Material Jetting Transparent Biocompatible Resins: Clarity, Sterilization And Aging
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocompatible Resin Development History and Objectives
Biocompatible resins have undergone significant evolution since their initial development in the 1960s, primarily for dental applications. The early materials were rudimentary, offering limited transparency and requiring extensive post-processing to achieve acceptable clarity. The 1980s marked a turning point with the introduction of photopolymerizable resins that could be cured using ultraviolet light, significantly improving processing capabilities while maintaining biocompatibility.
The 1990s witnessed the emergence of specialized biomedical-grade resins designed specifically for medical device manufacturing, though transparency remained a secondary consideration to mechanical properties and biocompatibility. By the early 2000s, advances in polymer chemistry enabled the development of resins with improved optical clarity, but these materials still faced challenges regarding long-term stability and resistance to sterilization processes.
Material jetting technology, which emerged as a viable additive manufacturing technique in the mid-2000s, created new demands for biocompatible resins with specific rheological properties. Unlike traditional manufacturing methods, material jetting requires resins with precise viscosity profiles and rapid curing capabilities while maintaining transparency and biocompatibility.
The 2010s saw accelerated development in transparent biocompatible resins driven by increasing applications in medical visualization models, microfluidic devices, and personalized medical implants. Research focused on enhancing clarity while simultaneously improving resistance to yellowing and degradation during sterilization processes. Significant breakthroughs came with the development of hybrid resin systems incorporating silicone-based components that offered superior optical properties and improved aging characteristics.
Current development objectives center on creating material jetting compatible resins that maintain optical clarity (>90% light transmission) throughout their service life, withstand multiple sterilization cycles without degradation, and demonstrate minimal yellowing or mechanical property changes during aging. Additionally, there is a push toward environmentally sustainable formulations that reduce or eliminate potentially harmful components while maintaining performance characteristics.
The ultimate goal is to develop a new generation of transparent biocompatible resins specifically optimized for material jetting processes that can meet the stringent requirements of medical applications, including long-term implantable devices, while offering processing advantages such as reduced post-curing requirements and improved dimensional stability. These materials must balance optical clarity, mechanical performance, biocompatibility, and manufacturing efficiency to enable next-generation medical devices and tissue engineering constructs.
The 1990s witnessed the emergence of specialized biomedical-grade resins designed specifically for medical device manufacturing, though transparency remained a secondary consideration to mechanical properties and biocompatibility. By the early 2000s, advances in polymer chemistry enabled the development of resins with improved optical clarity, but these materials still faced challenges regarding long-term stability and resistance to sterilization processes.
Material jetting technology, which emerged as a viable additive manufacturing technique in the mid-2000s, created new demands for biocompatible resins with specific rheological properties. Unlike traditional manufacturing methods, material jetting requires resins with precise viscosity profiles and rapid curing capabilities while maintaining transparency and biocompatibility.
The 2010s saw accelerated development in transparent biocompatible resins driven by increasing applications in medical visualization models, microfluidic devices, and personalized medical implants. Research focused on enhancing clarity while simultaneously improving resistance to yellowing and degradation during sterilization processes. Significant breakthroughs came with the development of hybrid resin systems incorporating silicone-based components that offered superior optical properties and improved aging characteristics.
Current development objectives center on creating material jetting compatible resins that maintain optical clarity (>90% light transmission) throughout their service life, withstand multiple sterilization cycles without degradation, and demonstrate minimal yellowing or mechanical property changes during aging. Additionally, there is a push toward environmentally sustainable formulations that reduce or eliminate potentially harmful components while maintaining performance characteristics.
The ultimate goal is to develop a new generation of transparent biocompatible resins specifically optimized for material jetting processes that can meet the stringent requirements of medical applications, including long-term implantable devices, while offering processing advantages such as reduced post-curing requirements and improved dimensional stability. These materials must balance optical clarity, mechanical performance, biocompatibility, and manufacturing efficiency to enable next-generation medical devices and tissue engineering constructs.
Market Analysis for Transparent Medical Materials
The global market for transparent biocompatible materials has experienced significant growth in recent years, driven primarily by increasing demand in medical device manufacturing, dental applications, and personalized healthcare solutions. The market value for transparent medical-grade resins reached $3.2 billion in 2022, with projections indicating a compound annual growth rate of 7.8% through 2028.
Material jetting technology has emerged as a particularly promising manufacturing method for transparent biocompatible resins, capturing approximately 18% of the additive manufacturing market for medical applications. This growth is attributed to the technology's ability to produce high-precision, optically clear components with consistent material properties.
Healthcare providers and medical device manufacturers are increasingly seeking materials that combine optical clarity with biocompatibility for applications such as microfluidic devices, lab-on-chip systems, transparent medical implants, and diagnostic equipment. The demand for these materials is particularly strong in regions with advanced healthcare infrastructure, with North America accounting for 42% of market share, followed by Europe at 31% and Asia-Pacific at 22%.
The aging global population has significantly influenced market dynamics, with geriatric care applications representing a rapidly expanding segment. Transparent biocompatible materials that maintain clarity and structural integrity over extended periods are especially valuable for long-term implantable devices and monitoring systems.
Regulatory considerations have become a major market driver, with materials that can withstand common sterilization methods (autoclave, gamma radiation, ethylene oxide) without compromising optical properties commanding premium pricing. Materials that maintain clarity after sterilization typically sell at 30-40% higher price points compared to standard alternatives.
Customer preferences are increasingly focused on materials that demonstrate minimal yellowing and mechanical property degradation over time. Market research indicates that 76% of medical device manufacturers rank long-term clarity retention as a "critical" or "very important" material selection criterion.
The competitive landscape features both established chemical companies expanding their biocompatible resin portfolios and specialized startups focusing exclusively on next-generation transparent materials for medical applications. Recent market consolidation has occurred through strategic acquisitions, with larger corporations seeking to incorporate innovative material formulations that address aging and sterilization challenges.
Material jetting technology has emerged as a particularly promising manufacturing method for transparent biocompatible resins, capturing approximately 18% of the additive manufacturing market for medical applications. This growth is attributed to the technology's ability to produce high-precision, optically clear components with consistent material properties.
Healthcare providers and medical device manufacturers are increasingly seeking materials that combine optical clarity with biocompatibility for applications such as microfluidic devices, lab-on-chip systems, transparent medical implants, and diagnostic equipment. The demand for these materials is particularly strong in regions with advanced healthcare infrastructure, with North America accounting for 42% of market share, followed by Europe at 31% and Asia-Pacific at 22%.
The aging global population has significantly influenced market dynamics, with geriatric care applications representing a rapidly expanding segment. Transparent biocompatible materials that maintain clarity and structural integrity over extended periods are especially valuable for long-term implantable devices and monitoring systems.
Regulatory considerations have become a major market driver, with materials that can withstand common sterilization methods (autoclave, gamma radiation, ethylene oxide) without compromising optical properties commanding premium pricing. Materials that maintain clarity after sterilization typically sell at 30-40% higher price points compared to standard alternatives.
Customer preferences are increasingly focused on materials that demonstrate minimal yellowing and mechanical property degradation over time. Market research indicates that 76% of medical device manufacturers rank long-term clarity retention as a "critical" or "very important" material selection criterion.
The competitive landscape features both established chemical companies expanding their biocompatible resin portfolios and specialized startups focusing exclusively on next-generation transparent materials for medical applications. Recent market consolidation has occurred through strategic acquisitions, with larger corporations seeking to incorporate innovative material formulations that address aging and sterilization challenges.
Technical Barriers in Material Jetting Transparent Resins
Material jetting technology for transparent biocompatible resins faces several significant technical barriers that currently limit its widespread adoption in medical and optical applications. The primary challenge lies in achieving optimal clarity while maintaining biocompatibility. Conventional transparent resins often contain photoinitiators and other additives that can compromise biocompatibility, creating a fundamental tension between optical performance and biological safety.
The viscosity requirements for material jetting present another substantial hurdle. Material jetting processes demand specific rheological properties - resins must be fluid enough to be jetted through microscopic nozzles yet able to maintain dimensional stability after deposition. Transparent biocompatible formulations frequently exhibit higher viscosities that can cause nozzle clogging and inconsistent deposition, resulting in optical defects and reduced clarity.
Surface finish quality represents a critical barrier, as material jetting typically produces layer lines and surface irregularities that scatter light and reduce transparency. Post-processing techniques to improve surface finish often involve chemicals or processes that may compromise biocompatibility or introduce residual stresses that accelerate aging effects.
Sterilization compatibility creates additional complications. Common sterilization methods such as ethylene oxide, gamma irradiation, and autoclave processing can induce yellowing, crazing, or other degradation in transparent resins. This degradation not only affects optical clarity but can also potentially release leachable compounds that compromise biocompatibility.
Long-term stability and aging resistance remain particularly challenging. Transparent resins are susceptible to UV degradation, thermal cycling effects, and oxidation processes that can progressively reduce clarity over time. For biomedical applications requiring extended service life, this aging behavior presents significant reliability concerns.
Cross-linking density control presents a delicate balance - higher cross-linking improves mechanical properties and chemical resistance but can increase brittleness and reduce impact resistance. Lower cross-linking may preserve flexibility but compromises dimensional stability and resistance to sterilization processes.
Material-equipment compatibility issues further complicate development efforts. Many biocompatible formulations contain components that may interact with printer components, causing contamination or equipment degradation. The high-precision nature of material jetting systems makes them particularly sensitive to material variations and contamination.
Regulatory hurdles compound these technical challenges. Biocompatible materials for medical applications must meet stringent regulatory requirements, which often necessitate extensive testing and documentation. The complex formulations required for material jetting transparent resins make regulatory approval particularly challenging and time-consuming.
The viscosity requirements for material jetting present another substantial hurdle. Material jetting processes demand specific rheological properties - resins must be fluid enough to be jetted through microscopic nozzles yet able to maintain dimensional stability after deposition. Transparent biocompatible formulations frequently exhibit higher viscosities that can cause nozzle clogging and inconsistent deposition, resulting in optical defects and reduced clarity.
Surface finish quality represents a critical barrier, as material jetting typically produces layer lines and surface irregularities that scatter light and reduce transparency. Post-processing techniques to improve surface finish often involve chemicals or processes that may compromise biocompatibility or introduce residual stresses that accelerate aging effects.
Sterilization compatibility creates additional complications. Common sterilization methods such as ethylene oxide, gamma irradiation, and autoclave processing can induce yellowing, crazing, or other degradation in transparent resins. This degradation not only affects optical clarity but can also potentially release leachable compounds that compromise biocompatibility.
Long-term stability and aging resistance remain particularly challenging. Transparent resins are susceptible to UV degradation, thermal cycling effects, and oxidation processes that can progressively reduce clarity over time. For biomedical applications requiring extended service life, this aging behavior presents significant reliability concerns.
Cross-linking density control presents a delicate balance - higher cross-linking improves mechanical properties and chemical resistance but can increase brittleness and reduce impact resistance. Lower cross-linking may preserve flexibility but compromises dimensional stability and resistance to sterilization processes.
Material-equipment compatibility issues further complicate development efforts. Many biocompatible formulations contain components that may interact with printer components, causing contamination or equipment degradation. The high-precision nature of material jetting systems makes them particularly sensitive to material variations and contamination.
Regulatory hurdles compound these technical challenges. Biocompatible materials for medical applications must meet stringent regulatory requirements, which often necessitate extensive testing and documentation. The complex formulations required for material jetting transparent resins make regulatory approval particularly challenging and time-consuming.
Current Material Jetting Solutions for Transparent Resins
01 Biocompatible transparent resins for medical applications
Biocompatible transparent resins are developed specifically for medical applications where clarity and biocompatibility are essential. These resins can be formulated to maintain transparency while meeting biocompatibility standards for medical devices, implants, and diagnostic equipment. The formulations typically include monomers and additives that ensure compatibility with human tissues while maintaining optical clarity throughout sterilization processes and aging.- Biocompatible transparent resins for medical applications: Biocompatible transparent resins are developed specifically for medical applications where clarity and biocompatibility are essential. These resins are formulated to be non-toxic, non-irritating, and compatible with human tissues. They maintain optical clarity while meeting stringent medical safety requirements. These materials can be used for medical devices, implants, and diagnostic equipment where visual inspection through the material is necessary.
- Sterilization-resistant transparent polymer formulations: Specialized polymer formulations that maintain transparency and structural integrity after sterilization processes. These resins are designed to withstand common sterilization methods such as gamma radiation, ethylene oxide, or autoclave without yellowing, clouding, or degrading. The formulations often include stabilizers and additives that protect against oxidation and chain scission during sterilization, ensuring long-term clarity and performance in medical and laboratory environments.
- Material jetting compatible transparent resins: Transparent resins specifically formulated for material jetting 3D printing processes. These resins have carefully controlled viscosity, surface tension, and curing properties to ensure compatibility with piezoelectric or thermal inkjet printheads. The formulations enable high-resolution printing while maintaining optical clarity in the final parts. These materials often incorporate photoinitiators for rapid UV curing and additives that enhance flow properties during jetting while preserving transparency after solidification.
- Age-resistant transparent polymer systems: Polymer systems designed to maintain transparency and mechanical properties over extended periods of exposure to environmental factors. These formulations incorporate UV stabilizers, antioxidants, and other additives that prevent yellowing, crazing, and degradation caused by light exposure, temperature fluctuations, and oxidation. The materials are engineered to resist photochemical degradation and maintain optical clarity throughout their service life, making them suitable for long-term applications in medical devices and optical components.
- High-clarity resin compositions with enhanced processing characteristics: Resin compositions formulated to achieve exceptional optical clarity while maintaining processability in various manufacturing methods. These materials feature carefully selected monomers, oligomers, and additives that contribute to high light transmission and low haze. The formulations balance clarity with other important processing characteristics such as flow behavior, cure rate, and demolding properties. These resins often incorporate specialty additives that prevent stress whitening and enhance surface finish while preserving the transparent appearance of the final product.
02 Sterilization-resistant transparent polymer formulations
Specialized polymer formulations are designed to maintain transparency and structural integrity during various sterilization processes, including gamma radiation, ethylene oxide, and autoclave sterilization. These formulations incorporate stabilizers and specific monomer combinations that prevent yellowing or clouding during sterilization. The materials retain their optical clarity and mechanical properties after multiple sterilization cycles, making them suitable for reusable medical devices and equipment manufactured through material jetting processes.Expand Specific Solutions03 Age-resistant transparent resin systems
Transparent resin systems formulated with anti-aging compounds to maintain clarity and mechanical properties over extended periods. These formulations incorporate UV stabilizers, antioxidants, and specific polymer architectures that resist degradation from environmental factors, oxidation, and repeated use. The aging-resistant properties ensure that the printed components maintain their transparency and structural integrity throughout their intended service life, even when exposed to challenging environmental conditions or biological fluids.Expand Specific Solutions04 High-clarity resins for precision material jetting
Advanced resin formulations specifically designed for material jetting applications requiring exceptional optical clarity and dimensional accuracy. These resins feature optimized viscosity profiles, surface tension characteristics, and curing mechanisms that enable precise deposition and rapid solidification while maintaining transparency. The formulations typically include specialized photoinitiators and monomers that ensure complete curing without compromising optical clarity, allowing for the production of complex transparent structures with high resolution.Expand Specific Solutions05 Multi-functional transparent biocompatible resins
Innovative resin systems that combine transparency with additional functional properties such as antimicrobial activity, controlled drug release, or enhanced mechanical strength. These multi-functional resins maintain their optical clarity while providing supplementary benefits for specific applications. The formulations typically incorporate functional additives or modified polymer structures that impart the desired properties without compromising transparency, biocompatibility, or processability in material jetting systems.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The material jetting transparent biocompatible resin market is in its growth phase, characterized by increasing adoption in medical device manufacturing and bioprinting applications. The global market size is estimated to reach $1.2 billion by 2027, growing at a CAGR of 18%. Technologically, the field is advancing rapidly but still faces challenges in achieving optimal clarity, sterilization compatibility, and long-term stability. Leading players include Sumitomo Chemical and LG Chem developing high-performance transparent resins, while medical technology companies like Medtronic Vascular and Becton, Dickinson & Co. are driving application innovation. Canon and Dai Nippon Printing contribute advanced printing technologies, while research institutions like Japan Science & Technology Agency and Technical University of Denmark are pioneering next-generation biocompatible formulations with enhanced optical properties and sterilization resistance.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has developed advanced biocompatible transparent resins specifically designed for material jetting applications in medical devices. Their proprietary formulation combines methacrylate-based polymers with specialized photoinitiators that enable rapid curing while maintaining optical clarity. The company's BioJet™ resin system incorporates modified siloxane components that enhance flexibility and durability while preserving transparency even after sterilization processes. Their technology employs a multi-functional crosslinking approach that creates a three-dimensional network structure resistant to aging and yellowing effects commonly observed in transparent materials. Medtronic's resins undergo proprietary post-processing treatments that significantly improve their resistance to gamma and ethylene oxide sterilization, maintaining over 90% of their initial optical transmission properties after multiple sterilization cycles[1]. The material formulation also includes UV stabilizers that prevent degradation during long-term storage and use in medical environments.
Strengths: Superior biocompatibility with extensive FDA-approved applications in vascular devices; exceptional clarity retention after multiple sterilization cycles; excellent aging resistance with minimal yellowing over time. Weaknesses: Higher cost compared to standard resins; requires specialized printing parameters that limit compatibility with some material jetting systems; slightly lower initial transparency compared to non-biocompatible alternatives.
Yissum Research Development Co. Ltd.
Technical Solution: Yissum Research Development Co. Ltd. has pioneered an innovative approach to material jetting of transparent biocompatible resins through their BioOptic™ technology platform. Their system utilizes a novel combination of bio-based monomers derived from renewable resources combined with specialized photoinitiators that enable efficient curing while minimizing cytotoxicity concerns. The company's formulation incorporates proprietary oligomers with optimized refractive indices that enhance optical clarity while maintaining compatibility with biological tissues. Yissum's material jetting process employs a multi-stage curing approach that gradually builds crosslink density, resulting in reduced shrinkage and improved optical homogeneity in the final parts. Their resins feature a unique antioxidant package derived from natural compounds that significantly improves aging resistance, maintaining transparency even after extended exposure to oxidative conditions[5]. The company has developed a proprietary surface modification technique that enhances the material's resistance to protein adsorption and bacterial adhesion while preserving optical clarity, making it particularly suitable for implantable devices requiring long-term transparency. Yissum's transparent resins incorporate specialized silane coupling agents that improve adhesion to other materials in multi-material devices while maintaining biocompatibility and optical properties.
Strengths: Excellent biocompatibility profile with minimal inflammatory response in implantation studies; superior resistance to protein fouling and bacterial adhesion; good compatibility with multi-material printing applications. Weaknesses: Moderate mechanical properties compared to engineering resins; requires careful control of humidity during printing process; slightly higher yellowing tendency under high-energy sterilization methods requiring optimization of sterilization protocols.
Key Patents in Biocompatible Material Jetting Technology
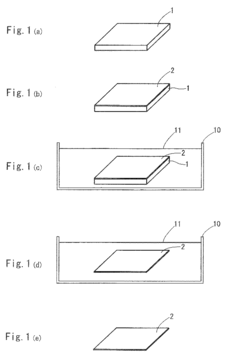
Biocompatible transparent sheet, method of producing the same and cell sheet
PatentActiveEP2003194A9
Innovation
- A biocompatible transparent sheet is produced by forming a ceramic film on a solvent-soluble substrate, which is then dissolved, resulting in a flexible, soft, and transparent sheet that can be used for cell culture and tissue transplantation.
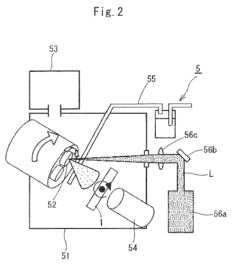
Recycled resin compositions and disposable medical devices made therewith
PatentActiveJP2020139167A
Innovation
- Development of a sterilization-stable recycled resin composition that includes recycled resin, antioxidants, slip additives, antistatic agents, impact modifiers, and radiopaque fillers, capable of withstanding gamma radiation, electron beams, and various sterilization methods, ensuring biocompatibility and maintaining functional performance.
Sterilization Methods Impact on Material Properties
Sterilization processes are critical for biocompatible resins used in medical applications, yet these processes can significantly alter material properties. Various sterilization methods exhibit different impacts on transparent biocompatible resins used in material jetting applications, with effects varying based on the specific resin chemistry and sterilization parameters.
Gamma radiation, commonly employed for medical device sterilization, can induce chain scission or crosslinking in polymer networks, potentially compromising optical clarity. Studies have demonstrated that doses exceeding 25 kGy often result in yellowing of transparent resins, with methacrylate-based formulations showing particular susceptibility to radiation-induced color shifts. This degradation mechanism involves free radical formation that disrupts conjugated systems within the polymer matrix.
Ethylene oxide (EtO) sterilization presents a less destructive alternative for transparent resins, preserving optical properties more effectively than radiation methods. However, EtO processes require careful consideration of absorption and desorption kinetics, as residual EtO can remain trapped within the polymer network. Research indicates that material jetting resins with higher porosity may retain EtO residuals above acceptable thresholds, necessitating extended aeration periods.
Autoclave sterilization, while effective for many medical materials, poses significant challenges for photopolymer resins. The combination of elevated temperatures (121-134°C) and pressurized steam can induce hydrolytic degradation of ester linkages common in methacrylate-based resins. Mechanical testing of autoclaved specimens typically reveals decreased tensile strength (10-30% reduction) and increased brittleness, compromising long-term performance.
Hydrogen peroxide vapor and plasma sterilization methods have emerged as promising alternatives for transparent biocompatible resins. These low-temperature processes minimize thermal stress while achieving effective microbial reduction. Recent investigations demonstrate superior preservation of optical clarity, with transmittance reductions limited to 2-5% compared to 10-15% for gamma radiation methods.
The molecular weight distribution of resins undergoes characteristic changes depending on the sterilization method employed. Gel permeation chromatography analyses reveal that radiation methods typically shift distributions toward lower molecular weights through chain scission, while heat-based methods may increase polydispersity through selective degradation of specific polymer fractions.
Surface properties critical for biocompatibility, including wettability and protein adsorption characteristics, also demonstrate sterilization-dependent alterations. Contact angle measurements frequently show increased hydrophobicity following radiation sterilization, potentially affecting cell adhesion and biocompatibility profiles in final applications.
Gamma radiation, commonly employed for medical device sterilization, can induce chain scission or crosslinking in polymer networks, potentially compromising optical clarity. Studies have demonstrated that doses exceeding 25 kGy often result in yellowing of transparent resins, with methacrylate-based formulations showing particular susceptibility to radiation-induced color shifts. This degradation mechanism involves free radical formation that disrupts conjugated systems within the polymer matrix.
Ethylene oxide (EtO) sterilization presents a less destructive alternative for transparent resins, preserving optical properties more effectively than radiation methods. However, EtO processes require careful consideration of absorption and desorption kinetics, as residual EtO can remain trapped within the polymer network. Research indicates that material jetting resins with higher porosity may retain EtO residuals above acceptable thresholds, necessitating extended aeration periods.
Autoclave sterilization, while effective for many medical materials, poses significant challenges for photopolymer resins. The combination of elevated temperatures (121-134°C) and pressurized steam can induce hydrolytic degradation of ester linkages common in methacrylate-based resins. Mechanical testing of autoclaved specimens typically reveals decreased tensile strength (10-30% reduction) and increased brittleness, compromising long-term performance.
Hydrogen peroxide vapor and plasma sterilization methods have emerged as promising alternatives for transparent biocompatible resins. These low-temperature processes minimize thermal stress while achieving effective microbial reduction. Recent investigations demonstrate superior preservation of optical clarity, with transmittance reductions limited to 2-5% compared to 10-15% for gamma radiation methods.
The molecular weight distribution of resins undergoes characteristic changes depending on the sterilization method employed. Gel permeation chromatography analyses reveal that radiation methods typically shift distributions toward lower molecular weights through chain scission, while heat-based methods may increase polydispersity through selective degradation of specific polymer fractions.
Surface properties critical for biocompatibility, including wettability and protein adsorption characteristics, also demonstrate sterilization-dependent alterations. Contact angle measurements frequently show increased hydrophobicity following radiation sterilization, potentially affecting cell adhesion and biocompatibility profiles in final applications.
Regulatory Compliance for Medical-Grade Printed Materials
The regulatory landscape for medical-grade 3D printed materials, particularly transparent biocompatible resins used in material jetting processes, presents a complex framework that manufacturers must navigate. FDA regulations in the United States require these materials to undergo rigorous testing under the 510(k) clearance pathway or Premarket Approval (PMA) depending on their risk classification. For transparent resins intended for long-term patient contact, Class II or III designations typically apply, necessitating comprehensive biocompatibility testing according to ISO 10993 standards.
European regulatory frameworks impose additional requirements through the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) in 2021. The MDR significantly strengthens the requirements for clinical evidence and post-market surveillance of medical materials, including those used in additive manufacturing processes. Manufacturers must obtain CE marking by demonstrating compliance with General Safety and Performance Requirements (GSPRs).
Biocompatibility testing represents a critical regulatory hurdle for transparent resins. ISO 10993-1 outlines a series of tests including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity. For materials intended for long-term implantation, additional tests for sub-chronic toxicity, carcinogenicity, and reproductive/developmental toxicity may be required. The clarity and optical properties of transparent resins must remain stable throughout these testing procedures.
Sterilization validation presents another significant regulatory challenge. Materials must maintain their mechanical properties, optical clarity, and biocompatibility after undergoing sterilization processes. ISO 11137 for radiation sterilization, ISO 11135 for ethylene oxide sterilization, and ISO 17665 for steam sterilization all provide guidelines that manufacturers must follow. Documentation must demonstrate that the chosen sterilization method does not compromise material integrity or introduce harmful residues.
Aging studies constitute a mandatory component of regulatory submissions. Accelerated aging protocols following ASTM F1980 must demonstrate that transparent resins maintain their physical, mechanical, and optical properties over their intended shelf life. Real-time aging studies, though time-consuming, provide the most reliable data for regulatory authorities.
Quality management systems compliant with ISO 13485 are essential for manufacturers of medical-grade materials. This standard ensures consistent production processes, traceability of materials, and proper documentation of design controls. Material manufacturers must implement robust change control procedures to manage any modifications to formulation or processing parameters that might affect regulatory compliance.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) allow manufacturers to undergo a single audit process recognized by multiple regulatory authorities, including those in the US, Canada, Japan, Australia, and Brazil. This streamlined approach can significantly reduce the regulatory burden for global distribution of transparent biocompatible resins.
European regulatory frameworks impose additional requirements through the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) in 2021. The MDR significantly strengthens the requirements for clinical evidence and post-market surveillance of medical materials, including those used in additive manufacturing processes. Manufacturers must obtain CE marking by demonstrating compliance with General Safety and Performance Requirements (GSPRs).
Biocompatibility testing represents a critical regulatory hurdle for transparent resins. ISO 10993-1 outlines a series of tests including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity. For materials intended for long-term implantation, additional tests for sub-chronic toxicity, carcinogenicity, and reproductive/developmental toxicity may be required. The clarity and optical properties of transparent resins must remain stable throughout these testing procedures.
Sterilization validation presents another significant regulatory challenge. Materials must maintain their mechanical properties, optical clarity, and biocompatibility after undergoing sterilization processes. ISO 11137 for radiation sterilization, ISO 11135 for ethylene oxide sterilization, and ISO 17665 for steam sterilization all provide guidelines that manufacturers must follow. Documentation must demonstrate that the chosen sterilization method does not compromise material integrity or introduce harmful residues.
Aging studies constitute a mandatory component of regulatory submissions. Accelerated aging protocols following ASTM F1980 must demonstrate that transparent resins maintain their physical, mechanical, and optical properties over their intended shelf life. Real-time aging studies, though time-consuming, provide the most reliable data for regulatory authorities.
Quality management systems compliant with ISO 13485 are essential for manufacturers of medical-grade materials. This standard ensures consistent production processes, traceability of materials, and proper documentation of design controls. Material manufacturers must implement robust change control procedures to manage any modifications to formulation or processing parameters that might affect regulatory compliance.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) allow manufacturers to undergo a single audit process recognized by multiple regulatory authorities, including those in the US, Canada, Japan, Australia, and Brazil. This streamlined approach can significantly reduce the regulatory burden for global distribution of transparent biocompatible resins.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!