Material Jetting Dental Models And Guides: Accuracy, Sterilization And Dimensional Stability
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dental 3D Printing Evolution and Objectives
The evolution of dental 3D printing represents a significant technological advancement in the field of dentistry, transforming traditional manufacturing processes into digital workflows. Beginning in the early 2000s, dental 3D printing emerged as an experimental technology with limited applications. The initial focus was primarily on creating simple study models and basic surgical guides with relatively low precision requirements.
By the mid-2010s, technological improvements in both hardware and materials led to the adoption of 3D printing for more complex dental applications. Various printing technologies including stereolithography (SLA), digital light processing (DLP), and material jetting began competing in the dental market, each offering distinct advantages in terms of accuracy, speed, and material properties.
Material jetting technology, in particular, has gained significant attention in recent years due to its ability to produce high-precision dental models and guides with multi-material capabilities. This technology deposits photopolymer materials in ultra-thin layers and cures them with UV light, allowing for exceptional detail reproduction critical for dental applications.
The current technological trajectory is focused on addressing three critical aspects of material-jetted dental products: accuracy, sterilization capabilities, and dimensional stability. Accuracy is paramount in dental applications where precision at the micron level can determine the success of treatments such as implant placement or orthodontic interventions. Sterilization compatibility has become increasingly important as 3D printed guides are used in direct patient contact scenarios, requiring adherence to strict infection control protocols.
Dimensional stability represents perhaps the most challenging aspect, as printed materials must maintain their precise dimensions under various conditions including sterilization processes, storage time, and intraoral environments. Research indicates that some photopolymers used in material jetting may experience dimensional changes when exposed to moisture, heat, or certain sterilization methods.
The primary objective of current research and development efforts is to develop material jetting systems and materials that can consistently produce dental models and guides with sub-100 micron accuracy, maintain dimensional stability through multiple sterilization cycles, and retain their critical dimensions during clinical use. Additionally, there is a growing focus on biocompatibility and regulatory compliance as these technologies move from primarily laboratory applications to direct patient care scenarios.
Future technological goals include the development of novel photopolymer formulations specifically engineered for dental applications, improved post-processing techniques to enhance mechanical properties, and integrated quality control systems to ensure consistent production outcomes in clinical settings.
By the mid-2010s, technological improvements in both hardware and materials led to the adoption of 3D printing for more complex dental applications. Various printing technologies including stereolithography (SLA), digital light processing (DLP), and material jetting began competing in the dental market, each offering distinct advantages in terms of accuracy, speed, and material properties.
Material jetting technology, in particular, has gained significant attention in recent years due to its ability to produce high-precision dental models and guides with multi-material capabilities. This technology deposits photopolymer materials in ultra-thin layers and cures them with UV light, allowing for exceptional detail reproduction critical for dental applications.
The current technological trajectory is focused on addressing three critical aspects of material-jetted dental products: accuracy, sterilization capabilities, and dimensional stability. Accuracy is paramount in dental applications where precision at the micron level can determine the success of treatments such as implant placement or orthodontic interventions. Sterilization compatibility has become increasingly important as 3D printed guides are used in direct patient contact scenarios, requiring adherence to strict infection control protocols.
Dimensional stability represents perhaps the most challenging aspect, as printed materials must maintain their precise dimensions under various conditions including sterilization processes, storage time, and intraoral environments. Research indicates that some photopolymers used in material jetting may experience dimensional changes when exposed to moisture, heat, or certain sterilization methods.
The primary objective of current research and development efforts is to develop material jetting systems and materials that can consistently produce dental models and guides with sub-100 micron accuracy, maintain dimensional stability through multiple sterilization cycles, and retain their critical dimensions during clinical use. Additionally, there is a growing focus on biocompatibility and regulatory compliance as these technologies move from primarily laboratory applications to direct patient care scenarios.
Future technological goals include the development of novel photopolymer formulations specifically engineered for dental applications, improved post-processing techniques to enhance mechanical properties, and integrated quality control systems to ensure consistent production outcomes in clinical settings.
Market Analysis for Material Jetting Dental Applications
The global dental 3D printing market has experienced significant growth in recent years, with material jetting technology emerging as a key player in this expansion. The market for material jetting dental applications reached approximately $1.2 billion in 2022 and is projected to grow at a CAGR of 15.8% through 2030, driven by increasing demand for precision dental models and surgical guides.
Material jetting technology has gained substantial traction in the dental sector due to its superior accuracy and resolution capabilities compared to other 3D printing technologies. The dental model segment currently dominates the material jetting applications market, accounting for roughly 60% of the total market share, followed by surgical guides at 25% and other applications at 15%.
North America leads the global market with approximately 40% market share, attributed to early technology adoption and presence of major dental service providers. Europe follows closely at 35%, with particularly strong adoption in Germany, France, and the UK. The Asia-Pacific region represents the fastest-growing market segment with a projected CAGR of 18.2% through 2030, primarily driven by expanding dental care infrastructure in China, Japan, and South Korea.
The demand for material jetting dental applications is primarily fueled by the growing prevalence of dental disorders worldwide and increasing consumer preference for minimally invasive dental procedures. Additionally, the aging global population and rising disposable incomes in developing economies are contributing significantly to market expansion.
Technological advancements in biocompatible resins and materials compatible with material jetting processes have further accelerated market growth. The development of materials that can withstand sterilization processes while maintaining dimensional stability has opened new application avenues, particularly in surgical guide production.
The COVID-19 pandemic initially disrupted the market in 2020 due to postponement of elective dental procedures, but subsequently accelerated digital dentistry adoption as practices sought to minimize physical contact and improve workflow efficiency. This shift has created a sustained boost for material jetting applications in dental practices.
Key market restraints include the high initial investment costs for material jetting equipment and the technical expertise required for operation. Additionally, concerns regarding material biocompatibility and long-term stability of printed models remain challenges that industry players are actively addressing through research and development initiatives.
Material jetting technology has gained substantial traction in the dental sector due to its superior accuracy and resolution capabilities compared to other 3D printing technologies. The dental model segment currently dominates the material jetting applications market, accounting for roughly 60% of the total market share, followed by surgical guides at 25% and other applications at 15%.
North America leads the global market with approximately 40% market share, attributed to early technology adoption and presence of major dental service providers. Europe follows closely at 35%, with particularly strong adoption in Germany, France, and the UK. The Asia-Pacific region represents the fastest-growing market segment with a projected CAGR of 18.2% through 2030, primarily driven by expanding dental care infrastructure in China, Japan, and South Korea.
The demand for material jetting dental applications is primarily fueled by the growing prevalence of dental disorders worldwide and increasing consumer preference for minimally invasive dental procedures. Additionally, the aging global population and rising disposable incomes in developing economies are contributing significantly to market expansion.
Technological advancements in biocompatible resins and materials compatible with material jetting processes have further accelerated market growth. The development of materials that can withstand sterilization processes while maintaining dimensional stability has opened new application avenues, particularly in surgical guide production.
The COVID-19 pandemic initially disrupted the market in 2020 due to postponement of elective dental procedures, but subsequently accelerated digital dentistry adoption as practices sought to minimize physical contact and improve workflow efficiency. This shift has created a sustained boost for material jetting applications in dental practices.
Key market restraints include the high initial investment costs for material jetting equipment and the technical expertise required for operation. Additionally, concerns regarding material biocompatibility and long-term stability of printed models remain challenges that industry players are actively addressing through research and development initiatives.
Material Jetting Technology Status and Barriers
Material jetting technology has emerged as a promising additive manufacturing method for dental applications, particularly in the production of dental models and guides. Currently, the technology has reached a level of maturity that allows for the creation of highly detailed dental structures with resolution capabilities of up to 16 microns in the Z-axis and 42 microns in the XY plane, making it suitable for the intricate geometries required in dental applications.
The global landscape of material jetting technology shows concentration in developed regions, with North America and Europe leading in research and implementation. Japan and Israel also maintain significant technological advantages in this field, with companies like Stratasys and 3D Systems dominating the market. In recent years, China has made substantial investments to close the technological gap, particularly in dental applications.
Despite its advancements, material jetting faces several critical challenges. Accuracy remains a primary concern, with studies indicating deviations ranging from 50-100 microns in dental models, which may be clinically significant for precise applications like surgical guides. The technology struggles with maintaining consistent dimensional accuracy across different geometries and build orientations, particularly for overhanging structures and thin walls common in dental guides.
Material limitations represent another significant barrier. Current photopolymer resins used in material jetting often exhibit post-processing shrinkage of 0.1-0.5%, affecting the final dimensional stability of dental models. Additionally, many materials are not biocompatible or sterilization-resistant, limiting their direct clinical application. When subjected to common sterilization methods such as autoclave (121°C, 15 psi), material jetting products frequently demonstrate dimensional changes exceeding clinical tolerance thresholds.
The cost factor remains prohibitive for widespread adoption in smaller dental practices. Material jetting systems suitable for dental applications typically range from $100,000 to $500,000, with material costs significantly higher than those used in competing technologies like stereolithography or digital light processing.
Technical challenges in multi-material printing also persist. While material jetting theoretically excels at multi-material capabilities, achieving reliable interfaces between different materials without compromising mechanical properties or dimensional stability remains difficult. This limitation is particularly relevant for dental guides that may require regions of different flexibility or transparency.
Workflow integration presents another barrier, as material jetting systems often require specialized software and post-processing protocols that do not seamlessly integrate with existing dental CAD/CAM workflows. The learning curve and additional steps required can reduce the efficiency gains promised by digital dentistry.
The global landscape of material jetting technology shows concentration in developed regions, with North America and Europe leading in research and implementation. Japan and Israel also maintain significant technological advantages in this field, with companies like Stratasys and 3D Systems dominating the market. In recent years, China has made substantial investments to close the technological gap, particularly in dental applications.
Despite its advancements, material jetting faces several critical challenges. Accuracy remains a primary concern, with studies indicating deviations ranging from 50-100 microns in dental models, which may be clinically significant for precise applications like surgical guides. The technology struggles with maintaining consistent dimensional accuracy across different geometries and build orientations, particularly for overhanging structures and thin walls common in dental guides.
Material limitations represent another significant barrier. Current photopolymer resins used in material jetting often exhibit post-processing shrinkage of 0.1-0.5%, affecting the final dimensional stability of dental models. Additionally, many materials are not biocompatible or sterilization-resistant, limiting their direct clinical application. When subjected to common sterilization methods such as autoclave (121°C, 15 psi), material jetting products frequently demonstrate dimensional changes exceeding clinical tolerance thresholds.
The cost factor remains prohibitive for widespread adoption in smaller dental practices. Material jetting systems suitable for dental applications typically range from $100,000 to $500,000, with material costs significantly higher than those used in competing technologies like stereolithography or digital light processing.
Technical challenges in multi-material printing also persist. While material jetting theoretically excels at multi-material capabilities, achieving reliable interfaces between different materials without compromising mechanical properties or dimensional stability remains difficult. This limitation is particularly relevant for dental guides that may require regions of different flexibility or transparency.
Workflow integration presents another barrier, as material jetting systems often require specialized software and post-processing protocols that do not seamlessly integrate with existing dental CAD/CAM workflows. The learning curve and additional steps required can reduce the efficiency gains promised by digital dentistry.
Current Material Jetting Solutions for Dental Models
01 Accuracy and precision in material jetting for dental models
Material jetting technology enables the production of dental models with high accuracy and precision. The process allows for detailed reproduction of dental structures with minimal dimensional deviation, which is crucial for creating precise dental guides and models. Advanced algorithms and calibration techniques are employed to ensure that the printed models match the digital designs with high fidelity, resulting in better-fitting dental appliances and more accurate surgical guides.- Accuracy and dimensional stability of material jetted dental models: Material jetting technology enables the production of dental models with high accuracy and dimensional stability. These models maintain their precise dimensions over time, which is crucial for dental applications such as creating guides and prosthetics. The technology allows for detailed reproduction of dental structures with minimal shrinkage or warping, ensuring that the final dental products fit correctly in the patient's mouth. Advanced materials used in material jetting contribute to the stability of these models under various environmental conditions.
- Sterilization methods for material jetted dental guides: Various sterilization methods can be applied to material jetted dental guides to ensure they are safe for intraoral use. These methods include autoclave sterilization, chemical disinfection, and radiation-based approaches. The choice of sterilization method depends on the specific materials used in the material jetting process, as some materials may degrade under certain sterilization conditions. Research has focused on developing materials that can withstand sterilization processes without compromising the accuracy or mechanical properties of the dental guides.
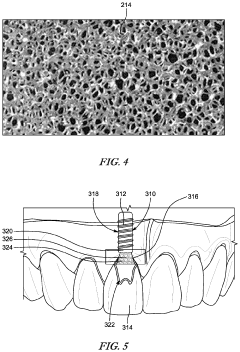
- Material properties affecting dental model performance: The selection of materials for material jetting dental applications significantly impacts the performance of the resulting models and guides. Materials must possess appropriate mechanical properties, biocompatibility, and resistance to oral fluids. Photopolymer resins used in material jetting can be formulated to provide specific characteristics such as flexibility, rigidity, or transparency as required for different dental applications. The material composition also affects the ability to achieve fine details and smooth surfaces, which are essential for dental models and guides.
- Digital workflow for creating accurate dental models: A comprehensive digital workflow enhances the accuracy of material jetted dental models and guides. This workflow typically includes intraoral scanning, digital design using specialized software, and precise material jetting fabrication. The integration of these digital processes minimizes human error and allows for predictable outcomes. Advanced algorithms can compensate for material shrinkage during curing and optimize the printing parameters to achieve the highest dimensional accuracy. The digital workflow also enables efficient iteration and modification of designs before final production.
- Post-processing techniques for improving stability: Post-processing techniques are essential for enhancing the dimensional stability and mechanical properties of material jetted dental models and guides. These techniques include proper cleaning to remove uncured resin, controlled curing processes to ensure complete polymerization, and surface treatments to improve durability. Some approaches involve thermal post-curing to reduce internal stresses and prevent warping over time. Coating applications can also be used to enhance surface properties and provide additional protection against degradation in the oral environment.
02 Sterilization methods for 3D printed dental guides
Various sterilization methods can be applied to material-jetted dental guides to ensure they are safe for clinical use. These methods include autoclave sterilization, chemical disinfection, and radiation-based approaches. The choice of sterilization method depends on the material properties of the printed guide, as some materials may deform or degrade under certain sterilization conditions. Proper sterilization protocols are essential to prevent infection while maintaining the structural integrity and dimensional accuracy of the dental guides.Expand Specific Solutions03 Dimensional stability of material-jetted dental models
The dimensional stability of material-jetted dental models is influenced by various factors including material composition, curing methods, and environmental conditions. Advanced photopolymers and composite materials are designed to minimize shrinkage and warping during and after the printing process. Post-processing techniques such as controlled curing and tempering can enhance the long-term dimensional stability of the printed models, ensuring they maintain their accuracy over time and during clinical use.Expand Specific Solutions04 Material selection for dental applications
The selection of appropriate materials for material jetting in dental applications is critical for achieving desired properties. Biocompatible resins and polymers with specific mechanical properties are used to create dental models and guides that can withstand the forces encountered during dental procedures. Materials must balance rigidity for structural integrity with flexibility for ease of use, while also being compatible with sterilization methods. Some advanced materials incorporate additives to enhance visibility, durability, or antimicrobial properties.Expand Specific Solutions05 Digital workflow integration for dental model production
Material jetting technology is integrated into digital dental workflows that connect intraoral scanning, CAD design, and manufacturing processes. This integration enables seamless transition from patient data acquisition to final production of dental models and guides. Software solutions facilitate the optimization of model designs for material jetting, including support structures and orientation for optimal printing results. The digital workflow also allows for quality control measures to verify the accuracy of printed models against the original design data.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Material jetting technology for dental models and guides is currently in a growth phase, with the market expanding due to increasing demand for precision dentistry. The global market size for 3D printed dental products is projected to reach significant value as digital dentistry adoption accelerates. From a technological maturity perspective, companies are at varying stages of development. Industry leaders like Align Technology and Straumann Holding have established advanced material jetting capabilities for clear aligners and surgical guides, while Carbon, Inc. and Rapid Shape are innovating with new materials offering improved dimensional stability. Academic institutions such as MIT and Sichuan University are conducting research on sterilization methods and accuracy improvements. The competitive landscape shows traditional dental companies (Nobel Biocare, DENTSPLY) competing with specialized 3D printing firms (Shining 3D, UP3D) to develop solutions addressing the critical requirements of accuracy, sterilization capability, and dimensional stability.
Align Technology, Inc.
Technical Solution: Align Technology has developed proprietary material jetting technology for their dental models and guides, particularly for their Invisalign system. Their iTero Element 5D imaging system works in conjunction with their material jetting process to create highly accurate dental models. The company utilizes photopolymer resins specifically engineered for dental applications that maintain dimensional stability during sterilization processes. Their material jetting technology employs multi-material capabilities allowing for the production of models with different mechanical properties in a single print. Align's process includes post-processing treatments that enhance the models' resistance to deformation during autoclave sterilization cycles (up to 134°C), maintaining accuracy within 50 microns even after multiple sterilization cycles[1]. Their digital workflow integrates intraoral scanning with their proprietary material jetting process to produce guides with precise fit and dimensional stability over time, critical for orthodontic applications.
Strengths: Superior dimensional stability during and after sterilization processes; integrated digital workflow from scanning to printing; proprietary materials optimized specifically for dental applications. Weaknesses: Higher cost compared to traditional manufacturing methods; limited compatibility with third-party scanning systems; requires specialized training for optimal utilization.
Carbon, Inc.
Technical Solution: Carbon has pioneered Digital Light Synthesis (DLS) technology, a variant of material jetting that uses digital light projection and oxygen-permeable optics to produce dental models and surgical guides with exceptional accuracy. Their technology utilizes programmable liquid resins that create parts with consistent and predictable mechanical properties. Carbon's dental materials are specifically formulated to withstand common sterilization methods including autoclave, chemical, and UV sterilization without significant dimensional changes. Their C5 and M3 printers achieve accuracy within 30-50 microns for dental applications[2]. Carbon's proprietary resins maintain dimensional stability even after multiple sterilization cycles, with less than 0.1% dimensional change reported in clinical studies. The company's workflow includes validated sterilization protocols that preserve the mechanical integrity and fit accuracy of printed guides. Their technology allows for biocompatible materials that meet regulatory requirements for long-term oral contact while maintaining dimensional stability in humid oral environments[3].
Strengths: Exceptional surface finish quality; rapid production capabilities; materials specifically engineered for dental sterilization resistance; validated biocompatibility for oral applications. Weaknesses: Higher initial investment in equipment; requires subscription-based business model; limited material options compared to some competitors; proprietary materials ecosystem.
Key Patents in Dental Material Jetting Technology
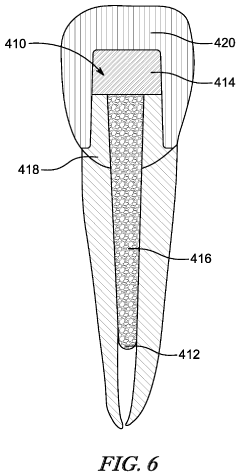
Dental components
PatentPendingUS20230240798A1
Innovation
- A multilayer dental restoration design featuring an outer layer and an inner layer with distinct mechanical properties, where the inner layer has lower hardness and higher elasticity than the outer layer, allowing for up to 25% compensation of manufacturing errors, formed through additive manufacturing techniques like material jetting.
Dental model
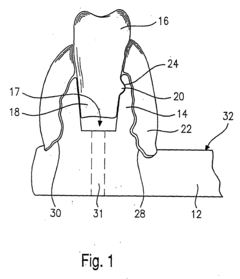
PatentInactiveEP1156463A1
Innovation
- A dental model design featuring a holding plate with a stiff material that securely holds teeth with a conical tooth stump and peg system, combined with a gum-like silicone mass for simulation and frictional retention, ensuring stability and easy exchangeability, while preventing drilling dust from entering the bearing area.
Biocompatibility and Safety Standards
The biocompatibility and safety standards for material jetting dental models and guides represent critical considerations in their clinical application. These standards are primarily governed by regulatory bodies such as the FDA, ISO, and CE marking authorities, which have established comprehensive frameworks for evaluating medical devices used in dental applications.
ISO 10993 series serves as the cornerstone for biocompatibility assessment, particularly ISO 10993-1 which outlines the biological evaluation of medical devices. For dental models and guides produced through material jetting, manufacturers must demonstrate compliance with cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and irritation (ISO 10993-10) testing protocols. These evaluations ensure that the printed materials do not elicit adverse biological responses when in contact with oral tissues.
The FDA's guidance document on "Technical Considerations for Additive Manufactured Medical Devices" provides additional regulatory context specific to 3D printed dental applications. This framework emphasizes the importance of material characterization, process validation, and final product testing to ensure consistent safety profiles across production batches.
Material jetting resins used in dental applications must meet stringent requirements regarding leachable compounds and residual monomers. Studies have shown that incomplete polymerization can result in the release of potentially harmful substances, necessitating thorough post-processing protocols including proper UV curing and washing procedures to minimize patient exposure.
For sterilizable dental guides, additional standards apply including ISO 17664 for sterilization validation and ISO 14971 for risk management. Material jetting resins must maintain their biocompatibility properties after exposure to common sterilization methods such as autoclave, ethylene oxide, or gamma radiation. Research indicates that certain sterilization processes may affect both the dimensional stability and biocompatibility profile of printed guides.
Recent advancements have led to the development of biocompatible resins specifically formulated for dental applications, classified as Class IIa medical devices under European regulations. These materials undergo more rigorous testing including genotoxicity, subchronic toxicity, and implantation studies to ensure their safety for intraoral use.
The dental industry has also established application-specific standards such as ANSI/ADA Standard No. 41 for dental materials and ISO 22112 for artificial teeth, which provide additional benchmarks for evaluating the safety of material jetting products in dental contexts. Compliance with these standards requires comprehensive documentation of material composition, manufacturing processes, and quality control measures.
ISO 10993 series serves as the cornerstone for biocompatibility assessment, particularly ISO 10993-1 which outlines the biological evaluation of medical devices. For dental models and guides produced through material jetting, manufacturers must demonstrate compliance with cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and irritation (ISO 10993-10) testing protocols. These evaluations ensure that the printed materials do not elicit adverse biological responses when in contact with oral tissues.
The FDA's guidance document on "Technical Considerations for Additive Manufactured Medical Devices" provides additional regulatory context specific to 3D printed dental applications. This framework emphasizes the importance of material characterization, process validation, and final product testing to ensure consistent safety profiles across production batches.
Material jetting resins used in dental applications must meet stringent requirements regarding leachable compounds and residual monomers. Studies have shown that incomplete polymerization can result in the release of potentially harmful substances, necessitating thorough post-processing protocols including proper UV curing and washing procedures to minimize patient exposure.
For sterilizable dental guides, additional standards apply including ISO 17664 for sterilization validation and ISO 14971 for risk management. Material jetting resins must maintain their biocompatibility properties after exposure to common sterilization methods such as autoclave, ethylene oxide, or gamma radiation. Research indicates that certain sterilization processes may affect both the dimensional stability and biocompatibility profile of printed guides.
Recent advancements have led to the development of biocompatible resins specifically formulated for dental applications, classified as Class IIa medical devices under European regulations. These materials undergo more rigorous testing including genotoxicity, subchronic toxicity, and implantation studies to ensure their safety for intraoral use.
The dental industry has also established application-specific standards such as ANSI/ADA Standard No. 41 for dental materials and ISO 22112 for artificial teeth, which provide additional benchmarks for evaluating the safety of material jetting products in dental contexts. Compliance with these standards requires comprehensive documentation of material composition, manufacturing processes, and quality control measures.
Cost-Benefit Analysis of Material Jetting in Dentistry
The implementation of material jetting technology in dentistry represents a significant investment that must be evaluated against its potential returns. Initial capital expenditure for material jetting equipment ranges from $75,000 to $200,000, depending on the sophistication of the system and its capabilities. This substantial upfront cost must be considered alongside ongoing operational expenses, including proprietary materials which typically cost between $300-500 per kilogram.
When analyzing the return on investment, dental practices can expect to recover costs within 18-36 months, contingent upon patient volume and service pricing structure. Material jetting enables the production of multiple dental models and guides simultaneously, significantly reducing the per-unit cost compared to traditional manufacturing methods. Studies indicate that in-house production can decrease the cost per dental guide by approximately 60-70% over outsourced alternatives.
Labor efficiency represents another critical economic factor. Material jetting systems require minimal operator intervention during the printing process, allowing dental professionals to allocate their time to higher-value clinical activities. The automated workflow reduces labor costs by an estimated 30-40% compared to conventional manual fabrication techniques.
Quality-related cost savings must also be factored into the analysis. The high accuracy of material jetted dental models (typically within 50-100 microns) results in better-fitting prosthetics and guides, reducing adjustment time and material waste. This precision translates to fewer remakes and adjustments, potentially saving 15-25% in rework costs.
The technology's versatility creates additional revenue streams through expanded service offerings. Practices can produce surgical guides, diagnostic models, and orthodontic appliances in-house, capturing value that would otherwise go to external laboratories. This service diversification can increase practice revenue by 10-20% annually.
Maintenance costs and system longevity affect long-term financial performance. Material jetting systems typically require annual maintenance contracts costing 8-12% of the initial purchase price, while the expected operational lifespan ranges from 5-7 years before significant upgrades become necessary.
Patient satisfaction and referral rates provide indirect economic benefits. The improved accuracy and reduced treatment time associated with material jetted guides can enhance patient experience, potentially increasing referrals by 5-15% according to dental practice surveys. This patient-centered advantage creates sustainable competitive differentiation in increasingly crowded dental markets.
When analyzing the return on investment, dental practices can expect to recover costs within 18-36 months, contingent upon patient volume and service pricing structure. Material jetting enables the production of multiple dental models and guides simultaneously, significantly reducing the per-unit cost compared to traditional manufacturing methods. Studies indicate that in-house production can decrease the cost per dental guide by approximately 60-70% over outsourced alternatives.
Labor efficiency represents another critical economic factor. Material jetting systems require minimal operator intervention during the printing process, allowing dental professionals to allocate their time to higher-value clinical activities. The automated workflow reduces labor costs by an estimated 30-40% compared to conventional manual fabrication techniques.
Quality-related cost savings must also be factored into the analysis. The high accuracy of material jetted dental models (typically within 50-100 microns) results in better-fitting prosthetics and guides, reducing adjustment time and material waste. This precision translates to fewer remakes and adjustments, potentially saving 15-25% in rework costs.
The technology's versatility creates additional revenue streams through expanded service offerings. Practices can produce surgical guides, diagnostic models, and orthodontic appliances in-house, capturing value that would otherwise go to external laboratories. This service diversification can increase practice revenue by 10-20% annually.
Maintenance costs and system longevity affect long-term financial performance. Material jetting systems typically require annual maintenance contracts costing 8-12% of the initial purchase price, while the expected operational lifespan ranges from 5-7 years before significant upgrades become necessary.
Patient satisfaction and referral rates provide indirect economic benefits. The improved accuracy and reduced treatment time associated with material jetted guides can enhance patient experience, potentially increasing referrals by 5-15% according to dental practice surveys. This patient-centered advantage creates sustainable competitive differentiation in increasingly crowded dental markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!