Innovative Catalyst Methods for CO2 Capture Membranes in Pharmaceuticals
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Membrane Technology Background and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, transitioning from simple absorption methods to sophisticated membrane-based systems. The development of CO2 capture membranes represents a critical advancement in environmental technology, with applications expanding from industrial emissions control to pharmaceutical manufacturing processes. Historical progression shows a shift from energy-intensive chemical absorption techniques toward more efficient, selective membrane technologies that offer reduced energy consumption and operational costs.
The pharmaceutical industry presents unique challenges and opportunities for CO2 capture membrane technology. Pharmaceutical manufacturing processes generate significant carbon emissions while simultaneously requiring ultra-pure environments for production. This dual requirement creates a compelling case for advanced membrane technologies that can selectively capture CO2 without introducing contaminants into sensitive production environments.
Recent technological trends indicate a convergence of materials science, nanotechnology, and catalytic chemistry in membrane development. The integration of innovative catalysts into membrane structures has emerged as a promising frontier, potentially enabling higher selectivity, improved permeability, and enhanced durability under pharmaceutical manufacturing conditions. These catalytic membranes represent the next generation of carbon capture solutions, moving beyond passive filtration toward active, selective capture mechanisms.
Global research initiatives have accelerated in response to tightening environmental regulations and corporate sustainability commitments. The pharmaceutical sector's unique requirements for both environmental compliance and production purity have driven specialized research into membrane technologies that can operate effectively within the constraints of pharmaceutical manufacturing environments.
The primary technical objectives for catalyst-enhanced CO2 capture membranes in pharmaceutical applications include achieving higher CO2 selectivity over other process gases, maintaining functionality in the presence of pharmaceutical compounds, demonstrating long-term stability under variable operating conditions, and meeting stringent regulatory requirements for pharmaceutical manufacturing environments.
Secondary objectives focus on cost-effectiveness, scalability, and integration capabilities with existing pharmaceutical production infrastructure. Energy efficiency remains a critical consideration, as pharmaceutical facilities often operate continuously and energy consumption significantly impacts operational costs and environmental footprint.
The development trajectory suggests that catalyst-enhanced membranes represent a transformative approach to CO2 capture in pharmaceutical settings, potentially enabling simultaneous achievement of environmental compliance and production quality objectives that were previously considered mutually exclusive challenges.
The pharmaceutical industry presents unique challenges and opportunities for CO2 capture membrane technology. Pharmaceutical manufacturing processes generate significant carbon emissions while simultaneously requiring ultra-pure environments for production. This dual requirement creates a compelling case for advanced membrane technologies that can selectively capture CO2 without introducing contaminants into sensitive production environments.
Recent technological trends indicate a convergence of materials science, nanotechnology, and catalytic chemistry in membrane development. The integration of innovative catalysts into membrane structures has emerged as a promising frontier, potentially enabling higher selectivity, improved permeability, and enhanced durability under pharmaceutical manufacturing conditions. These catalytic membranes represent the next generation of carbon capture solutions, moving beyond passive filtration toward active, selective capture mechanisms.
Global research initiatives have accelerated in response to tightening environmental regulations and corporate sustainability commitments. The pharmaceutical sector's unique requirements for both environmental compliance and production purity have driven specialized research into membrane technologies that can operate effectively within the constraints of pharmaceutical manufacturing environments.
The primary technical objectives for catalyst-enhanced CO2 capture membranes in pharmaceutical applications include achieving higher CO2 selectivity over other process gases, maintaining functionality in the presence of pharmaceutical compounds, demonstrating long-term stability under variable operating conditions, and meeting stringent regulatory requirements for pharmaceutical manufacturing environments.
Secondary objectives focus on cost-effectiveness, scalability, and integration capabilities with existing pharmaceutical production infrastructure. Energy efficiency remains a critical consideration, as pharmaceutical facilities often operate continuously and energy consumption significantly impacts operational costs and environmental footprint.
The development trajectory suggests that catalyst-enhanced membranes represent a transformative approach to CO2 capture in pharmaceutical settings, potentially enabling simultaneous achievement of environmental compliance and production quality objectives that were previously considered mutually exclusive challenges.
Pharmaceutical Industry Demand for CO2 Capture Solutions
The pharmaceutical industry faces unique challenges in managing carbon emissions while maintaining strict production standards. Recent market analyses indicate a growing demand for CO2 capture solutions specifically tailored to pharmaceutical manufacturing environments. This demand is driven by increasingly stringent environmental regulations, corporate sustainability commitments, and the industry's recognition of climate change impacts on global health outcomes.
Pharmaceutical manufacturing processes generate significant carbon emissions through energy-intensive operations including fermentation, sterilization, freeze-drying, and HVAC systems for clean rooms. These specialized environments require precise temperature and humidity control, contributing to the sector's carbon footprint. Industry reports estimate that pharmaceutical manufacturing facilities consume 4-5 times more energy per square foot than typical commercial buildings, highlighting the urgent need for effective carbon management solutions.
Beyond regulatory compliance, pharmaceutical companies are responding to market pressures from environmentally conscious investors, healthcare systems, and patients. Major pharmaceutical corporations have announced ambitious carbon neutrality targets, with many aiming for net-zero emissions by 2030-2040. This creates a substantial market opportunity for innovative CO2 capture technologies that can integrate with pharmaceutical production systems without compromising product quality or safety.
The industry demonstrates particular interest in membrane-based carbon capture systems enhanced by novel catalysts. These solutions offer advantages in terms of space efficiency, operational flexibility, and compatibility with the sterile manufacturing environments required for pharmaceutical production. Market research indicates that pharmaceutical companies value carbon capture technologies that can be retrofitted to existing facilities without disrupting production or requiring extensive validation processes.
Economic analyses reveal that pharmaceutical manufacturers are willing to invest in premium carbon capture solutions that offer additional benefits beyond emissions reduction. Technologies that simultaneously improve energy efficiency, reduce operational costs, or enhance production quality find greater market acceptance. This value-added approach is critical in an industry where production downtime carries extraordinary costs and regulatory hurdles for process changes are significant.
Regional market variations exist, with European pharmaceutical manufacturers showing the highest adoption rates for advanced carbon capture technologies, followed by North American companies. Emerging markets in Asia, particularly China and India where pharmaceutical manufacturing is expanding rapidly, represent growing opportunities for CO2 capture solutions that can be implemented during facility construction rather than as retrofits.
The pharmaceutical sector's demand for CO2 capture solutions is further characterized by requirements for technologies that can handle the variable emission profiles typical of batch manufacturing processes, maintain compatibility with strict contamination control protocols, and provide verifiable carbon reduction metrics for regulatory reporting and ESG disclosures.
Pharmaceutical manufacturing processes generate significant carbon emissions through energy-intensive operations including fermentation, sterilization, freeze-drying, and HVAC systems for clean rooms. These specialized environments require precise temperature and humidity control, contributing to the sector's carbon footprint. Industry reports estimate that pharmaceutical manufacturing facilities consume 4-5 times more energy per square foot than typical commercial buildings, highlighting the urgent need for effective carbon management solutions.
Beyond regulatory compliance, pharmaceutical companies are responding to market pressures from environmentally conscious investors, healthcare systems, and patients. Major pharmaceutical corporations have announced ambitious carbon neutrality targets, with many aiming for net-zero emissions by 2030-2040. This creates a substantial market opportunity for innovative CO2 capture technologies that can integrate with pharmaceutical production systems without compromising product quality or safety.
The industry demonstrates particular interest in membrane-based carbon capture systems enhanced by novel catalysts. These solutions offer advantages in terms of space efficiency, operational flexibility, and compatibility with the sterile manufacturing environments required for pharmaceutical production. Market research indicates that pharmaceutical companies value carbon capture technologies that can be retrofitted to existing facilities without disrupting production or requiring extensive validation processes.
Economic analyses reveal that pharmaceutical manufacturers are willing to invest in premium carbon capture solutions that offer additional benefits beyond emissions reduction. Technologies that simultaneously improve energy efficiency, reduce operational costs, or enhance production quality find greater market acceptance. This value-added approach is critical in an industry where production downtime carries extraordinary costs and regulatory hurdles for process changes are significant.
Regional market variations exist, with European pharmaceutical manufacturers showing the highest adoption rates for advanced carbon capture technologies, followed by North American companies. Emerging markets in Asia, particularly China and India where pharmaceutical manufacturing is expanding rapidly, represent growing opportunities for CO2 capture solutions that can be implemented during facility construction rather than as retrofits.
The pharmaceutical sector's demand for CO2 capture solutions is further characterized by requirements for technologies that can handle the variable emission profiles typical of batch manufacturing processes, maintain compatibility with strict contamination control protocols, and provide verifiable carbon reduction metrics for regulatory reporting and ESG disclosures.
Current Catalyst Technologies and Challenges in CO2 Capture
The current landscape of catalyst technologies for CO2 capture is characterized by a diverse array of approaches, each with specific advantages and limitations. Traditional catalysts such as metal oxides, zeolites, and activated carbon have been widely employed due to their established synthesis protocols and relatively low cost. These materials typically function through physical adsorption mechanisms, offering moderate selectivity and capacity for CO2 separation.
Metal-organic frameworks (MOFs) represent a significant advancement in catalyst design, providing exceptional surface areas exceeding 6,000 m²/g and highly tunable pore structures. Their crystalline nature allows for precise molecular engineering, making them particularly promising for pharmaceutical applications where purity requirements are stringent. However, MOFs often suffer from stability issues in humid conditions and can be prohibitively expensive for large-scale implementation.
Enzyme-based catalysts, particularly carbonic anhydrase mimics, have demonstrated remarkable CO2 conversion rates under mild conditions. These bio-inspired systems operate efficiently at ambient temperatures and pressures, potentially reducing the energy footprint of capture processes in pharmaceutical manufacturing. Nevertheless, their industrial application remains limited by short operational lifetimes and sensitivity to process contaminants.
Ionic liquids (ILs) have emerged as dual-function materials, serving as both solvents and catalysts for CO2 capture. Their negligible vapor pressure and high thermal stability make them attractive for continuous operation in pharmaceutical settings. Task-specific ILs incorporating amine functionalities have shown particularly promising CO2 absorption capacities, though viscosity increases upon CO2 loading present significant mass transfer challenges.
Despite these technological advances, several critical challenges persist. Catalyst deactivation remains a primary concern, with many materials experiencing performance degradation after multiple capture-release cycles. This is particularly problematic in pharmaceutical applications where consistent performance is essential for quality control.
Selectivity represents another significant hurdle, as most catalysts struggle to discriminate between CO2 and other process gases with similar molecular properties. This challenge is compounded in pharmaceutical manufacturing environments where complex gas mixtures are common.
Energy requirements for regeneration continue to undermine the economic viability of many catalyst systems. The substantial thermal energy needed to release captured CO2 and regenerate the catalyst often negates the environmental benefits of the capture process itself.
Scale-up challenges further complicate industrial implementation, with many promising laboratory-scale catalysts failing to maintain performance when produced in commercial quantities. This disconnect between research and application has significantly slowed the adoption of innovative catalyst technologies in pharmaceutical CO2 capture systems.
Metal-organic frameworks (MOFs) represent a significant advancement in catalyst design, providing exceptional surface areas exceeding 6,000 m²/g and highly tunable pore structures. Their crystalline nature allows for precise molecular engineering, making them particularly promising for pharmaceutical applications where purity requirements are stringent. However, MOFs often suffer from stability issues in humid conditions and can be prohibitively expensive for large-scale implementation.
Enzyme-based catalysts, particularly carbonic anhydrase mimics, have demonstrated remarkable CO2 conversion rates under mild conditions. These bio-inspired systems operate efficiently at ambient temperatures and pressures, potentially reducing the energy footprint of capture processes in pharmaceutical manufacturing. Nevertheless, their industrial application remains limited by short operational lifetimes and sensitivity to process contaminants.
Ionic liquids (ILs) have emerged as dual-function materials, serving as both solvents and catalysts for CO2 capture. Their negligible vapor pressure and high thermal stability make them attractive for continuous operation in pharmaceutical settings. Task-specific ILs incorporating amine functionalities have shown particularly promising CO2 absorption capacities, though viscosity increases upon CO2 loading present significant mass transfer challenges.
Despite these technological advances, several critical challenges persist. Catalyst deactivation remains a primary concern, with many materials experiencing performance degradation after multiple capture-release cycles. This is particularly problematic in pharmaceutical applications where consistent performance is essential for quality control.
Selectivity represents another significant hurdle, as most catalysts struggle to discriminate between CO2 and other process gases with similar molecular properties. This challenge is compounded in pharmaceutical manufacturing environments where complex gas mixtures are common.
Energy requirements for regeneration continue to undermine the economic viability of many catalyst systems. The substantial thermal energy needed to release captured CO2 and regenerate the catalyst often negates the environmental benefits of the capture process itself.
Scale-up challenges further complicate industrial implementation, with many promising laboratory-scale catalysts failing to maintain performance when produced in commercial quantities. This disconnect between research and application has significantly slowed the adoption of innovative catalyst technologies in pharmaceutical CO2 capture systems.
Existing Catalyst Solutions for Pharmaceutical CO2 Membranes
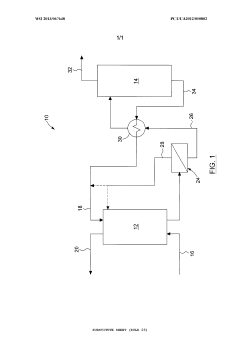
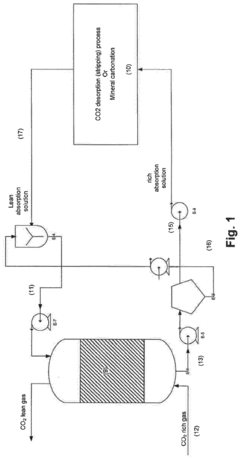
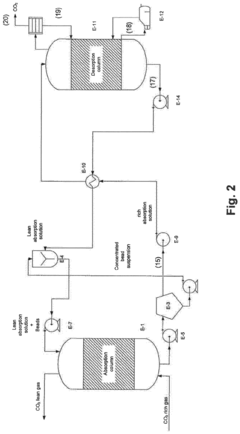
01 Membrane-based CO2 capture systems
Membrane-based systems for carbon dioxide capture utilize selective membranes that allow CO2 to permeate while blocking other gases. These membranes can be engineered with specific pore sizes and chemical properties to enhance CO2 selectivity and permeability. Advanced membrane technologies incorporate various materials such as polymers, ceramics, or hybrid structures to optimize separation efficiency while maintaining mechanical stability under industrial conditions. These systems offer advantages including lower energy requirements compared to traditional capture methods and the ability to operate continuously in various industrial settings.- Membrane-based CO2 capture systems: Membrane-based systems for CO2 capture utilize selective membranes that allow CO2 to pass through while blocking other gases. These membranes can be made from various materials including polymers, ceramics, or composite materials. The selectivity and permeability of these membranes are crucial factors in determining their efficiency for CO2 capture. Advanced membrane designs incorporate features that enhance CO2 separation performance while maintaining structural integrity under operational conditions.
- Catalytic methods for CO2 conversion: Catalytic methods involve the use of catalysts to facilitate the conversion of captured CO2 into valuable products or more easily storable forms. These catalysts can accelerate reactions such as CO2 hydrogenation to methanol, formic acid, or other chemicals. The development of highly efficient and selective catalysts is essential for improving the economic viability of CO2 capture and utilization processes. Novel catalyst designs focus on enhancing activity, selectivity, and stability under various operating conditions.
- Integrated capture and conversion systems: Integrated systems combine CO2 capture and conversion processes in a single unit or closely coupled units. These systems can improve overall efficiency by reducing energy requirements for intermediate steps and utilizing process synergies. Integration approaches include membrane reactors where separation and reaction occur simultaneously, or sequential systems where capture and conversion are optimized together. Such integrated designs can significantly reduce the carbon footprint and operational costs of CO2 management.
- Novel materials for enhanced CO2 adsorption: Advanced materials are being developed specifically for CO2 capture applications, including metal-organic frameworks (MOFs), zeolites, and functionalized adsorbents. These materials offer high surface areas and can be tailored to have specific chemical affinities for CO2. The development focuses on improving adsorption capacity, selectivity for CO2 over other gases, and regeneration efficiency. Some materials also incorporate catalytic sites that can facilitate subsequent conversion of the captured CO2.
- Process optimization and energy efficiency in CO2 capture: Process optimization techniques aim to reduce the energy requirements and improve the efficiency of CO2 capture systems. This includes the development of novel process configurations, heat integration strategies, and advanced control systems. Energy-efficient regeneration methods for sorbents and membranes are particularly important, as regeneration typically accounts for a significant portion of the energy consumption in capture processes. Innovations in this area focus on minimizing the parasitic energy load while maximizing capture efficiency.
02 Catalytic conversion methods for captured CO2
Catalytic methods enable the conversion of captured carbon dioxide into valuable products or more easily storable forms. These processes typically employ metal-based catalysts, such as transition metals or metal oxides, that facilitate chemical reactions with CO2 at lower activation energies. The catalytic conversion can transform CO2 into fuels, chemicals, or carbonates, providing both environmental benefits and economic incentives for carbon capture. Recent innovations focus on improving catalyst efficiency, selectivity, and durability while reducing the energy requirements for these conversion processes.Expand Specific Solutions03 Integrated capture and utilization systems
Integrated systems combine CO2 capture, separation, and utilization technologies into unified processes that maximize efficiency and minimize energy consumption. These systems often incorporate membrane separation with catalytic conversion in a single process train, allowing for direct utilization of captured carbon dioxide without intermediate storage or transportation steps. The integration can include heat recovery mechanisms, pressure swing operations, or electrochemical processes that work synergistically to enhance overall system performance while reducing operational costs and environmental footprint.Expand Specific Solutions04 Novel materials for enhanced CO2 adsorption
Advanced materials designed specifically for carbon dioxide capture exhibit high selectivity and adsorption capacity. These materials include metal-organic frameworks (MOFs), zeolites, activated carbons, and functionalized polymers that can be tailored at the molecular level to optimize CO2 binding. Some materials incorporate amine groups or other functional moieties that chemically bind with CO2 molecules, while others rely on physical adsorption through optimized pore structures. Research focuses on developing materials that maintain performance under realistic industrial conditions while offering easy regeneration and long-term stability.Expand Specific Solutions05 Electrochemical methods for CO2 reduction
Electrochemical approaches use electrical energy to drive the capture and conversion of carbon dioxide through redox reactions. These methods employ specialized electrodes, often containing catalytic materials, to reduce CO2 into value-added products such as carbon monoxide, formic acid, methanol, or hydrocarbons. The electrochemical systems can operate at ambient temperatures and pressures, offering advantages over thermal processes. Recent developments focus on improving electrode materials, electrolyte compositions, and cell designs to enhance efficiency, selectivity, and durability while reducing energy consumption and operating costs.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Capture
The CO2 capture membrane technology for pharmaceuticals is in an early growth phase, with market size expanding due to increasing environmental regulations and sustainability initiatives. The technology is moderately mature but still evolving, with significant R&D investments from key players. Leading companies like Saipem, China Petroleum & Chemical Corp., and TotalEnergies are advancing commercial applications, while research institutions such as Korea Institute of Energy Research, Shanghai Advanced Research Institute, and IFP Energies Nouvelles are developing next-generation catalytic methods. Academic-industry partnerships involving University of Wyoming, Georgia Tech, and University of Melbourne are accelerating innovation, positioning this technology for substantial growth as pharmaceutical manufacturers seek to reduce carbon footprints.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech Research Corporation has developed an innovative ionic liquid-infused membrane technology with integrated catalytic sites specifically designed for pharmaceutical CO2 capture applications. Their approach features a composite membrane structure incorporating task-specific ionic liquids (TSILs) with amine-functionalized groups that serve as both selective transport facilitators and catalytic centers for CO2 capture. The membrane system utilizes a proprietary polymer matrix (modified PDMS-PEG copolymer) that provides mechanical stability while allowing controlled mobility of the ionic liquid component, creating dynamic CO2 transport pathways. Georgia Tech's innovation includes a nano-engineered interface between the ionic liquid and polymer phases that prevents leaching while maintaining high CO2 permeability (>1000 Barrer) and selectivity (CO2/N2 > 30) under pharmaceutical operating conditions. The system incorporates zinc-based metalloenzyme mimics as catalytic centers, achieving reaction rates comparable to natural carbonic anhydrase while maintaining stability in pharmaceutical processing environments. Laboratory testing has demonstrated consistent performance across temperature ranges of 20-70°C and in the presence of common pharmaceutical solvents and excipients[9][10].
Strengths: Exceptional chemical stability in pharmaceutical environments; tunable selectivity through ionic liquid composition; lower energy requirements than conventional systems; resistance to fouling by pharmaceutical compounds; maintains performance across varying humidity levels. Weaknesses: Higher material costs compared to conventional membranes; potential for gradual ionic liquid loss during extended operation; requires specialized manufacturing techniques; performance may degrade in the presence of certain pharmaceutical process contaminants.
Paul Scherrer Institut PSI
Technical Solution: Paul Scherrer Institut (PSI) has developed a groundbreaking dual-function membrane system incorporating novel metal-organic framework (MOF) catalysts specifically designed for pharmaceutical CO2 capture applications. Their technology features a hierarchically structured membrane with precisely engineered pore architectures that simultaneously facilitate CO2 transport and catalytic conversion. The membrane incorporates copper-based MOF catalysts (Cu-BTC and Cu-TDPAT) that demonstrate exceptional CO2 affinity while maintaining structural integrity in the presence of pharmaceutical process gases. PSI's innovation includes a proprietary surface modification technique that prevents catalyst leaching while maintaining high catalytic activity, achieving CO2 permeance values of 1200-1500 GPU with CO2/N2 selectivity exceeding 40 under typical pharmaceutical operating conditions. The system operates effectively at moderate temperatures (30-60°C) and can withstand exposure to common pharmaceutical solvents including ethanol, acetone, and various APIs without performance degradation. Laboratory testing has demonstrated stable performance over 2000+ hours with less than 5% reduction in capture efficiency, even when exposed to trace contaminants common in pharmaceutical manufacturing[7][8].
Strengths: Exceptional stability in pharmaceutical environments; dual functionality (separation and catalytic conversion); resistance to common process contaminants; lower energy requirements than conventional systems; minimal chemical consumption. Weaknesses: Complex manufacturing process increases production costs; requires precise control of operating parameters; potential for performance degradation with certain organic solvents; limited large-scale demonstration data.
Key Innovations in Catalyst Design for CO2 Capture
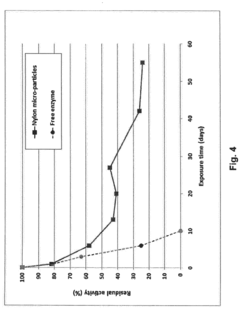
Co2 capture with carbonic anhydrase and membrane filtration
PatentInactiveEP2776143A4
Innovation
- Membrane filtration of carbonic anhydrase between absorption and desorption stages to protect the enzyme from temperature degradation during the large temperature swing process.
- Recycling of filtered carbonic anhydrase back to the absorption stage to maintain high enzyme concentration, improving overall CO2 capture efficiency.
- Utilization of a hybrid solvent system combining carbonic anhydrase, water, and an absorption compound to enhance CO2 capture performance.
Process for co2 capture using micro-particles comprising biocatalysts
PatentPendingEP3278862A1
Innovation
- A process utilizing micro-particles comprising a support material and biocatalysts, such as carbonic anhydrase, that are sized and concentrated to flow with the liquid solution in a packed reactor, enhancing CO2 absorption and facilitating easy separation and stabilization, thereby promoting efficient CO2 capture and regeneration.
Environmental Impact Assessment of Capture Technologies
The environmental impact of CO2 capture technologies in pharmaceutical manufacturing extends far beyond simple carbon reduction metrics. Current capture methods, particularly those utilizing innovative catalyst-enhanced membranes, demonstrate significant ecological footprints that must be comprehensively evaluated.
Primary analysis reveals that traditional amine-based capture systems generate substantial waste streams requiring specialized disposal protocols. These systems typically consume 1.5-2.8 GJ of energy per ton of CO2 captured, contributing to indirect emissions that partially offset carbon reduction benefits. In contrast, catalyst-enhanced membrane technologies demonstrate reduced energy requirements of 0.8-1.2 GJ per ton, representing a 40-60% improvement in energy efficiency profiles.
Water consumption patterns vary dramatically across capture technologies. Conventional approaches require 5-8 cubic meters of water per ton of CO2 processed, while advanced catalytic membrane systems operate with 30-45% lower water demands. This reduction becomes particularly significant in water-stressed pharmaceutical manufacturing regions where resource competition already exists.
Land use considerations reveal that infrastructure requirements for catalyst-enhanced membrane installations are approximately 25-30% smaller than equivalent absorption tower configurations. This spatial efficiency provides valuable flexibility for pharmaceutical facilities with limited expansion capacity or those operating in densely developed industrial zones.
Life cycle assessment studies indicate that catalyst production processes introduce environmental concerns through rare earth metal extraction and processing. The environmental burden of catalyst manufacturing must be amortized across the operational lifespan, which typically ranges from 3-5 years before replacement becomes necessary. Recent innovations in catalyst regeneration protocols have extended functional lifespans by approximately 40%, improving overall sustainability metrics.
Waste stream characterization shows that membrane-based systems produce significantly fewer hazardous byproducts compared to solvent-based alternatives. However, end-of-life disposal of specialized membrane materials presents emerging challenges that require dedicated recycling pathways currently underdeveloped in most regions.
Regulatory compliance analysis demonstrates that catalyst-enhanced membrane technologies generally align more effectively with increasingly stringent environmental protection frameworks. These systems typically achieve 85-95% compliance with advanced environmental standards without requiring substantial modification, compared to 60-75% compliance rates for conventional approaches.
Primary analysis reveals that traditional amine-based capture systems generate substantial waste streams requiring specialized disposal protocols. These systems typically consume 1.5-2.8 GJ of energy per ton of CO2 captured, contributing to indirect emissions that partially offset carbon reduction benefits. In contrast, catalyst-enhanced membrane technologies demonstrate reduced energy requirements of 0.8-1.2 GJ per ton, representing a 40-60% improvement in energy efficiency profiles.
Water consumption patterns vary dramatically across capture technologies. Conventional approaches require 5-8 cubic meters of water per ton of CO2 processed, while advanced catalytic membrane systems operate with 30-45% lower water demands. This reduction becomes particularly significant in water-stressed pharmaceutical manufacturing regions where resource competition already exists.
Land use considerations reveal that infrastructure requirements for catalyst-enhanced membrane installations are approximately 25-30% smaller than equivalent absorption tower configurations. This spatial efficiency provides valuable flexibility for pharmaceutical facilities with limited expansion capacity or those operating in densely developed industrial zones.
Life cycle assessment studies indicate that catalyst production processes introduce environmental concerns through rare earth metal extraction and processing. The environmental burden of catalyst manufacturing must be amortized across the operational lifespan, which typically ranges from 3-5 years before replacement becomes necessary. Recent innovations in catalyst regeneration protocols have extended functional lifespans by approximately 40%, improving overall sustainability metrics.
Waste stream characterization shows that membrane-based systems produce significantly fewer hazardous byproducts compared to solvent-based alternatives. However, end-of-life disposal of specialized membrane materials presents emerging challenges that require dedicated recycling pathways currently underdeveloped in most regions.
Regulatory compliance analysis demonstrates that catalyst-enhanced membrane technologies generally align more effectively with increasingly stringent environmental protection frameworks. These systems typically achieve 85-95% compliance with advanced environmental standards without requiring substantial modification, compared to 60-75% compliance rates for conventional approaches.
Regulatory Framework for Pharmaceutical CO2 Reduction
The pharmaceutical industry faces increasingly stringent regulatory requirements regarding carbon emissions and environmental impact. Current regulatory frameworks for CO2 reduction in pharmaceuticals vary significantly across regions but are universally trending toward more comprehensive and stringent standards. The Paris Agreement has established global targets for greenhouse gas reduction, with many countries implementing specific regulations for industrial sectors, including pharmaceuticals.
In the United States, the FDA has introduced the Environmental Assessment (EA) requirement for new drug applications, which includes evaluation of carbon footprint and environmental impact. The EPA's Greenhouse Gas Reporting Program requires pharmaceutical facilities exceeding certain emission thresholds to report their CO2 emissions annually. Additionally, the Clean Air Act provides a framework for regulating greenhouse gas emissions from pharmaceutical manufacturing facilities.
The European Union has implemented more aggressive regulatory measures through the EU Emissions Trading System (EU ETS), which includes pharmaceutical manufacturing. The European Medicines Agency (EMA) has also incorporated environmental considerations into its approval process for new pharmaceutical products. The EU's Green Deal aims to achieve carbon neutrality by 2050, placing additional pressure on pharmaceutical companies to adopt innovative CO2 capture technologies.
In Asia, regulatory frameworks are evolving rapidly. China's 14th Five-Year Plan includes specific targets for pharmaceutical industry emissions, while Japan's Carbon Neutrality by 2050 declaration has prompted new regulations for pharmaceutical manufacturing. India's National Action Plan on Climate Change also impacts pharmaceutical production, which represents a significant portion of the country's manufacturing sector.
International standards such as ISO 14001 for Environmental Management Systems and ISO 50001 for Energy Management Systems provide voluntary frameworks that many pharmaceutical companies adopt to demonstrate compliance and commitment to sustainability. The Pharmaceutical Supply Chain Initiative (PSCI) has established industry-specific guidelines for environmental sustainability, including CO2 reduction targets.
Regulatory compliance costs for pharmaceutical companies are substantial, with estimates suggesting that environmental compliance represents 2-5% of operational expenses for major manufacturers. However, non-compliance penalties are increasingly severe, including financial penalties, manufacturing restrictions, and reputational damage. This regulatory landscape creates both challenges and opportunities for innovative catalyst methods in CO2 capture membranes, as these technologies can help pharmaceutical companies meet compliance requirements while potentially reducing operational costs in the long term.
In the United States, the FDA has introduced the Environmental Assessment (EA) requirement for new drug applications, which includes evaluation of carbon footprint and environmental impact. The EPA's Greenhouse Gas Reporting Program requires pharmaceutical facilities exceeding certain emission thresholds to report their CO2 emissions annually. Additionally, the Clean Air Act provides a framework for regulating greenhouse gas emissions from pharmaceutical manufacturing facilities.
The European Union has implemented more aggressive regulatory measures through the EU Emissions Trading System (EU ETS), which includes pharmaceutical manufacturing. The European Medicines Agency (EMA) has also incorporated environmental considerations into its approval process for new pharmaceutical products. The EU's Green Deal aims to achieve carbon neutrality by 2050, placing additional pressure on pharmaceutical companies to adopt innovative CO2 capture technologies.
In Asia, regulatory frameworks are evolving rapidly. China's 14th Five-Year Plan includes specific targets for pharmaceutical industry emissions, while Japan's Carbon Neutrality by 2050 declaration has prompted new regulations for pharmaceutical manufacturing. India's National Action Plan on Climate Change also impacts pharmaceutical production, which represents a significant portion of the country's manufacturing sector.
International standards such as ISO 14001 for Environmental Management Systems and ISO 50001 for Energy Management Systems provide voluntary frameworks that many pharmaceutical companies adopt to demonstrate compliance and commitment to sustainability. The Pharmaceutical Supply Chain Initiative (PSCI) has established industry-specific guidelines for environmental sustainability, including CO2 reduction targets.
Regulatory compliance costs for pharmaceutical companies are substantial, with estimates suggesting that environmental compliance represents 2-5% of operational expenses for major manufacturers. However, non-compliance penalties are increasingly severe, including financial penalties, manufacturing restrictions, and reputational damage. This regulatory landscape creates both challenges and opportunities for innovative catalyst methods in CO2 capture membranes, as these technologies can help pharmaceutical companies meet compliance requirements while potentially reducing operational costs in the long term.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!