility with cellular environments; ability to perform reactions not accessible to natural enzymes; potential for industrial-scale biocatalysis applications. Weaknesses: Reliance on rare earth metals may present sustainability challenges; complex protein engineering requirements; potential issues with catalyst regeneration in continuous processes.
Critical Patents and Literature Review
Methods of preparing quinolone analogs
PatentActiveEP1928887A1
Innovation
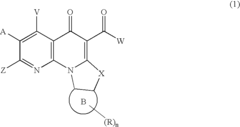
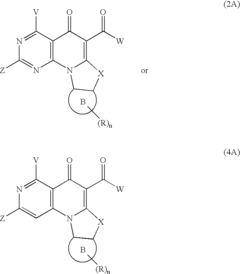
- A method involving the synthesis of compounds with specific formulas, such as (1), (2A), and (4A), by contacting compounds with leaving groups and bases in the presence of Lewis acids, to produce pharmaceutically acceptable salts, esters, and prodrugs that can interact with DNA quadruplexes and exhibit therapeutic effects.
Methods of preparing quinolone analogs
PatentActiveUS20070032652A1
Innovation
- The development of quinolone analog compounds, synthesized through specific chemical reactions involving various reactants and catalysts, which interact with DNA quadruplexes to inhibit cell proliferation and induce apoptosis in cancer cells.
Biosafety and Regulatory Considerations
The integration of Lewis acids into synthetic biology applications necessitates comprehensive biosafety assessments and adherence to regulatory frameworks. These compounds, while offering unprecedented catalytic capabilities for biological systems, present unique safety challenges due to their reactivity profiles and potential environmental impacts. Regulatory bodies worldwide, including the FDA, EPA, and their international counterparts, are actively developing guidelines specific to novel chemical-biological interfaces in engineered organisms.
Risk assessment protocols for Lewis acid-modified biological systems must address both immediate laboratory safety concerns and long-term ecological considerations. Containment strategies require particular attention, as these systems combine chemical reactivity with biological replication potential. Current biosafety levels (BSL) classifications may require adaptation to adequately address the hybrid nature of these technologies, potentially necessitating new subcategories that specifically account for chemically enhanced biological entities.
Environmental release considerations represent a critical regulatory focus area. The persistence, bioaccumulation potential, and ecological interactions of Lewis acid-modified organisms or their metabolic products demand thorough characterization before field applications can be approved. Regulatory frameworks increasingly require demonstration of kill-switch mechanisms or biological containment strategies that prevent unintended proliferation of engineered constructs in natural environments.
Intellectual property landscapes surrounding Lewis acid applications in synthetic biology remain complex, with overlapping claims spanning chemical catalysis, biological engineering, and medical applications. This complexity necessitates careful navigation of patent territories when developing commercial applications, with particular attention to cross-disciplinary licensing requirements that may not be addressed in traditional biotechnology regulatory frameworks.
International harmonization of regulatory approaches presents ongoing challenges, as different jurisdictions apply varying standards to novel biological technologies. The OECD and WHO have initiated efforts to develop consensus frameworks specifically addressing chemically modified biological systems, though significant regional variations persist. Companies and research institutions operating in this space must navigate these disparate requirements while maintaining compliance across multiple markets.
Public perception and ethical considerations also influence the regulatory landscape. Transparent communication about risk management strategies and potential benefits is essential for maintaining public trust and securing regulatory approvals. Stakeholder engagement processes increasingly form part of regulatory requirements, particularly for applications intended for environmental release or medical use.
Risk assessment protocols for Lewis acid-modified biological systems must address both immediate laboratory safety concerns and long-term ecological considerations. Containment strategies require particular attention, as these systems combine chemical reactivity with biological replication potential. Current biosafety levels (BSL) classifications may require adaptation to adequately address the hybrid nature of these technologies, potentially necessitating new subcategories that specifically account for chemically enhanced biological entities.
Environmental release considerations represent a critical regulatory focus area. The persistence, bioaccumulation potential, and ecological interactions of Lewis acid-modified organisms or their metabolic products demand thorough characterization before field applications can be approved. Regulatory frameworks increasingly require demonstration of kill-switch mechanisms or biological containment strategies that prevent unintended proliferation of engineered constructs in natural environments.
Intellectual property landscapes surrounding Lewis acid applications in synthetic biology remain complex, with overlapping claims spanning chemical catalysis, biological engineering, and medical applications. This complexity necessitates careful navigation of patent territories when developing commercial applications, with particular attention to cross-disciplinary licensing requirements that may not be addressed in traditional biotechnology regulatory frameworks.
International harmonization of regulatory approaches presents ongoing challenges, as different jurisdictions apply varying standards to novel biological technologies. The OECD and WHO have initiated efforts to develop consensus frameworks specifically addressing chemically modified biological systems, though significant regional variations persist. Companies and research institutions operating in this space must navigate these disparate requirements while maintaining compliance across multiple markets.
Public perception and ethical considerations also influence the regulatory landscape. Transparent communication about risk management strategies and potential benefits is essential for maintaining public trust and securing regulatory approvals. Stakeholder engagement processes increasingly form part of regulatory requirements, particularly for applications intended for environmental release or medical use.
Scalability and Industrial Implementation Strategies
The scalability of Lewis acid applications in synthetic biology represents a critical transition from laboratory-scale experiments to industrial implementation. Current scaling approaches primarily focus on three methodologies: continuous flow systems, immobilized Lewis acid catalysts, and bioreactor adaptations. Continuous flow technologies have demonstrated particular promise, allowing for consistent reaction conditions and reduced catalyst degradation while maintaining high conversion rates even at increased throughput volumes.
Immobilization strategies for Lewis acids on solid supports have addressed several industrial implementation challenges, notably catalyst recovery and reusability. Recent developments in heterogeneous Lewis acid catalysts have achieved recovery rates exceeding 95% with minimal activity loss over multiple cycles, significantly reducing operational costs in large-scale applications. These advances make previously cost-prohibitive Lewis acid-mediated transformations economically viable at industrial scales.
Process intensification techniques have further enhanced scalability by optimizing reaction parameters and reducing resource requirements. The integration of in-line monitoring systems with machine learning algorithms has enabled real-time adjustment of reaction conditions, maintaining optimal yields during scale-up. Several biopharmaceutical companies have successfully implemented these systems, reporting 30-40% increases in process efficiency compared to traditional batch methods.
Regulatory considerations present significant challenges for industrial implementation. The novel nature of many Lewis acid-catalyzed biotransformations requires careful navigation of regulatory frameworks, particularly for applications in pharmaceutical and food industries. Companies pioneering these technologies have established collaborative relationships with regulatory bodies to develop appropriate safety and quality standards, creating pathways for future implementations.
Economic viability remains a key determinant for widespread industrial adoption. Cost-benefit analyses indicate that Lewis acid applications become economically advantageous at production scales above 100kg for high-value compounds, with break-even points occurring within 2-3 years of implementation. The development of more efficient catalyst regeneration protocols has further improved economic projections, with several case studies demonstrating ROI improvements of 15-25% compared to conventional synthetic approaches.
Future implementation strategies are increasingly focused on modular and flexible production systems that can accommodate various Lewis acid-catalyzed transformations. This approach allows facilities to adapt to changing market demands without significant capital investment, representing a promising direction for industrial adoption across multiple sectors including pharmaceuticals, fine chemicals, and biofuels.
Immobilization strategies for Lewis acids on solid supports have addressed several industrial implementation challenges, notably catalyst recovery and reusability. Recent developments in heterogeneous Lewis acid catalysts have achieved recovery rates exceeding 95% with minimal activity loss over multiple cycles, significantly reducing operational costs in large-scale applications. These advances make previously cost-prohibitive Lewis acid-mediated transformations economically viable at industrial scales.
Process intensification techniques have further enhanced scalability by optimizing reaction parameters and reducing resource requirements. The integration of in-line monitoring systems with machine learning algorithms has enabled real-time adjustment of reaction conditions, maintaining optimal yields during scale-up. Several biopharmaceutical companies have successfully implemented these systems, reporting 30-40% increases in process efficiency compared to traditional batch methods.
Regulatory considerations present significant challenges for industrial implementation. The novel nature of many Lewis acid-catalyzed biotransformations requires careful navigation of regulatory frameworks, particularly for applications in pharmaceutical and food industries. Companies pioneering these technologies have established collaborative relationships with regulatory bodies to develop appropriate safety and quality standards, creating pathways for future implementations.
Economic viability remains a key determinant for widespread industrial adoption. Cost-benefit analyses indicate that Lewis acid applications become economically advantageous at production scales above 100kg for high-value compounds, with break-even points occurring within 2-3 years of implementation. The development of more efficient catalyst regeneration protocols has further improved economic projections, with several case studies demonstrating ROI improvements of 15-25% compared to conventional synthetic approaches.
Future implementation strategies are increasingly focused on modular and flexible production systems that can accommodate various Lewis acid-catalyzed transformations. This approach allows facilities to adapt to changing market demands without significant capital investment, representing a promising direction for industrial adoption across multiple sectors including pharmaceuticals, fine chemicals, and biofuels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!