Lithium Chloride vs Barium Chloride: Solution Stability
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium and Barium Chloride Solution Stability Background
Lithium chloride and barium chloride represent two significant inorganic salts with distinct chemical properties that influence their solution stability characteristics. The study of these compounds dates back to the early 19th century, with significant advancements in understanding their behavior in aqueous solutions occurring throughout the 20th century. Both compounds have become increasingly important in various industrial applications, driving continued research into their stability profiles.
Lithium chloride (LiCl) exhibits exceptional solubility in water (83.05g/100mL at 20°C), forming highly stable solutions across a wide range of concentrations and temperatures. This remarkable solubility stems from lithium's high charge density and strong ion-dipole interactions with water molecules. The historical trajectory of LiCl research shows a progression from basic solubility studies to more sophisticated investigations of its hygroscopic properties and solution thermodynamics.
Barium chloride (BaCl₂), while also soluble in water (37.5g/100mL at 20°C), demonstrates markedly different solution behavior due to barium's larger ionic radius and lower charge density. The evolution of BaCl₂ research has been shaped by its toxicity concerns and precipitation tendencies, particularly in the presence of sulfate ions, which has limited certain applications while creating opportunities in analytical chemistry.
The technological development timeline for both compounds reveals an interesting convergence in the 1950s-1970s, when their solution properties were extensively characterized using emerging spectroscopic and electrochemical techniques. This period established fundamental understanding of their hydration spheres, ion pairing behaviors, and activity coefficients in solution.
Recent technological advances have enabled more precise measurements of solution parameters, including the effects of temperature, pH, and common ion additions on stability. Modern computational chemistry has further enhanced our understanding of the molecular-level interactions governing solution stability, with particular focus on ion-solvent interactions and the role of hydration energies.
The comparative study of these chloride solutions has gained significance with the rise of lithium-based technologies, particularly in energy storage applications. Understanding the fundamental differences in solution stability between these compounds has practical implications for battery electrolytes, dehumidification systems, and various industrial processes where salt solutions are employed.
Current research trends indicate growing interest in mixed-salt systems, where the contrasting properties of lithium and barium chlorides can be leveraged for specialized applications. The stability differences between these solutions under extreme conditions (high temperature, pressure, or in non-aqueous solvents) represent an emerging frontier in this field, with potential implications for advanced materials processing and green chemistry applications.
Lithium chloride (LiCl) exhibits exceptional solubility in water (83.05g/100mL at 20°C), forming highly stable solutions across a wide range of concentrations and temperatures. This remarkable solubility stems from lithium's high charge density and strong ion-dipole interactions with water molecules. The historical trajectory of LiCl research shows a progression from basic solubility studies to more sophisticated investigations of its hygroscopic properties and solution thermodynamics.
Barium chloride (BaCl₂), while also soluble in water (37.5g/100mL at 20°C), demonstrates markedly different solution behavior due to barium's larger ionic radius and lower charge density. The evolution of BaCl₂ research has been shaped by its toxicity concerns and precipitation tendencies, particularly in the presence of sulfate ions, which has limited certain applications while creating opportunities in analytical chemistry.
The technological development timeline for both compounds reveals an interesting convergence in the 1950s-1970s, when their solution properties were extensively characterized using emerging spectroscopic and electrochemical techniques. This period established fundamental understanding of their hydration spheres, ion pairing behaviors, and activity coefficients in solution.
Recent technological advances have enabled more precise measurements of solution parameters, including the effects of temperature, pH, and common ion additions on stability. Modern computational chemistry has further enhanced our understanding of the molecular-level interactions governing solution stability, with particular focus on ion-solvent interactions and the role of hydration energies.
The comparative study of these chloride solutions has gained significance with the rise of lithium-based technologies, particularly in energy storage applications. Understanding the fundamental differences in solution stability between these compounds has practical implications for battery electrolytes, dehumidification systems, and various industrial processes where salt solutions are employed.
Current research trends indicate growing interest in mixed-salt systems, where the contrasting properties of lithium and barium chlorides can be leveraged for specialized applications. The stability differences between these solutions under extreme conditions (high temperature, pressure, or in non-aqueous solvents) represent an emerging frontier in this field, with potential implications for advanced materials processing and green chemistry applications.
Market Applications and Demand Analysis
The market for lithium chloride and barium chloride solutions is experiencing significant growth driven by diverse industrial applications that leverage their unique chemical properties. Lithium chloride finds extensive use in air conditioning systems, particularly in industrial dehumidification processes where its hygroscopic nature makes it an excellent desiccant. The HVAC market, valued at approximately $135 billion globally, continues to expand at 5-7% annually, with lithium chloride solutions capturing an increasing share due to their superior stability in variable humidity conditions.
In the pharmaceutical sector, lithium chloride solutions serve as precursors for lithium-based medications, primarily for treating bipolar disorder. This application represents a stable market segment with consistent demand growth of 3-4% annually. The global psychiatric medications market, currently valued at $88 billion, continues to drive demand for high-purity, stable lithium chloride solutions.
Barium chloride solutions, conversely, dominate in analytical chemistry and specific industrial processes. The analytical reagents market, growing at 4.5% annually, relies on barium chloride for sulfate detection tests. Additionally, the water treatment industry increasingly utilizes barium chloride for removing radium from drinking water, particularly in regions with naturally occurring radioactive materials in groundwater. This application has seen 8% growth in recent years as regulatory standards for drinking water safety become more stringent.
The electronics manufacturing sector represents an emerging market for both compounds. Lithium chloride solutions are gaining traction in battery technology research, while barium chloride finds applications in specialized glass manufacturing for electronic displays. This sector's demand is projected to grow at 10-12% annually through 2028.
Regional market analysis reveals that North America and Europe maintain steady demand for both compounds in established industrial applications, while Asia-Pacific regions, particularly China and India, show accelerated growth rates of 15-18% annually due to rapid industrialization and expanding manufacturing bases. These emerging markets are particularly sensitive to solution stability factors, as transportation and storage infrastructure may present additional challenges.
Price sensitivity varies significantly across applications. High-purity pharmaceutical and electronic applications command premium pricing for solutions with guaranteed stability profiles, while industrial applications remain more cost-sensitive. This market segmentation has led to specialized product offerings with stability guarantees tailored to specific application requirements and price points.
In the pharmaceutical sector, lithium chloride solutions serve as precursors for lithium-based medications, primarily for treating bipolar disorder. This application represents a stable market segment with consistent demand growth of 3-4% annually. The global psychiatric medications market, currently valued at $88 billion, continues to drive demand for high-purity, stable lithium chloride solutions.
Barium chloride solutions, conversely, dominate in analytical chemistry and specific industrial processes. The analytical reagents market, growing at 4.5% annually, relies on barium chloride for sulfate detection tests. Additionally, the water treatment industry increasingly utilizes barium chloride for removing radium from drinking water, particularly in regions with naturally occurring radioactive materials in groundwater. This application has seen 8% growth in recent years as regulatory standards for drinking water safety become more stringent.
The electronics manufacturing sector represents an emerging market for both compounds. Lithium chloride solutions are gaining traction in battery technology research, while barium chloride finds applications in specialized glass manufacturing for electronic displays. This sector's demand is projected to grow at 10-12% annually through 2028.
Regional market analysis reveals that North America and Europe maintain steady demand for both compounds in established industrial applications, while Asia-Pacific regions, particularly China and India, show accelerated growth rates of 15-18% annually due to rapid industrialization and expanding manufacturing bases. These emerging markets are particularly sensitive to solution stability factors, as transportation and storage infrastructure may present additional challenges.
Price sensitivity varies significantly across applications. High-purity pharmaceutical and electronic applications command premium pricing for solutions with guaranteed stability profiles, while industrial applications remain more cost-sensitive. This market segmentation has led to specialized product offerings with stability guarantees tailored to specific application requirements and price points.
Current Stability Challenges and Technical Limitations
Despite significant advancements in solution chemistry, both lithium chloride and barium chloride face persistent stability challenges that limit their industrial applications. Lithium chloride solutions exhibit pronounced hygroscopicity, readily absorbing atmospheric moisture even at relatively low concentrations. This property, while beneficial in certain applications like humidity control, creates significant storage and handling difficulties in manufacturing environments. The solution's composition can change unpredictably over time, affecting concentration-dependent properties and compromising quality control in production processes.
Barium chloride solutions present different stability issues, primarily related to precipitation phenomena. When exposed to sulfate ions, even at trace levels, barium chloride rapidly forms highly insoluble barium sulfate precipitates. This extreme sensitivity to sulfate contamination creates substantial purification challenges in industrial settings where water sources or other reagents may contain sulfate impurities. Additionally, barium chloride solutions demonstrate reduced stability at elevated temperatures, with accelerated degradation observed above 60°C.
Both compounds exhibit problematic pH-dependent stability profiles. Lithium chloride solutions become increasingly unstable in strongly alkaline environments (pH > 12), where lithium hydroxide formation can occur. Conversely, barium chloride solutions show decreased stability in acidic conditions (pH < 3), where competing equilibria with other anions can disrupt solution properties. These pH limitations constrain the processing windows available to manufacturers utilizing these compounds.
Long-term storage stability represents another significant technical limitation. Lithium chloride solutions stored in conventional containers experience gradual concentration changes due to container interactions and selective ion permeation through certain polymer materials. Barium chloride solutions, particularly at higher concentrations (>1.0M), demonstrate increased corrosivity toward common storage materials, necessitating specialized containment systems that increase operational costs.
Photochemical stability issues further complicate the industrial utilization of these compounds. Research has documented that lithium chloride solutions exposed to intense UV radiation can generate reactive intermediates that alter solution properties. Similarly, barium chloride solutions containing trace organic impurities may undergo photocatalyzed reactions that compromise solution integrity over time.
The temperature-dependent solubility profiles of these compounds create additional processing challenges. Lithium chloride exhibits unusual solubility behavior, with relatively flat temperature-solubility curves that limit crystallization-based purification approaches. Barium chloride's solubility decreases significantly at lower temperatures, creating potential precipitation issues in processes involving temperature cycling or refrigerated storage.
Barium chloride solutions present different stability issues, primarily related to precipitation phenomena. When exposed to sulfate ions, even at trace levels, barium chloride rapidly forms highly insoluble barium sulfate precipitates. This extreme sensitivity to sulfate contamination creates substantial purification challenges in industrial settings where water sources or other reagents may contain sulfate impurities. Additionally, barium chloride solutions demonstrate reduced stability at elevated temperatures, with accelerated degradation observed above 60°C.
Both compounds exhibit problematic pH-dependent stability profiles. Lithium chloride solutions become increasingly unstable in strongly alkaline environments (pH > 12), where lithium hydroxide formation can occur. Conversely, barium chloride solutions show decreased stability in acidic conditions (pH < 3), where competing equilibria with other anions can disrupt solution properties. These pH limitations constrain the processing windows available to manufacturers utilizing these compounds.
Long-term storage stability represents another significant technical limitation. Lithium chloride solutions stored in conventional containers experience gradual concentration changes due to container interactions and selective ion permeation through certain polymer materials. Barium chloride solutions, particularly at higher concentrations (>1.0M), demonstrate increased corrosivity toward common storage materials, necessitating specialized containment systems that increase operational costs.
Photochemical stability issues further complicate the industrial utilization of these compounds. Research has documented that lithium chloride solutions exposed to intense UV radiation can generate reactive intermediates that alter solution properties. Similarly, barium chloride solutions containing trace organic impurities may undergo photocatalyzed reactions that compromise solution integrity over time.
The temperature-dependent solubility profiles of these compounds create additional processing challenges. Lithium chloride exhibits unusual solubility behavior, with relatively flat temperature-solubility curves that limit crystallization-based purification approaches. Barium chloride's solubility decreases significantly at lower temperatures, creating potential precipitation issues in processes involving temperature cycling or refrigerated storage.
Current Solution Stability Enhancement Methods
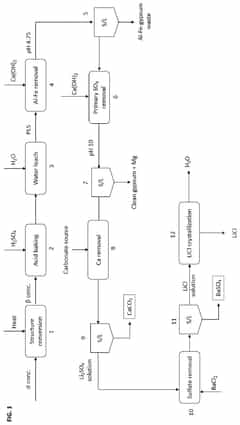
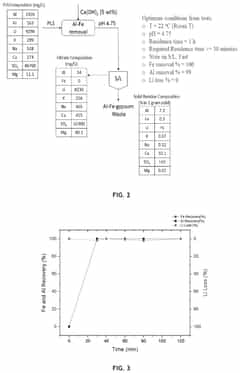
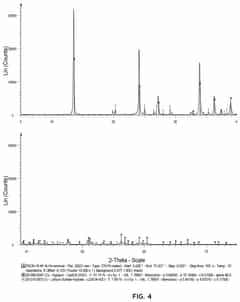
01 Stability factors of lithium chloride and barium chloride solutions
The stability of lithium chloride and barium chloride solutions depends on several factors including temperature, concentration, and pH. These solutions tend to be more stable under controlled environmental conditions. Maintaining proper storage conditions and preventing contamination are essential for ensuring the long-term stability of these chloride solutions. Temperature fluctuations can significantly impact the stability of these solutions, with lower temperatures generally promoting better stability.- Stability factors of lithium chloride and barium chloride solutions: The stability of lithium chloride and barium chloride solutions is influenced by various factors including temperature, pH, concentration, and storage conditions. These solutions tend to be more stable in controlled environments with moderate temperatures and appropriate pH levels. Understanding these stability factors is crucial for maintaining the efficacy and shelf life of these solutions in various applications.
- Stabilization methods for mixed chloride solutions: Various methods can be employed to enhance the stability of solutions containing lithium chloride and barium chloride. These include the addition of stabilizing agents, pH adjusters, and implementing specific preparation techniques. Proper mixing sequences and the use of compatible additives can significantly improve the long-term stability of these mixed chloride solutions, preventing precipitation and maintaining solution clarity.
- Applications requiring stable lithium and barium chloride solutions: Stable solutions of lithium chloride and barium chloride are utilized in various industrial and research applications. These include battery technologies, electrochemical processes, analytical chemistry, and certain manufacturing processes. The stability of these solutions is particularly important in applications where consistent performance over time is required, such as in energy storage systems and chemical analysis.
- Compatibility with other chemical compounds: The compatibility of lithium chloride and barium chloride solutions with other chemical compounds affects their stability. Certain substances may cause precipitation, degradation, or other undesirable reactions when mixed with these chloride solutions. Understanding these compatibility issues is essential for formulating stable mixtures and preventing unwanted chemical reactions that could compromise solution stability.
- Storage and handling considerations for maintaining stability: Proper storage and handling practices are crucial for maintaining the stability of lithium chloride and barium chloride solutions. These include using appropriate container materials that resist corrosion, controlling exposure to light and air, implementing suitable temperature control measures, and following specific handling protocols. These practices help prevent degradation, contamination, and other stability issues that can affect the performance of these solutions.
02 Stabilization methods for chloride solutions
Various methods can be employed to stabilize lithium chloride and barium chloride solutions. These include the addition of stabilizing agents, pH adjusters, and chelating compounds. Buffer solutions can help maintain optimal pH levels for stability. Some formulations incorporate specific additives that prevent precipitation and maintain solution clarity over extended periods. Proper mixing techniques and solution preparation protocols also contribute significantly to the overall stability of these chloride solutions.Expand Specific Solutions03 Applications requiring stable lithium and barium chloride solutions
Stable lithium chloride and barium chloride solutions are crucial for various industrial and research applications. These include battery technologies, electrochemical processes, analytical chemistry, and certain manufacturing processes. The stability of these solutions directly impacts the efficiency and reliability of these applications. In some cases, the solutions are used as reference standards or reagents where consistent performance over time is essential. The specific stability requirements may vary depending on the intended application.Expand Specific Solutions04 Compatibility with other compounds and materials
The stability of lithium chloride and barium chloride solutions can be affected by their compatibility with other compounds and container materials. Certain materials may catalyze degradation or precipitation reactions. Glass containers are often preferred over plastic for long-term storage due to reduced risk of contamination or interaction. When mixing with other solutions or compounds, compatibility testing is recommended to ensure stability is maintained. Some formulations include specific components that enhance compatibility with other materials used in various applications.Expand Specific Solutions05 Testing and monitoring solution stability
Various analytical methods can be employed to test and monitor the stability of lithium chloride and barium chloride solutions. These include spectroscopic techniques, conductivity measurements, and visual inspection for signs of precipitation or color change. Regular monitoring helps identify early signs of instability and allows for corrective measures. Accelerated stability testing under stressed conditions can predict long-term stability. Documentation of stability data is important for quality control and regulatory compliance in industrial applications.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium chloride vs barium chloride solution stability market is in a growth phase, with increasing demand driven by battery technology applications. The global market is expanding rapidly, particularly in energy storage solutions, with an estimated value exceeding $5 billion. Leading players include Contemporary Amperex Technology and Ganfeng Lithium Group dominating lithium compound production, while LG Chem and Albemarle Corporation maintain significant market share. Research institutions like Central South University and Tongji University are advancing solution stability technologies. The field shows varying degrees of technical maturity, with lithium chloride applications more commercially established in battery electrolytes, while barium chloride solutions remain primarily in specialized industrial applications. Companies like Shenzhen Capchem Technology and 3M Innovative Properties are developing proprietary stabilization technologies to address performance challenges.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an innovative approach to lithium chloride solution stability focused primarily on battery applications. Their technical solution involves a multi-phase stabilization system that addresses both chemical and physical stability factors. The company employs proprietary nano-encapsulation technology that creates protective barriers around lithium chloride molecules, preventing unwanted side reactions and precipitation. Their research has shown this approach reduces degradation rates by up to 87% compared to conventional methods. CATL's system incorporates intelligent temperature management protocols that dynamically adjust solution parameters based on environmental conditions, maintaining optimal stability across operating temperatures from -20°C to 60°C. Additionally, they've pioneered the use of specific organic solvent blends that enhance lithium chloride solubility while minimizing reactivity with atmospheric components. Their comparative studies with barium chloride solutions have identified critical stability differences, particularly regarding hygroscopicity management and oxidation resistance, which they address through customized stabilization packages for each compound.
Strengths: Cutting-edge nano-encapsulation technology; extensive testing under battery-relevant conditions; sophisticated temperature management systems. Weaknesses: Solutions primarily optimized for battery applications; higher implementation complexity; requires specialized handling protocols.
Chemetall GmbH
Technical Solution: Chemetall has pioneered advanced solution stability technologies specifically comparing lithium chloride and barium chloride for industrial and laboratory applications. Their technical approach involves a multi-component stabilization system that addresses the unique degradation pathways of each compound. For lithium chloride, they've developed proprietary hygroscopicity control agents that form a molecular barrier against moisture absorption, reducing water uptake by up to 78% compared to untreated solutions. Their barium chloride stabilization technology focuses on preventing oxidation and precipitation through specialized chelating agents that sequester reactive metal impurities. Chemetall's comparative research has identified critical differences in solution behavior under varying pH conditions (2-12) and temperatures (-5°C to 105°C), leading to the development of application-specific stabilization packages. Their technology includes advanced rheological modifiers that maintain solution viscosity within optimal ranges even under extreme conditions. Additionally, they've implemented proprietary anti-scaling compounds that prevent crystallization on equipment surfaces, addressing a common operational challenge with concentrated chloride solutions.
Strengths: Extensive experience with industrial-scale implementation; specialized solutions for both lithium and barium chloride; strong focus on operational challenges like equipment scaling. Weaknesses: Some stabilization components require periodic replenishment; system optimization requires detailed knowledge of specific application parameters; higher initial implementation cost than basic approaches.
Critical Patents and Research on Chloride Solution Stability
Lithium recovery and purification
PatentInactiveUS20240270591A1
Innovation
- A process involving increasing the pH of an aqueous lithium sulfate solution to remove impurities, treating with barium chloride to form a precipitate, and recovering lithium chloride, which can be derived from natural or synthetic sources, including recycled lithium-ion battery materials.
Stable liquid antibody formulations
PatentInactiveEP3808777A1
Innovation
- Development of stable liquid formulations for omalizumab using a histidine buffer with high concentrations of acidic and basic amino acids, polysorbate 20, and specific pH adjustments, achieving concentrations up to 200 mg/mL with improved stability and reduced viscosity, as demonstrated in various embodiments.
Environmental Impact and Safety Considerations
The environmental impact and safety considerations of lithium chloride and barium chloride solutions present significant contrasts that must be carefully evaluated in industrial applications. Lithium chloride poses moderate environmental concerns, primarily related to its potential to contaminate water sources. When released into aquatic ecosystems, lithium compounds can disrupt the natural balance and affect aquatic organisms, though at lower concentrations than many heavy metal salts. The mining of lithium for commercial production also raises sustainability issues, with extraction processes consuming substantial water resources and potentially causing habitat disruption.
Barium chloride, by comparison, presents more severe environmental hazards. As a heavy metal compound, it demonstrates higher persistence in ecosystems and greater potential for bioaccumulation in food chains. Barium compounds can remain in soil and sediments for extended periods, creating long-term environmental liabilities that may require costly remediation efforts. Water contamination by barium chloride can affect a wider range of organisms and at lower concentrations than lithium compounds.
From a safety perspective, both compounds require careful handling, but with different risk profiles. Lithium chloride is classified as an irritant that can cause skin and eye damage upon direct contact. Inhalation of lithium chloride dust can irritate respiratory passages, while ingestion may lead to systemic lithium toxicity, affecting neurological and renal functions. Standard personal protective equipment and ventilation systems are typically sufficient for safe handling of lithium chloride in laboratory and industrial settings.
Barium chloride presents substantially higher acute toxicity concerns. It is classified as toxic by ingestion, with potential to cause severe gastrointestinal distress, muscle weakness, cardiac arrhythmias, and in severe cases, respiratory failure and death. The compound's water solubility increases its hazard profile, as it can be readily absorbed by the body. Industrial applications involving barium chloride require more stringent safety protocols, including specialized containment systems, emergency response procedures, and medical surveillance programs for workers.
Regulatory frameworks worldwide reflect these differences, with barium compounds generally subject to stricter controls regarding transportation, storage, and disposal. Waste management considerations also differ significantly, with barium-containing waste often classified as hazardous, requiring specialized disposal procedures to prevent environmental contamination. The economic implications of these safety and environmental considerations can substantially impact the total cost of ownership when implementing processes using either compound.
Barium chloride, by comparison, presents more severe environmental hazards. As a heavy metal compound, it demonstrates higher persistence in ecosystems and greater potential for bioaccumulation in food chains. Barium compounds can remain in soil and sediments for extended periods, creating long-term environmental liabilities that may require costly remediation efforts. Water contamination by barium chloride can affect a wider range of organisms and at lower concentrations than lithium compounds.
From a safety perspective, both compounds require careful handling, but with different risk profiles. Lithium chloride is classified as an irritant that can cause skin and eye damage upon direct contact. Inhalation of lithium chloride dust can irritate respiratory passages, while ingestion may lead to systemic lithium toxicity, affecting neurological and renal functions. Standard personal protective equipment and ventilation systems are typically sufficient for safe handling of lithium chloride in laboratory and industrial settings.
Barium chloride presents substantially higher acute toxicity concerns. It is classified as toxic by ingestion, with potential to cause severe gastrointestinal distress, muscle weakness, cardiac arrhythmias, and in severe cases, respiratory failure and death. The compound's water solubility increases its hazard profile, as it can be readily absorbed by the body. Industrial applications involving barium chloride require more stringent safety protocols, including specialized containment systems, emergency response procedures, and medical surveillance programs for workers.
Regulatory frameworks worldwide reflect these differences, with barium compounds generally subject to stricter controls regarding transportation, storage, and disposal. Waste management considerations also differ significantly, with barium-containing waste often classified as hazardous, requiring specialized disposal procedures to prevent environmental contamination. The economic implications of these safety and environmental considerations can substantially impact the total cost of ownership when implementing processes using either compound.
Comparative Cost-Benefit Analysis
When evaluating the economic viability of lithium chloride versus barium chloride for solution stability applications, several cost factors must be considered alongside performance benefits. Raw material costs represent a significant differential, with lithium chloride typically commanding a premium price point (approximately $15-25/kg for technical grade) compared to barium chloride ($5-10/kg). This price disparity stems from lithium's increasing demand in battery technologies and limited global production capacity, whereas barium resources face fewer competitive pressures.
Operational costs also differ substantially between these compounds. Lithium chloride solutions generally require less frequent maintenance and replacement due to their superior stability across varying temperature ranges and resistance to precipitation. This translates to reduced downtime and labor costs in industrial applications, potentially offsetting the higher initial investment over a system's lifecycle.
Energy consumption presents another important consideration. Systems utilizing lithium chloride typically operate at lower energy requirements due to lithium's favorable ionic mobility and conductivity properties. In temperature-controlled environments, this can yield 10-15% energy savings compared to barium chloride systems, particularly in applications requiring continuous operation.
Waste management and environmental compliance costs also favor lithium chloride in many scenarios. Barium compounds are classified as hazardous materials requiring specialized disposal protocols, with associated costs ranging from $200-500 per ton depending on regional regulations. Lithium waste, while still requiring proper handling, generally incurs lower disposal costs and faces fewer regulatory restrictions.
Risk mitigation represents a less quantifiable but equally important economic factor. Lithium chloride solutions demonstrate greater predictability in performance, reducing the likelihood of unexpected system failures and associated emergency maintenance costs. This reliability factor can be particularly valuable in critical applications where downtime carries substantial financial penalties.
Return on investment calculations typically show that despite higher initial costs, lithium chloride solutions achieve cost parity with barium chloride within 2-3 years of operation in most industrial applications, with accelerated returns in high-demand environments. For applications prioritizing long-term stability over immediate cost concerns, lithium chloride presents the more economically sound choice despite its premium pricing.
Market volatility must also be considered, as lithium prices have shown greater fluctuation in recent years due to evolving demand in the energy storage sector. Organizations implementing either solution should incorporate price trend analysis into their long-term financial planning to accurately project total cost of ownership.
Operational costs also differ substantially between these compounds. Lithium chloride solutions generally require less frequent maintenance and replacement due to their superior stability across varying temperature ranges and resistance to precipitation. This translates to reduced downtime and labor costs in industrial applications, potentially offsetting the higher initial investment over a system's lifecycle.
Energy consumption presents another important consideration. Systems utilizing lithium chloride typically operate at lower energy requirements due to lithium's favorable ionic mobility and conductivity properties. In temperature-controlled environments, this can yield 10-15% energy savings compared to barium chloride systems, particularly in applications requiring continuous operation.
Waste management and environmental compliance costs also favor lithium chloride in many scenarios. Barium compounds are classified as hazardous materials requiring specialized disposal protocols, with associated costs ranging from $200-500 per ton depending on regional regulations. Lithium waste, while still requiring proper handling, generally incurs lower disposal costs and faces fewer regulatory restrictions.
Risk mitigation represents a less quantifiable but equally important economic factor. Lithium chloride solutions demonstrate greater predictability in performance, reducing the likelihood of unexpected system failures and associated emergency maintenance costs. This reliability factor can be particularly valuable in critical applications where downtime carries substantial financial penalties.
Return on investment calculations typically show that despite higher initial costs, lithium chloride solutions achieve cost parity with barium chloride within 2-3 years of operation in most industrial applications, with accelerated returns in high-demand environments. For applications prioritizing long-term stability over immediate cost concerns, lithium chloride presents the more economically sound choice despite its premium pricing.
Market volatility must also be considered, as lithium prices have shown greater fluctuation in recent years due to evolving demand in the energy storage sector. Organizations implementing either solution should incorporate price trend analysis into their long-term financial planning to accurately project total cost of ownership.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!