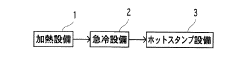
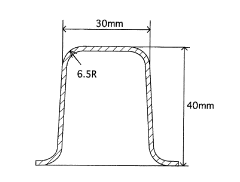
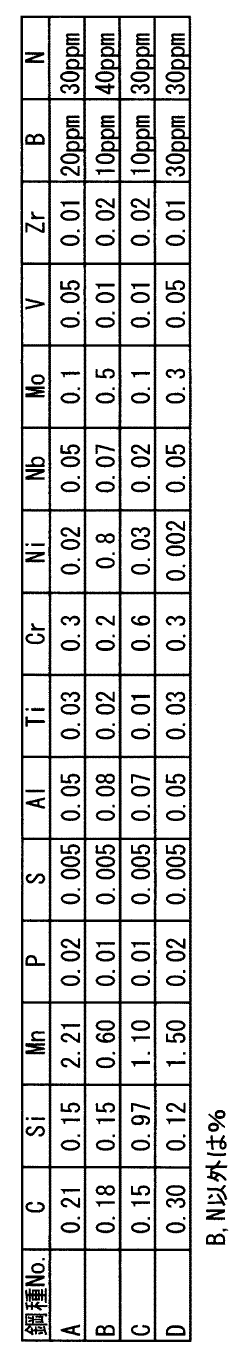
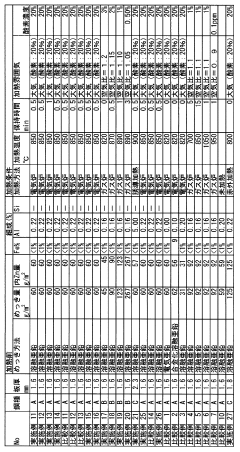
Measure Corrosion Resistance of Galvanized Steel in Acidic Environments
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Galvanized Steel Corrosion Background and Objectives
Galvanized steel has been a cornerstone material in various industries for over 150 years, providing essential corrosion protection through its zinc coating. The evolution of galvanization technology has progressed from early hot-dip methods to modern continuous galvanizing processes, enabling widespread application across construction, automotive, and infrastructure sectors. Recent technological advancements have focused on enhancing zinc coating adhesion, uniformity, and corrosion resistance properties, particularly in challenging environments.
The performance of galvanized steel in acidic environments represents a critical area of concern as industrial emissions, acid rain, and specific application environments continue to expose these materials to increasingly aggressive conditions. Historical data indicates that while galvanized coatings offer substantial protection in neutral environments, their behavior in acidic media varies significantly based on pH levels, acid types, temperature, and exposure duration.
Current industry standards for measuring corrosion resistance primarily rely on salt spray tests (ASTM B117), electrochemical impedance spectroscopy, and weight loss measurements. However, these methods often fail to accurately simulate real-world acidic exposure conditions or provide reliable predictive data for long-term performance. This technological gap has created significant challenges for engineers and materials scientists attempting to specify appropriate materials for acidic environments.
The primary objective of this technical research is to develop and validate improved methodologies for measuring and predicting the corrosion resistance of galvanized steel specifically in acidic environments. These methods must accurately simulate real-world conditions while providing reproducible, quantifiable results that correlate with actual field performance. Additionally, we aim to establish standardized testing protocols that can be widely adopted across industries.
Secondary objectives include identifying critical factors affecting corrosion resistance in acidic media, establishing performance benchmarks for different galvanizing methods, and developing predictive models that can estimate service life based on laboratory measurements. These objectives align with growing industry demands for more reliable materials performance data in increasingly diverse and challenging application environments.
The technological trajectory suggests potential for significant advancements in both measurement techniques and galvanized coating formulations specifically designed for acid resistance. Recent innovations in surface analysis techniques, including advanced microscopy and spectroscopy methods, offer promising approaches for more detailed characterization of corrosion mechanisms and rates in acidic environments.
Understanding these corrosion processes at a fundamental level will enable the development of next-generation galvanized products with enhanced performance characteristics, potentially extending service life and expanding application possibilities in previously unsuitable environments.
The performance of galvanized steel in acidic environments represents a critical area of concern as industrial emissions, acid rain, and specific application environments continue to expose these materials to increasingly aggressive conditions. Historical data indicates that while galvanized coatings offer substantial protection in neutral environments, their behavior in acidic media varies significantly based on pH levels, acid types, temperature, and exposure duration.
Current industry standards for measuring corrosion resistance primarily rely on salt spray tests (ASTM B117), electrochemical impedance spectroscopy, and weight loss measurements. However, these methods often fail to accurately simulate real-world acidic exposure conditions or provide reliable predictive data for long-term performance. This technological gap has created significant challenges for engineers and materials scientists attempting to specify appropriate materials for acidic environments.
The primary objective of this technical research is to develop and validate improved methodologies for measuring and predicting the corrosion resistance of galvanized steel specifically in acidic environments. These methods must accurately simulate real-world conditions while providing reproducible, quantifiable results that correlate with actual field performance. Additionally, we aim to establish standardized testing protocols that can be widely adopted across industries.
Secondary objectives include identifying critical factors affecting corrosion resistance in acidic media, establishing performance benchmarks for different galvanizing methods, and developing predictive models that can estimate service life based on laboratory measurements. These objectives align with growing industry demands for more reliable materials performance data in increasingly diverse and challenging application environments.
The technological trajectory suggests potential for significant advancements in both measurement techniques and galvanized coating formulations specifically designed for acid resistance. Recent innovations in surface analysis techniques, including advanced microscopy and spectroscopy methods, offer promising approaches for more detailed characterization of corrosion mechanisms and rates in acidic environments.
Understanding these corrosion processes at a fundamental level will enable the development of next-generation galvanized products with enhanced performance characteristics, potentially extending service life and expanding application possibilities in previously unsuitable environments.
Market Analysis for Acid-Resistant Galvanized Steel
The global market for acid-resistant galvanized steel continues to expand significantly, driven by increasing industrial applications requiring materials that can withstand harsh chemical environments. Current market valuations indicate the acid-resistant metal coatings sector reached approximately 3.2 billion USD in 2022, with galvanized steel products specifically designed for acidic environments representing about 18% of this market.
Growth projections remain strong, with analysts forecasting a compound annual growth rate of 5.7% through 2028. This growth is primarily fueled by expanding applications in chemical processing, wastewater treatment, and automotive manufacturing sectors where exposure to acidic conditions is unavoidable.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for nearly 45% of global consumption. This dominance stems from rapid industrialization in China and India, coupled with extensive manufacturing operations requiring corrosion-resistant materials. North America and Europe follow with market shares of 27% and 22% respectively, where stringent environmental regulations drive adoption of advanced corrosion-resistant materials.
Industry segmentation shows chemical processing as the largest end-user segment (34%), followed by water treatment (23%), automotive (19%), construction (14%), and other applications (10%). The chemical processing sector's dominance is attributed to the constant exposure of equipment to various acids during production processes.
Customer demand patterns indicate a growing preference for galvanized steel products with documented corrosion resistance metrics in specific acidic environments. Buyers increasingly request standardized testing data comparing performance across different pH levels and acid types, suggesting a market shift toward evidence-based purchasing decisions.
Price sensitivity analysis reveals that while customers prioritize corrosion resistance, they remain cost-conscious. Premium pricing is sustainable only when backed by quantifiable performance data demonstrating extended service life and reduced maintenance costs. The average price premium for acid-resistant galvanized steel ranges between 15-30% above standard galvanized products, depending on performance specifications.
Market barriers include competition from alternative materials such as specialized polymers, fiber-reinforced plastics, and higher-grade stainless steels. However, galvanized steel maintains competitive advantage through its favorable strength-to-cost ratio and established fabrication infrastructure.
Emerging market opportunities exist in developing specialized galvanized coatings for specific industrial acids and concentrations, particularly for sulfuric, hydrochloric, and organic acid environments where current solutions show performance limitations.
Growth projections remain strong, with analysts forecasting a compound annual growth rate of 5.7% through 2028. This growth is primarily fueled by expanding applications in chemical processing, wastewater treatment, and automotive manufacturing sectors where exposure to acidic conditions is unavoidable.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for nearly 45% of global consumption. This dominance stems from rapid industrialization in China and India, coupled with extensive manufacturing operations requiring corrosion-resistant materials. North America and Europe follow with market shares of 27% and 22% respectively, where stringent environmental regulations drive adoption of advanced corrosion-resistant materials.
Industry segmentation shows chemical processing as the largest end-user segment (34%), followed by water treatment (23%), automotive (19%), construction (14%), and other applications (10%). The chemical processing sector's dominance is attributed to the constant exposure of equipment to various acids during production processes.
Customer demand patterns indicate a growing preference for galvanized steel products with documented corrosion resistance metrics in specific acidic environments. Buyers increasingly request standardized testing data comparing performance across different pH levels and acid types, suggesting a market shift toward evidence-based purchasing decisions.
Price sensitivity analysis reveals that while customers prioritize corrosion resistance, they remain cost-conscious. Premium pricing is sustainable only when backed by quantifiable performance data demonstrating extended service life and reduced maintenance costs. The average price premium for acid-resistant galvanized steel ranges between 15-30% above standard galvanized products, depending on performance specifications.
Market barriers include competition from alternative materials such as specialized polymers, fiber-reinforced plastics, and higher-grade stainless steels. However, galvanized steel maintains competitive advantage through its favorable strength-to-cost ratio and established fabrication infrastructure.
Emerging market opportunities exist in developing specialized galvanized coatings for specific industrial acids and concentrations, particularly for sulfuric, hydrochloric, and organic acid environments where current solutions show performance limitations.
Current Challenges in Acidic Corrosion Testing
Despite significant advancements in corrosion testing methodologies, evaluating the corrosion resistance of galvanized steel in acidic environments presents several persistent challenges that impede accurate assessment and standardization. The primary difficulty lies in replicating real-world acidic exposure conditions within laboratory settings. Industrial environments often feature complex combinations of acids at varying concentrations, temperatures, and humidity levels that fluctuate over time—conditions exceedingly difficult to simulate consistently in accelerated testing protocols.
The time-dependent nature of corrosion processes creates another substantial hurdle. Accelerated testing methods, while necessary for timely results, may trigger different corrosion mechanisms than those occurring during natural exposure. This discrepancy frequently leads to misleading predictions about long-term performance, as the relationship between accelerated and natural corrosion is rarely linear, particularly in acidic environments where reaction kinetics can shift dramatically with slight changes in conditions.
Standardization issues further complicate testing procedures. Current international standards for acid corrosion testing of galvanized steel exhibit significant variations in methodologies, evaluation criteria, and result interpretation. This lack of uniformity makes cross-comparison between different research studies or manufacturer specifications problematic, hindering industry-wide benchmarking and quality assurance efforts.
The complex nature of galvanized coatings themselves introduces additional testing complications. Modern galvanized steel often incorporates multiple metallic layers or passivation treatments specifically designed to enhance acid resistance. These sophisticated coating systems respond differently to various acids, creating interactions that conventional testing methods struggle to characterize comprehensively. The zinc-iron alloy layers formed during galvanization exhibit distinct electrochemical behaviors that vary with acid type and concentration.
Measurement and quantification challenges persist in determining corrosion rates accurately. Traditional weight loss methods provide limited insight into localized corrosion phenomena, while electrochemical techniques may be influenced by solution resistance and polarization effects in highly acidic media. Advanced techniques like electrochemical impedance spectroscopy require complex interpretation and specialized expertise not universally available in testing facilities.
Environmental and safety concerns impose further constraints on testing protocols. Many acidic test solutions present significant handling hazards, requiring specialized containment systems and safety protocols that limit test duration or monitoring capabilities. Additionally, waste disposal regulations for spent acidic solutions have become increasingly stringent, restricting the scale and frequency of comprehensive testing programs.
The correlation between laboratory results and field performance remains perhaps the most significant challenge. Validation studies comparing accelerated acid testing with actual service performance often reveal discrepancies that undermine confidence in predictive models, particularly for newer galvanized coating systems with limited field history in aggressive acidic environments.
The time-dependent nature of corrosion processes creates another substantial hurdle. Accelerated testing methods, while necessary for timely results, may trigger different corrosion mechanisms than those occurring during natural exposure. This discrepancy frequently leads to misleading predictions about long-term performance, as the relationship between accelerated and natural corrosion is rarely linear, particularly in acidic environments where reaction kinetics can shift dramatically with slight changes in conditions.
Standardization issues further complicate testing procedures. Current international standards for acid corrosion testing of galvanized steel exhibit significant variations in methodologies, evaluation criteria, and result interpretation. This lack of uniformity makes cross-comparison between different research studies or manufacturer specifications problematic, hindering industry-wide benchmarking and quality assurance efforts.
The complex nature of galvanized coatings themselves introduces additional testing complications. Modern galvanized steel often incorporates multiple metallic layers or passivation treatments specifically designed to enhance acid resistance. These sophisticated coating systems respond differently to various acids, creating interactions that conventional testing methods struggle to characterize comprehensively. The zinc-iron alloy layers formed during galvanization exhibit distinct electrochemical behaviors that vary with acid type and concentration.
Measurement and quantification challenges persist in determining corrosion rates accurately. Traditional weight loss methods provide limited insight into localized corrosion phenomena, while electrochemical techniques may be influenced by solution resistance and polarization effects in highly acidic media. Advanced techniques like electrochemical impedance spectroscopy require complex interpretation and specialized expertise not universally available in testing facilities.
Environmental and safety concerns impose further constraints on testing protocols. Many acidic test solutions present significant handling hazards, requiring specialized containment systems and safety protocols that limit test duration or monitoring capabilities. Additionally, waste disposal regulations for spent acidic solutions have become increasingly stringent, restricting the scale and frequency of comprehensive testing programs.
The correlation between laboratory results and field performance remains perhaps the most significant challenge. Validation studies comparing accelerated acid testing with actual service performance often reveal discrepancies that undermine confidence in predictive models, particularly for newer galvanized coating systems with limited field history in aggressive acidic environments.
Established Methods for Acidic Corrosion Assessment
01 Surface coating compositions for galvanized steel
Various coating compositions can be applied to galvanized steel to enhance its corrosion resistance. These include chromate-free coatings, polymer-based protective layers, and specialized surface treatments that form a barrier against corrosive elements. These coatings provide additional protection beyond the zinc layer and can significantly extend the service life of galvanized steel in harsh environments.- Zinc coating composition and structure: The composition and structure of zinc coatings significantly affect the corrosion resistance of galvanized steel. Various alloying elements can be added to the zinc coating to enhance its protective properties. The microstructure of the coating, including grain size and distribution, plays a crucial role in determining how effectively it can resist corrosion in different environments. Optimizing these factors can lead to substantial improvements in the longevity and performance of galvanized steel products.
- Surface treatment methods: Various surface treatment methods can be applied to galvanized steel to enhance its corrosion resistance. These include chromate treatments, phosphate coatings, and other chemical conversion processes that create a protective layer on the zinc surface. Post-galvanizing treatments can significantly improve the steel's ability to withstand corrosive environments by forming additional barrier layers or by modifying the surface chemistry of the zinc coating to make it less reactive with environmental factors.
- Alloy galvanizing techniques: Alloy galvanizing involves incorporating other metals such as aluminum, magnesium, or nickel into the zinc coating to create zinc alloy layers with superior corrosion resistance. These alloys can provide enhanced protection through mechanisms such as more stable oxide formation or reduced galvanic activity. The specific composition and processing parameters of these alloys are critical in determining their effectiveness in protecting the underlying steel from corrosive attack.
- Multilayer coating systems: Multilayer coating systems combine galvanization with additional protective layers to provide enhanced corrosion resistance. These systems may include organic coatings, such as paints or polymers, applied over the zinc layer, or multiple metallic layers with different compositions. The synergistic effect of these layers can provide superior protection compared to single-layer zinc coatings, especially in aggressive environments where zinc alone might be quickly consumed.
- Environmental factors and testing methods: Understanding how environmental factors affect galvanized steel corrosion is essential for improving resistance. Factors such as humidity, temperature, presence of pollutants, and exposure to marine environments can significantly impact corrosion rates. Various testing methods have been developed to evaluate and predict the corrosion resistance of galvanized steel under different conditions, allowing for the optimization of coating systems for specific applications and environments.
02 Zinc alloy composition modifications
The corrosion resistance of galvanized steel can be improved by modifying the composition of the zinc coating through alloying with other metals. Common alloying elements include aluminum, magnesium, nickel, and rare earth metals, which can enhance the protective properties of the zinc layer. These modified zinc alloys form more stable and durable protective layers that resist corrosion better than pure zinc coatings.Expand Specific Solutions03 Surface treatment processes
Various surface treatment processes can be applied to galvanized steel to enhance its corrosion resistance. These include passivation treatments, chemical conversion coatings, and specialized post-galvanizing processes. These treatments modify the surface properties of the zinc coating, creating a more corrosion-resistant layer that provides better protection against environmental factors that cause degradation.Expand Specific Solutions04 Multi-layer protection systems
Multi-layer protection systems combine different protective mechanisms to enhance the corrosion resistance of galvanized steel. These systems typically include a zinc base layer, intermediate barrier layers, and top coatings with specific protective properties. The combination of different protective layers provides synergistic effects, offering superior corrosion protection compared to single-layer systems, especially in aggressive environments.Expand Specific Solutions05 Corrosion inhibitors and additives
Specific corrosion inhibitors and additives can be incorporated into galvanized coatings or applied as supplementary treatments to enhance corrosion resistance. These include organic and inorganic compounds that interact with the zinc surface to form protective complexes. These additives can significantly improve the performance of galvanized steel by providing additional protection mechanisms that complement the sacrificial protection offered by the zinc coating.Expand Specific Solutions
Leading Players in Corrosion Testing Industry
The galvanized steel corrosion resistance market in acidic environments is currently in a growth phase, driven by increasing infrastructure development and industrial applications. The market size is expanding steadily, with major steel manufacturers like NIPPON STEEL, JFE Steel, POSCO Holdings, and Baoshan Iron & Steel competing for market share. Technical maturity varies across solutions, with established players such as Kobe Steel and Wuhan Iron & Steel offering conventional protection methods, while companies like Akzo Nobel Coatings and 3M Innovative Properties are advancing specialized coating technologies. Research institutions including Swansea University and the Central Research Institute of Electric Power Industry are collaborating with industry leaders to develop next-generation corrosion-resistant materials for increasingly demanding acidic environments.
JFE Steel Corp.
Technical Solution: JFE Steel has developed advanced galvanized steel products with enhanced corrosion resistance specifically designed for acidic environments. Their technology involves a multi-layer coating approach that combines conventional hot-dip galvanizing with specialized chromium-free passivation treatments. The company's proprietary JFE-ECOGAL® coating system incorporates aluminum and magnesium into the zinc coating, creating a more stable and durable protective layer that forms complex oxide structures when exposed to acidic conditions. Their research has demonstrated that this Al-Mg-Zn alloy coating provides up to 10 times greater corrosion resistance in acidic environments compared to conventional galvanized steel. JFE's testing protocols include cyclic corrosion testing that simulates industrial and marine environments with pH levels as low as 2.5, and electrochemical impedance spectroscopy to quantify the protective performance of their coatings over time.
Strengths: Superior long-term protection in highly acidic industrial environments; environmentally friendly chromium-free formulations; excellent scratch and damage resistance with self-healing properties. Weaknesses: Higher production costs compared to standard galvanizing; requires specialized application equipment; performance may vary depending on specific acid type and concentration.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL has pioneered the SuperDyma® galvanized steel technology specifically engineered for superior corrosion resistance in acidic environments. This innovative coating consists of zinc alloyed with 11% aluminum, 3% magnesium, and 0.2% silicon, creating a robust protective barrier that significantly outperforms conventional galvanized products. Their testing methodology employs comprehensive electrochemical techniques including potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) to quantify corrosion behavior in various acidic media. The company has developed specialized accelerated testing protocols that correlate with real-world performance, including cyclic salt spray testing modified with acidic solutions ranging from pH 2 to 5. Research data shows their SuperDyma® coating provides 5-8 times longer protection than conventional hot-dip galvanized steel in sulfuric and hydrochloric acid environments. NIPPON STEEL also employs advanced surface analysis techniques including glow discharge optical emission spectroscopy (GDOES) to characterize the protective oxide layers that form on their galvanized products during exposure to acidic conditions.
Strengths: Exceptional durability in industrial environments with acid rain exposure; superior edge protection compared to conventional galvanized products; maintains structural integrity even after prolonged acid exposure. Weaknesses: Premium pricing structure limits adoption in cost-sensitive applications; requires specialized welding techniques; slightly reduced formability compared to standard galvanized steel.
Key Technologies in Galvanized Steel Protection
High-strength hardened body having excellent corrosion resistance and fatigue resistance
PatentActiveJP2011117086A
Innovation
- A method involving rapid cooling and controlled heating of galvanized steel to form a dense Zn-Fe alloy layer with specific Al and Si content, suppressing Zn diffusion and ensuring a sufficient Zn-Fe alloy layer for enhanced corrosion resistance and fatigue resistance, using a zinc-based plated steel material.
Environmental Impact of Corrosion Testing Procedures
Corrosion testing procedures, while essential for evaluating galvanized steel performance in acidic environments, carry significant environmental implications that warrant careful consideration. Traditional testing methods often involve hazardous chemicals such as sulfuric acid, hydrochloric acid, and various salt solutions that pose environmental risks when improperly handled or disposed of.
The acidic solutions used in accelerated corrosion testing contribute to potential soil and water contamination if released into the environment without proper neutralization. These solutions typically maintain pH levels between 2.0 and 4.0, creating conditions that can mobilize heavy metals from test specimens, including zinc, iron, and trace elements present in galvanized coatings.
Waste management represents a critical environmental concern in corrosion testing facilities. The testing process generates substantial volumes of contaminated solutions containing dissolved metals, particularly zinc from galvanized coatings. These solutions require specialized treatment before disposal to prevent ecological damage and comply with increasingly stringent environmental regulations worldwide.
Energy consumption constitutes another significant environmental impact factor. Accelerated testing chambers that simulate harsh acidic environments often operate continuously for extended periods, sometimes weeks or months, consuming considerable electrical power. This energy footprint contributes to carbon emissions when non-renewable energy sources power testing facilities.
Water usage in corrosion testing procedures presents additional sustainability challenges. Many protocols require regular solution replacement or continuous flow systems that consume substantial water resources. A typical comprehensive corrosion resistance evaluation program for galvanized steel products may utilize hundreds of liters of water throughout the testing cycle.
Recent advancements have focused on developing more environmentally responsible testing methodologies. These include closed-loop systems that recycle test solutions, miniaturized testing approaches that reduce chemical consumption, and computational modeling techniques that can partially substitute physical testing, thereby minimizing environmental impact while maintaining testing efficacy.
Regulatory frameworks increasingly address the environmental aspects of laboratory testing procedures. Organizations such as ISO and ASTM have begun incorporating environmental considerations into their standard testing protocols for corrosion resistance, encouraging adoption of more sustainable practices throughout the materials testing industry.
The life cycle assessment of corrosion testing must balance the environmental costs of testing against the environmental benefits of developing more corrosion-resistant galvanized steel products. Improved corrosion resistance extends product lifespans, reducing resource consumption and waste generation associated with premature replacement of corroded infrastructure components.
The acidic solutions used in accelerated corrosion testing contribute to potential soil and water contamination if released into the environment without proper neutralization. These solutions typically maintain pH levels between 2.0 and 4.0, creating conditions that can mobilize heavy metals from test specimens, including zinc, iron, and trace elements present in galvanized coatings.
Waste management represents a critical environmental concern in corrosion testing facilities. The testing process generates substantial volumes of contaminated solutions containing dissolved metals, particularly zinc from galvanized coatings. These solutions require specialized treatment before disposal to prevent ecological damage and comply with increasingly stringent environmental regulations worldwide.
Energy consumption constitutes another significant environmental impact factor. Accelerated testing chambers that simulate harsh acidic environments often operate continuously for extended periods, sometimes weeks or months, consuming considerable electrical power. This energy footprint contributes to carbon emissions when non-renewable energy sources power testing facilities.
Water usage in corrosion testing procedures presents additional sustainability challenges. Many protocols require regular solution replacement or continuous flow systems that consume substantial water resources. A typical comprehensive corrosion resistance evaluation program for galvanized steel products may utilize hundreds of liters of water throughout the testing cycle.
Recent advancements have focused on developing more environmentally responsible testing methodologies. These include closed-loop systems that recycle test solutions, miniaturized testing approaches that reduce chemical consumption, and computational modeling techniques that can partially substitute physical testing, thereby minimizing environmental impact while maintaining testing efficacy.
Regulatory frameworks increasingly address the environmental aspects of laboratory testing procedures. Organizations such as ISO and ASTM have begun incorporating environmental considerations into their standard testing protocols for corrosion resistance, encouraging adoption of more sustainable practices throughout the materials testing industry.
The life cycle assessment of corrosion testing must balance the environmental costs of testing against the environmental benefits of developing more corrosion-resistant galvanized steel products. Improved corrosion resistance extends product lifespans, reducing resource consumption and waste generation associated with premature replacement of corroded infrastructure components.
Standards and Certification Requirements
The measurement of corrosion resistance in galvanized steel requires adherence to established standards and certification requirements to ensure reliability, reproducibility, and industry acceptance of test results. The American Society for Testing and Materials (ASTM) provides several key standards specifically for evaluating galvanized steel in acidic environments, including ASTM B117 for salt spray testing, ASTM G31 for immersion corrosion testing, and ASTM G1 for preparing, cleaning, and evaluating corrosion test specimens.
International Organization for Standardization (ISO) has developed complementary standards such as ISO 9227 for salt spray tests and ISO 8407 for removal of corrosion products from corrosion test specimens. These standards define precise methodologies, equipment specifications, and environmental parameters that must be maintained during testing to ensure valid results.
For galvanized steel intended for specific industrial applications, additional industry-specific standards may apply. The American Association of State Highway and Transportation Officials (AASHTO) provides standards for highway and bridge applications, while NACE International (formerly National Association of Corrosion Engineers) offers standards specifically focused on corrosion testing in petrochemical environments.
Certification requirements typically involve laboratory accreditation to ISO/IEC 17025, which establishes general requirements for the competence of testing and calibration laboratories. Accredited laboratories must demonstrate technical competence, impartiality, and consistent operation according to the standard. This accreditation is often a prerequisite for test results to be accepted by regulatory bodies and industry stakeholders.
Quality management systems certification, such as ISO 9001, may also be required for organizations conducting corrosion testing. This ensures that the testing process is embedded within a broader quality management framework that emphasizes continuous improvement and customer satisfaction.
For products entering specific markets, regional certification requirements must be considered. The European Union requires CE marking for certain construction products, which may include corrosion resistance specifications for galvanized steel. Similarly, products entering North American markets may require certification to relevant ASTM or AASHTO standards.
Documentation requirements for certification typically include detailed test reports with specific information about test specimens, testing conditions, measurement methods, and results interpretation. These reports must be maintained according to record retention policies specified in the applicable standards and may be subject to audit by certification bodies.
International Organization for Standardization (ISO) has developed complementary standards such as ISO 9227 for salt spray tests and ISO 8407 for removal of corrosion products from corrosion test specimens. These standards define precise methodologies, equipment specifications, and environmental parameters that must be maintained during testing to ensure valid results.
For galvanized steel intended for specific industrial applications, additional industry-specific standards may apply. The American Association of State Highway and Transportation Officials (AASHTO) provides standards for highway and bridge applications, while NACE International (formerly National Association of Corrosion Engineers) offers standards specifically focused on corrosion testing in petrochemical environments.
Certification requirements typically involve laboratory accreditation to ISO/IEC 17025, which establishes general requirements for the competence of testing and calibration laboratories. Accredited laboratories must demonstrate technical competence, impartiality, and consistent operation according to the standard. This accreditation is often a prerequisite for test results to be accepted by regulatory bodies and industry stakeholders.
Quality management systems certification, such as ISO 9001, may also be required for organizations conducting corrosion testing. This ensures that the testing process is embedded within a broader quality management framework that emphasizes continuous improvement and customer satisfaction.
For products entering specific markets, regional certification requirements must be considered. The European Union requires CE marking for certain construction products, which may include corrosion resistance specifications for galvanized steel. Similarly, products entering North American markets may require certification to relevant ASTM or AASHTO standards.
Documentation requirements for certification typically include detailed test reports with specific information about test specimens, testing conditions, measurement methods, and results interpretation. These reports must be maintained according to record retention policies specified in the applicable standards and may be subject to audit by certification bodies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!