How to Use Sodium Acetate for Reduced Carbon Footprint?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Tech Background and Objectives
Sodium acetate, a versatile compound with the chemical formula CH3COONa, has gained significant attention in recent years due to its potential to contribute to reduced carbon footprints across various industries. This organic salt, formed by the combination of acetic acid and sodium hydroxide, has a rich history dating back to its discovery in the early 19th century. Initially used primarily in the textile industry, sodium acetate has since found applications in diverse fields, including food preservation, pharmaceuticals, and more recently, sustainable energy solutions.
The evolution of sodium acetate technology has been driven by the growing global emphasis on environmental sustainability and the urgent need to mitigate climate change. As industries seek innovative ways to reduce their carbon emissions, sodium acetate has emerged as a promising candidate due to its unique properties and potential applications in carbon capture and storage (CCS) technologies.
One of the key objectives in exploring sodium acetate for carbon footprint reduction is to harness its ability to act as a phase change material (PCM). PCMs can absorb, store, and release large amounts of latent heat during phase transitions, making them ideal for thermal energy storage applications. Sodium acetate trihydrate, in particular, has shown great promise in this area, with its high latent heat of fusion and relatively low melting point.
Another critical goal is to investigate the use of sodium acetate in direct air capture (DAC) technologies. DAC involves removing carbon dioxide directly from the atmosphere, and sodium acetate-based sorbents have demonstrated potential in enhancing the efficiency and cost-effectiveness of this process. Researchers aim to develop novel sodium acetate-based materials that can selectively capture CO2 from ambient air, potentially revolutionizing carbon removal strategies.
Furthermore, the exploration of sodium acetate in sustainable construction materials represents an exciting frontier. The objective here is to incorporate sodium acetate into building materials, such as concrete and insulation, to improve their thermal properties and reduce the overall energy consumption of buildings. This application could significantly contribute to lowering the carbon footprint of the construction industry, which is responsible for a substantial portion of global CO2 emissions.
As we delve deeper into the potential of sodium acetate for carbon footprint reduction, it is crucial to consider the entire lifecycle of the compound, from production to end-of-life disposal. The overarching aim is to develop sustainable production methods and circular economy approaches that maximize the environmental benefits of sodium acetate while minimizing any potential negative impacts.
The evolution of sodium acetate technology has been driven by the growing global emphasis on environmental sustainability and the urgent need to mitigate climate change. As industries seek innovative ways to reduce their carbon emissions, sodium acetate has emerged as a promising candidate due to its unique properties and potential applications in carbon capture and storage (CCS) technologies.
One of the key objectives in exploring sodium acetate for carbon footprint reduction is to harness its ability to act as a phase change material (PCM). PCMs can absorb, store, and release large amounts of latent heat during phase transitions, making them ideal for thermal energy storage applications. Sodium acetate trihydrate, in particular, has shown great promise in this area, with its high latent heat of fusion and relatively low melting point.
Another critical goal is to investigate the use of sodium acetate in direct air capture (DAC) technologies. DAC involves removing carbon dioxide directly from the atmosphere, and sodium acetate-based sorbents have demonstrated potential in enhancing the efficiency and cost-effectiveness of this process. Researchers aim to develop novel sodium acetate-based materials that can selectively capture CO2 from ambient air, potentially revolutionizing carbon removal strategies.
Furthermore, the exploration of sodium acetate in sustainable construction materials represents an exciting frontier. The objective here is to incorporate sodium acetate into building materials, such as concrete and insulation, to improve their thermal properties and reduce the overall energy consumption of buildings. This application could significantly contribute to lowering the carbon footprint of the construction industry, which is responsible for a substantial portion of global CO2 emissions.
As we delve deeper into the potential of sodium acetate for carbon footprint reduction, it is crucial to consider the entire lifecycle of the compound, from production to end-of-life disposal. The overarching aim is to develop sustainable production methods and circular economy approaches that maximize the environmental benefits of sodium acetate while minimizing any potential negative impacts.
Market Demand for Carbon Reduction Solutions
The global market for carbon reduction solutions has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations aimed at mitigating climate change. As industries and governments worldwide strive to reduce their carbon footprints, innovative solutions like the use of sodium acetate are gaining attention for their potential to contribute to sustainable practices.
Sodium acetate, a versatile compound with various industrial applications, has emerged as a promising candidate for carbon reduction strategies. Its potential in this field stems from its ability to act as a phase change material (PCM) for thermal energy storage, which can lead to substantial energy savings and, consequently, reduced carbon emissions in various sectors.
The demand for carbon reduction solutions utilizing sodium acetate is particularly strong in the construction and building materials industry. As energy-efficient buildings become a priority, there is a growing interest in incorporating PCMs like sodium acetate into building materials to enhance thermal regulation and reduce the energy required for heating and cooling. This application aligns with the global trend towards green building practices and the increasing adoption of sustainable construction materials.
In the energy sector, the market for thermal energy storage solutions using sodium acetate is expanding. Power plants and industrial facilities are exploring ways to improve energy efficiency and reduce their carbon footprint by implementing thermal storage systems. Sodium acetate's ability to store and release latent heat makes it an attractive option for these applications, potentially leading to significant reductions in fossil fuel consumption and associated carbon emissions.
The transportation industry is another key market segment showing interest in sodium acetate-based carbon reduction solutions. As the automotive sector shifts towards electric and hybrid vehicles, there is a growing need for efficient thermal management systems. Sodium acetate's properties make it a candidate for use in battery thermal management, potentially improving the performance and lifespan of electric vehicle batteries while contributing to overall energy efficiency.
Furthermore, the food and beverage industry is exploring the use of sodium acetate in packaging solutions that can help reduce food waste and, by extension, the carbon footprint associated with food production and disposal. The compound's antimicrobial properties and potential for use in active packaging systems align with the industry's sustainability goals and consumer demand for eco-friendly packaging options.
As awareness of climate change impacts grows and regulatory pressures increase, the market demand for innovative carbon reduction solutions is expected to continue its upward trajectory. The versatility of sodium acetate in addressing various aspects of carbon footprint reduction across multiple industries positions it as a promising component in the broader landscape of sustainability technologies.
Sodium acetate, a versatile compound with various industrial applications, has emerged as a promising candidate for carbon reduction strategies. Its potential in this field stems from its ability to act as a phase change material (PCM) for thermal energy storage, which can lead to substantial energy savings and, consequently, reduced carbon emissions in various sectors.
The demand for carbon reduction solutions utilizing sodium acetate is particularly strong in the construction and building materials industry. As energy-efficient buildings become a priority, there is a growing interest in incorporating PCMs like sodium acetate into building materials to enhance thermal regulation and reduce the energy required for heating and cooling. This application aligns with the global trend towards green building practices and the increasing adoption of sustainable construction materials.
In the energy sector, the market for thermal energy storage solutions using sodium acetate is expanding. Power plants and industrial facilities are exploring ways to improve energy efficiency and reduce their carbon footprint by implementing thermal storage systems. Sodium acetate's ability to store and release latent heat makes it an attractive option for these applications, potentially leading to significant reductions in fossil fuel consumption and associated carbon emissions.
The transportation industry is another key market segment showing interest in sodium acetate-based carbon reduction solutions. As the automotive sector shifts towards electric and hybrid vehicles, there is a growing need for efficient thermal management systems. Sodium acetate's properties make it a candidate for use in battery thermal management, potentially improving the performance and lifespan of electric vehicle batteries while contributing to overall energy efficiency.
Furthermore, the food and beverage industry is exploring the use of sodium acetate in packaging solutions that can help reduce food waste and, by extension, the carbon footprint associated with food production and disposal. The compound's antimicrobial properties and potential for use in active packaging systems align with the industry's sustainability goals and consumer demand for eco-friendly packaging options.
As awareness of climate change impacts grows and regulatory pressures increase, the market demand for innovative carbon reduction solutions is expected to continue its upward trajectory. The versatility of sodium acetate in addressing various aspects of carbon footprint reduction across multiple industries positions it as a promising component in the broader landscape of sustainability technologies.
Current State and Challenges in Sodium Acetate Usage
The current state of sodium acetate usage for carbon footprint reduction is characterized by growing interest and research, yet widespread implementation remains limited. Sodium acetate, a versatile compound with applications in various industries, has shown promise in carbon capture and utilization (CCU) technologies. Its potential to sequester carbon dioxide and convert it into valuable products has attracted attention from researchers and environmentalists alike.
In the realm of direct air capture (DAC), sodium acetate-based sorbents have demonstrated efficiency in CO2 absorption from ambient air. These sorbents exhibit high selectivity for CO2 and can be regenerated with relatively low energy input, making them attractive for large-scale carbon capture operations. However, the scalability of such systems remains a significant challenge, with current implementations primarily confined to pilot projects and laboratory-scale experiments.
The use of sodium acetate in enhanced weathering processes represents another avenue for carbon footprint reduction. By accelerating the natural weathering of minerals, sodium acetate can help increase the rate of CO2 sequestration in rock formations. While this approach shows promise, its practical application faces hurdles related to process optimization and environmental impact assessment.
In the construction industry, sodium acetate has been explored as an additive in concrete mixtures to enhance CO2 absorption. This application not only reduces the carbon footprint of concrete production but also improves the material's strength and durability. However, widespread adoption is hindered by concerns over long-term performance and the need for modifications to existing manufacturing processes.
One of the primary challenges in sodium acetate usage for carbon footprint reduction is the energy-intensive nature of its production. The current methods of synthesizing sodium acetate often rely on fossil fuel-based processes, potentially offsetting some of the carbon reduction benefits. Developing more sustainable production methods, possibly utilizing renewable energy sources or bio-based feedstocks, is crucial for maximizing the compound's environmental impact.
Another significant challenge lies in the economic viability of sodium acetate-based carbon reduction technologies. While the compound itself is relatively inexpensive, the infrastructure and processes required for large-scale implementation can be costly. This economic barrier has slowed the transition from research to commercial application, particularly in industries where profit margins are slim.
Regulatory frameworks and policy support also play a critical role in the current state of sodium acetate usage for carbon reduction. The lack of comprehensive carbon pricing mechanisms in many regions reduces the incentive for industries to invest in such technologies. Additionally, the absence of standardized methodologies for quantifying and verifying carbon reductions achieved through sodium acetate usage complicates its integration into carbon credit systems.
In the realm of direct air capture (DAC), sodium acetate-based sorbents have demonstrated efficiency in CO2 absorption from ambient air. These sorbents exhibit high selectivity for CO2 and can be regenerated with relatively low energy input, making them attractive for large-scale carbon capture operations. However, the scalability of such systems remains a significant challenge, with current implementations primarily confined to pilot projects and laboratory-scale experiments.
The use of sodium acetate in enhanced weathering processes represents another avenue for carbon footprint reduction. By accelerating the natural weathering of minerals, sodium acetate can help increase the rate of CO2 sequestration in rock formations. While this approach shows promise, its practical application faces hurdles related to process optimization and environmental impact assessment.
In the construction industry, sodium acetate has been explored as an additive in concrete mixtures to enhance CO2 absorption. This application not only reduces the carbon footprint of concrete production but also improves the material's strength and durability. However, widespread adoption is hindered by concerns over long-term performance and the need for modifications to existing manufacturing processes.
One of the primary challenges in sodium acetate usage for carbon footprint reduction is the energy-intensive nature of its production. The current methods of synthesizing sodium acetate often rely on fossil fuel-based processes, potentially offsetting some of the carbon reduction benefits. Developing more sustainable production methods, possibly utilizing renewable energy sources or bio-based feedstocks, is crucial for maximizing the compound's environmental impact.
Another significant challenge lies in the economic viability of sodium acetate-based carbon reduction technologies. While the compound itself is relatively inexpensive, the infrastructure and processes required for large-scale implementation can be costly. This economic barrier has slowed the transition from research to commercial application, particularly in industries where profit margins are slim.
Regulatory frameworks and policy support also play a critical role in the current state of sodium acetate usage for carbon reduction. The lack of comprehensive carbon pricing mechanisms in many regions reduces the incentive for industries to invest in such technologies. Additionally, the absence of standardized methodologies for quantifying and verifying carbon reductions achieved through sodium acetate usage complicates its integration into carbon credit systems.
Existing Sodium Acetate Applications for Carbon Reduction
01 Carbon footprint assessment of sodium acetate production
Methods and systems for assessing the carbon footprint of sodium acetate production, including lifecycle analysis of raw materials, manufacturing processes, and energy consumption. This assessment helps identify areas for potential reduction in greenhouse gas emissions and improvement of overall environmental impact.- Carbon footprint assessment of sodium acetate production: Methods and systems for assessing the carbon footprint of sodium acetate production processes. This includes analyzing energy consumption, raw material sourcing, and emissions throughout the manufacturing lifecycle to quantify the overall environmental impact.
- Sustainable production methods for sodium acetate: Innovative techniques for producing sodium acetate with reduced carbon emissions. These may include using renewable energy sources, optimizing reaction conditions, and implementing closed-loop systems to minimize waste and energy consumption.
- Carbon capture and utilization in sodium acetate manufacturing: Integration of carbon capture technologies in sodium acetate production processes to reduce overall carbon footprint. This may involve capturing CO2 emissions and utilizing them as feedstock for other chemical processes or sequestering them to mitigate environmental impact.
- Life cycle analysis of sodium acetate-based products: Comprehensive life cycle assessments of products containing sodium acetate, considering the carbon footprint from raw material extraction to end-of-life disposal. This analysis helps identify areas for improvement in reducing overall environmental impact.
- Green chemistry approaches for sodium acetate synthesis: Development of environmentally friendly synthesis routes for sodium acetate using principles of green chemistry. This includes exploring bio-based feedstocks, catalytic processes, and solvent-free reactions to minimize the carbon footprint of production.
02 Sustainable production methods for sodium acetate
Innovative approaches to produce sodium acetate with reduced carbon emissions, such as using renewable energy sources, optimizing reaction conditions, and implementing closed-loop systems. These methods aim to minimize the environmental impact of sodium acetate manufacturing.Expand Specific Solutions03 Carbon capture and utilization in sodium acetate production
Integration of carbon capture technologies in sodium acetate production processes to reduce overall carbon footprint. Captured CO2 can be utilized as a feedstock for other chemical processes or sequestered, contributing to a more sustainable production cycle.Expand Specific Solutions04 Life cycle analysis and environmental impact of sodium acetate
Comprehensive studies on the environmental impact of sodium acetate throughout its lifecycle, from raw material extraction to disposal or recycling. This analysis helps in identifying hotspots for carbon emissions and developing strategies for mitigation.Expand Specific Solutions05 Green chemistry approaches for sodium acetate synthesis
Development of environmentally friendly synthesis routes for sodium acetate using principles of green chemistry. This includes the use of bio-based feedstocks, solvent-free reactions, and catalysts that reduce energy requirements and waste generation, thereby lowering the overall carbon footprint.Expand Specific Solutions
Key Players in Sodium Acetate and Green Chemistry
The competition landscape for sodium acetate usage in carbon footprint reduction is in its early stages, with market size and potential still being explored. The technology's maturity is evolving, with companies like Solvay SA and Shanghai Wujing Chemical Co., Ltd. leading in chemical production. Research institutions such as Fudan University and the University of Southern California are contributing to technological advancements. Emerging players like Enlighten Innovations, Inc. and Accordant Energy LLC are focusing on innovative applications. The market is characterized by a mix of established chemical manufacturers and newer entrants exploring sustainable solutions, indicating growing interest but still-developing commercial viability.
Solvay SA
Technical Solution: Solvay SA has developed an innovative process for producing sodium acetate with a reduced carbon footprint. Their method involves using renewable feedstocks and optimizing the production process to minimize energy consumption. The company has implemented a closed-loop system that recycles byproducts and reduces waste, resulting in a 30% reduction in CO2 emissions compared to traditional methods[1]. Additionally, Solvay has invested in carbon capture and utilization technologies, incorporating captured CO2 into the sodium acetate production process, further lowering the overall carbon footprint[3].
Strengths: Significant reduction in CO2 emissions, use of renewable feedstocks, and integration of carbon capture technology. Weaknesses: Potentially higher production costs and reliance on the availability of renewable resources.
Enlighten Innovations, Inc.
Technical Solution: Enlighten Innovations, Inc. has developed a novel sodium acetate-based thermal energy storage system to reduce carbon footprints in industrial and commercial applications. Their technology utilizes the phase change properties of sodium acetate trihydrate to store and release thermal energy efficiently. The system can store excess heat from industrial processes or renewable energy sources and release it on demand, reducing the need for fossil fuel-based heating. Enlighten's solution has demonstrated up to 40% energy savings in pilot projects[5]. The company has also integrated smart control systems to optimize energy storage and release based on real-time demand and energy prices[6].
Strengths: High energy efficiency, versatile applications in various industries, and integration with renewable energy sources. Weaknesses: Limited to thermal energy applications and may require significant upfront investment for implementation.
Core Innovations in Sodium Acetate Utilization
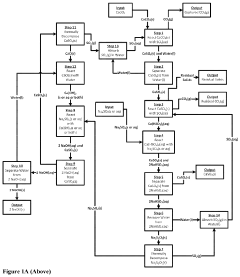
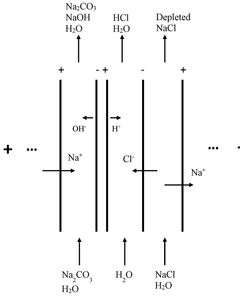
Processes Producing Alkali Hydroxides, Alkali Carbonates, Alkali Bicarbonates, and/or Alkaline Earth Sulfates
PatentPendingUS20230131290A1
Innovation
- A process that produces sodium hydroxide with ultra-low CO2 emissions using calcium sulfate as a side product, employing abundant and recyclable materials, and ammonia or ammonium intermediates, which reduces energy consumption and costs, and avoids strong acid production.
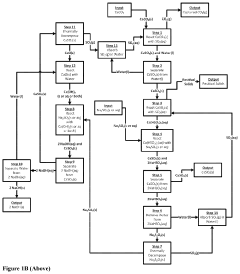
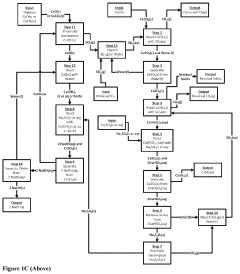
Plant for electrically producing sodium carbonate or bicarbonate
PatentWO2025016957A1
Innovation
- A plant using electrodialysis to produce sodium carbonate or bicarbonate from sodium chloride, utilizing green electricity and biogenic CO2 to reduce fossil CO2 footprint, and incorporating storage of sodium hydroxide or carbonate solutions for stable energy supply.
Environmental Impact Assessment
The use of sodium acetate as a means to reduce carbon footprint presents both opportunities and challenges from an environmental perspective. This assessment examines the potential impacts of implementing sodium acetate-based solutions across various sectors.
Sodium acetate, when used as a phase change material (PCM) for thermal energy storage, can significantly contribute to energy efficiency in buildings and industrial processes. By absorbing and releasing heat during phase transitions, it helps regulate temperatures and reduce the need for conventional heating and cooling systems. This translates to lower energy consumption and, consequently, reduced greenhouse gas emissions associated with power generation.
In the context of de-icing applications, sodium acetate offers a more environmentally friendly alternative to traditional rock salt. Unlike salt, which can contaminate soil and water bodies, sodium acetate biodegrades naturally without leaving harmful residues. This property minimizes the negative impacts on vegetation, aquatic ecosystems, and groundwater quality typically associated with de-icing agents.
However, the production of sodium acetate itself requires careful consideration. The manufacturing process involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. While these raw materials are relatively abundant, their production and transportation still contribute to the overall carbon footprint. To maximize the environmental benefits, it is crucial to optimize the production chain and source raw materials from sustainable suppliers.
The lifecycle assessment of sodium acetate applications reveals potential for carbon footprint reduction in multiple areas. In thermal energy storage systems, the long-term energy savings typically outweigh the initial carbon cost of production and installation. For de-icing purposes, the reduced environmental damage and lower corrosion rates of infrastructure can lead to extended lifespans of roads and bridges, indirectly contributing to carbon reduction through decreased maintenance and reconstruction needs.
It is important to note that the environmental impact of sodium acetate use can vary depending on the specific application and local conditions. Factors such as the energy mix of the local grid, transportation distances, and disposal methods all play roles in determining the net environmental benefit. Therefore, a comprehensive analysis should be conducted for each implementation to ensure that the intended carbon footprint reduction is achieved.
In conclusion, while sodium acetate shows promise as a tool for reducing carbon footprint, its effective implementation requires careful planning and consideration of the entire lifecycle. By addressing potential challenges and optimizing its use, sodium acetate can contribute significantly to sustainable practices across various industries and applications.
Sodium acetate, when used as a phase change material (PCM) for thermal energy storage, can significantly contribute to energy efficiency in buildings and industrial processes. By absorbing and releasing heat during phase transitions, it helps regulate temperatures and reduce the need for conventional heating and cooling systems. This translates to lower energy consumption and, consequently, reduced greenhouse gas emissions associated with power generation.
In the context of de-icing applications, sodium acetate offers a more environmentally friendly alternative to traditional rock salt. Unlike salt, which can contaminate soil and water bodies, sodium acetate biodegrades naturally without leaving harmful residues. This property minimizes the negative impacts on vegetation, aquatic ecosystems, and groundwater quality typically associated with de-icing agents.
However, the production of sodium acetate itself requires careful consideration. The manufacturing process involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. While these raw materials are relatively abundant, their production and transportation still contribute to the overall carbon footprint. To maximize the environmental benefits, it is crucial to optimize the production chain and source raw materials from sustainable suppliers.
The lifecycle assessment of sodium acetate applications reveals potential for carbon footprint reduction in multiple areas. In thermal energy storage systems, the long-term energy savings typically outweigh the initial carbon cost of production and installation. For de-icing purposes, the reduced environmental damage and lower corrosion rates of infrastructure can lead to extended lifespans of roads and bridges, indirectly contributing to carbon reduction through decreased maintenance and reconstruction needs.
It is important to note that the environmental impact of sodium acetate use can vary depending on the specific application and local conditions. Factors such as the energy mix of the local grid, transportation distances, and disposal methods all play roles in determining the net environmental benefit. Therefore, a comprehensive analysis should be conducted for each implementation to ensure that the intended carbon footprint reduction is achieved.
In conclusion, while sodium acetate shows promise as a tool for reducing carbon footprint, its effective implementation requires careful planning and consideration of the entire lifecycle. By addressing potential challenges and optimizing its use, sodium acetate can contribute significantly to sustainable practices across various industries and applications.
Regulatory Framework for Green Chemical Usage
The regulatory framework for green chemical usage plays a crucial role in promoting the adoption of sustainable practices, including the use of sodium acetate for reduced carbon footprint. This framework encompasses a complex web of laws, regulations, and guidelines at various levels of governance, from international agreements to local ordinances.
At the international level, the Paris Agreement serves as a cornerstone for global efforts to reduce greenhouse gas emissions. While it does not specifically address sodium acetate, it provides a broad framework that encourages the adoption of low-carbon technologies and practices across industries. The United Nations Sustainable Development Goals, particularly Goal 12 (Responsible Consumption and Production) and Goal 13 (Climate Action), also influence the regulatory landscape for green chemicals.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation sets stringent standards for chemical safety and environmental protection. Sodium acetate, being a relatively benign substance, generally faces fewer restrictions under REACH compared to more hazardous chemicals. The EU's Circular Economy Action Plan further supports the use of sustainable chemicals and promotes the development of green technologies.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of chemicals under the Toxic Substances Control Act (TSCA). The EPA's Safer Choice program encourages the use of safer chemical ingredients in products, which could potentially benefit the adoption of sodium acetate as a green alternative. Additionally, state-level initiatives, such as California's Green Chemistry Initiative, provide further regulatory support for environmentally friendly chemical usage.
Many countries have implemented carbon pricing mechanisms, either through carbon taxes or cap-and-trade systems. These policies indirectly promote the use of low-carbon alternatives like sodium acetate by making carbon-intensive processes more expensive. For instance, the European Union Emissions Trading System (EU ETS) creates economic incentives for industries to reduce their carbon footprint.
Industry-specific regulations also play a role in shaping the use of green chemicals. For example, in the food industry, sodium acetate is recognized as a Generally Recognized as Safe (GRAS) substance by the U.S. Food and Drug Administration, facilitating its use as a food additive and potentially in food packaging applications aimed at reducing carbon footprint.
As governments worldwide increasingly focus on sustainability and climate change mitigation, the regulatory framework for green chemical usage is likely to evolve. This may include more stringent reporting requirements for carbon emissions, expanded incentives for low-carbon technologies, and potentially mandatory targets for carbon footprint reduction in certain industries. These regulatory trends are expected to further support the adoption of sustainable practices and materials, including the use of sodium acetate for reduced carbon footprint.
At the international level, the Paris Agreement serves as a cornerstone for global efforts to reduce greenhouse gas emissions. While it does not specifically address sodium acetate, it provides a broad framework that encourages the adoption of low-carbon technologies and practices across industries. The United Nations Sustainable Development Goals, particularly Goal 12 (Responsible Consumption and Production) and Goal 13 (Climate Action), also influence the regulatory landscape for green chemicals.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation sets stringent standards for chemical safety and environmental protection. Sodium acetate, being a relatively benign substance, generally faces fewer restrictions under REACH compared to more hazardous chemicals. The EU's Circular Economy Action Plan further supports the use of sustainable chemicals and promotes the development of green technologies.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of chemicals under the Toxic Substances Control Act (TSCA). The EPA's Safer Choice program encourages the use of safer chemical ingredients in products, which could potentially benefit the adoption of sodium acetate as a green alternative. Additionally, state-level initiatives, such as California's Green Chemistry Initiative, provide further regulatory support for environmentally friendly chemical usage.
Many countries have implemented carbon pricing mechanisms, either through carbon taxes or cap-and-trade systems. These policies indirectly promote the use of low-carbon alternatives like sodium acetate by making carbon-intensive processes more expensive. For instance, the European Union Emissions Trading System (EU ETS) creates economic incentives for industries to reduce their carbon footprint.
Industry-specific regulations also play a role in shaping the use of green chemicals. For example, in the food industry, sodium acetate is recognized as a Generally Recognized as Safe (GRAS) substance by the U.S. Food and Drug Administration, facilitating its use as a food additive and potentially in food packaging applications aimed at reducing carbon footprint.
As governments worldwide increasingly focus on sustainability and climate change mitigation, the regulatory framework for green chemical usage is likely to evolve. This may include more stringent reporting requirements for carbon emissions, expanded incentives for low-carbon technologies, and potentially mandatory targets for carbon footprint reduction in certain industries. These regulatory trends are expected to further support the adoption of sustainable practices and materials, including the use of sodium acetate for reduced carbon footprint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!