Quantify Galvanized Steel’s Rust Inhibition under Saline Conditions
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Galvanized Steel Corrosion Protection Background and Objectives
Galvanized steel has emerged as a cornerstone material in numerous industries due to its exceptional corrosion resistance properties. The process of galvanization, which involves coating steel with a protective layer of zinc, dates back to the 19th century when it was first commercially developed. Since then, the technology has evolved significantly, with modern hot-dip galvanizing processes becoming standardized by the mid-20th century.
The evolution of galvanized steel technology has been driven by increasing demands for durable materials in harsh environments, particularly those with high saline content. Coastal infrastructure, marine applications, and road systems exposed to de-icing salts represent critical areas where understanding corrosion mechanisms becomes paramount for ensuring structural integrity and longevity.
Recent technological advancements have focused on enhancing the performance of zinc coatings through alloying elements and improved application methods. The addition of aluminum, magnesium, and silicon to zinc coatings has demonstrated promising results in extending service life under aggressive environmental conditions. Concurrently, developments in surface preparation techniques have significantly improved coating adhesion and uniformity.
The primary objective of quantifying galvanized steel's rust inhibition under saline conditions is to establish reliable predictive models that can accurately forecast service life across varying exposure scenarios. This endeavor requires comprehensive understanding of the electrochemical processes occurring at the zinc-steel interface when exposed to chloride ions, which are particularly aggressive corrosion accelerators.
Furthermore, this research aims to bridge the gap between laboratory testing and real-world performance by developing standardized methodologies that correlate accelerated testing results with actual field performance. Such correlations would enable more accurate life-cycle cost analyses and maintenance planning for critical infrastructure.
Another crucial goal is to optimize zinc coating thickness and composition for specific environmental conditions, moving beyond the one-size-fits-all approach currently prevalent in industry standards. This optimization would lead to more cost-effective and environmentally sustainable solutions by reducing zinc usage where possible while maintaining or improving performance.
The technological trajectory points toward smart corrosion protection systems that incorporate real-time monitoring capabilities, allowing for predictive maintenance rather than reactive repairs. These systems represent the next frontier in galvanized steel technology, potentially revolutionizing how we approach corrosion management in high-value assets exposed to saline environments.
The evolution of galvanized steel technology has been driven by increasing demands for durable materials in harsh environments, particularly those with high saline content. Coastal infrastructure, marine applications, and road systems exposed to de-icing salts represent critical areas where understanding corrosion mechanisms becomes paramount for ensuring structural integrity and longevity.
Recent technological advancements have focused on enhancing the performance of zinc coatings through alloying elements and improved application methods. The addition of aluminum, magnesium, and silicon to zinc coatings has demonstrated promising results in extending service life under aggressive environmental conditions. Concurrently, developments in surface preparation techniques have significantly improved coating adhesion and uniformity.
The primary objective of quantifying galvanized steel's rust inhibition under saline conditions is to establish reliable predictive models that can accurately forecast service life across varying exposure scenarios. This endeavor requires comprehensive understanding of the electrochemical processes occurring at the zinc-steel interface when exposed to chloride ions, which are particularly aggressive corrosion accelerators.
Furthermore, this research aims to bridge the gap between laboratory testing and real-world performance by developing standardized methodologies that correlate accelerated testing results with actual field performance. Such correlations would enable more accurate life-cycle cost analyses and maintenance planning for critical infrastructure.
Another crucial goal is to optimize zinc coating thickness and composition for specific environmental conditions, moving beyond the one-size-fits-all approach currently prevalent in industry standards. This optimization would lead to more cost-effective and environmentally sustainable solutions by reducing zinc usage where possible while maintaining or improving performance.
The technological trajectory points toward smart corrosion protection systems that incorporate real-time monitoring capabilities, allowing for predictive maintenance rather than reactive repairs. These systems represent the next frontier in galvanized steel technology, potentially revolutionizing how we approach corrosion management in high-value assets exposed to saline environments.
Market Analysis for Corrosion-Resistant Steel Products
The global market for corrosion-resistant steel products, particularly galvanized steel, has been experiencing steady growth driven by increasing demand from construction, automotive, and infrastructure sectors. The market size for corrosion-resistant steel reached approximately $152 billion in 2022 and is projected to grow at a CAGR of 5.7% through 2030, according to industry reports from Metal Market Research Institute.
Galvanized steel products specifically account for roughly 60% of the corrosion-resistant steel market, with hot-dip galvanized steel being the dominant segment due to its superior protection capabilities in saline environments. The construction industry remains the largest consumer, utilizing about 45% of all galvanized steel production, followed by automotive (25%) and industrial equipment (15%).
Regional analysis reveals that Asia-Pacific dominates the market with a 42% share, led by China's massive infrastructure development and manufacturing base. North America and Europe follow with 24% and 22% market shares respectively, where stringent environmental regulations and coastal infrastructure projects drive demand for highly corrosion-resistant materials.
The marine and coastal infrastructure segment represents the fastest-growing application area, expanding at 7.3% annually, as governments worldwide invest in resilient coastal structures capable of withstanding saline conditions. This segment particularly values quantifiable performance metrics regarding rust inhibition under varying saline concentrations.
Market research indicates a significant price premium for high-performance galvanized steel products with documented salt spray test results exceeding 1,000 hours. Products with scientifically validated corrosion resistance in high-salinity environments command 15-20% higher prices compared to standard galvanized offerings.
Customer surveys reveal that 78% of industrial buyers consider quantifiable corrosion resistance data as "very important" or "critical" in purchasing decisions for projects in coastal areas. The ability to predict service life under specific environmental conditions has become a key differentiator in the market.
Emerging market trends include increased demand for dual-coating systems combining galvanization with organic coatings for extreme environments, growing interest in zinc-aluminum-magnesium coatings offering enhanced performance in saline conditions, and the development of digital monitoring systems to track corrosion rates in real-time for critical infrastructure.
Competition is intensifying as steel manufacturers invest in research to quantify and improve their products' performance in saline environments, with major players establishing dedicated marine-grade product lines supported by comprehensive testing data and performance guarantees.
Galvanized steel products specifically account for roughly 60% of the corrosion-resistant steel market, with hot-dip galvanized steel being the dominant segment due to its superior protection capabilities in saline environments. The construction industry remains the largest consumer, utilizing about 45% of all galvanized steel production, followed by automotive (25%) and industrial equipment (15%).
Regional analysis reveals that Asia-Pacific dominates the market with a 42% share, led by China's massive infrastructure development and manufacturing base. North America and Europe follow with 24% and 22% market shares respectively, where stringent environmental regulations and coastal infrastructure projects drive demand for highly corrosion-resistant materials.
The marine and coastal infrastructure segment represents the fastest-growing application area, expanding at 7.3% annually, as governments worldwide invest in resilient coastal structures capable of withstanding saline conditions. This segment particularly values quantifiable performance metrics regarding rust inhibition under varying saline concentrations.
Market research indicates a significant price premium for high-performance galvanized steel products with documented salt spray test results exceeding 1,000 hours. Products with scientifically validated corrosion resistance in high-salinity environments command 15-20% higher prices compared to standard galvanized offerings.
Customer surveys reveal that 78% of industrial buyers consider quantifiable corrosion resistance data as "very important" or "critical" in purchasing decisions for projects in coastal areas. The ability to predict service life under specific environmental conditions has become a key differentiator in the market.
Emerging market trends include increased demand for dual-coating systems combining galvanization with organic coatings for extreme environments, growing interest in zinc-aluminum-magnesium coatings offering enhanced performance in saline conditions, and the development of digital monitoring systems to track corrosion rates in real-time for critical infrastructure.
Competition is intensifying as steel manufacturers invest in research to quantify and improve their products' performance in saline environments, with major players establishing dedicated marine-grade product lines supported by comprehensive testing data and performance guarantees.
Current Challenges in Saline Environment Corrosion Protection
Despite significant advancements in corrosion protection technologies, saline environments continue to present formidable challenges for galvanized steel protection systems. The aggressive nature of chloride ions in saline conditions accelerates corrosion processes through multiple mechanisms, creating a complex protection problem that current technologies struggle to fully address.
The primary challenge lies in the breakdown of the passive zinc oxide layer that typically forms on galvanized coatings. In saline environments, chloride ions penetrate this protective layer, initiating localized corrosion sites. This phenomenon, known as pitting corrosion, creates microscopic anodic areas that rapidly deteriorate, undermining the structural integrity of the galvanized coating system.
Temperature fluctuations in marine and coastal environments further complicate protection efforts. Higher temperatures accelerate electrochemical reactions, while temperature cycling causes expansion and contraction that can create microcracks in protective coatings. These physical defects provide additional pathways for chloride ion penetration, exacerbating the corrosion process.
Current quantification methods for corrosion resistance present another significant challenge. Traditional testing protocols often fail to accurately simulate real-world saline exposure conditions, leading to discrepancies between laboratory performance and field results. The lack of standardized accelerated testing methods specifically designed for saline environments hampers comparative analysis between different protection systems.
The zinc consumption rate in galvanized coatings exposed to saline conditions remains difficult to predict with precision. Variables such as salinity concentration, pH fluctuations, dissolved oxygen levels, and biological factors create a multidimensional problem that current mathematical models cannot fully capture. This unpredictability complicates service life estimations for critical infrastructure.
Existing coating thickness measurement techniques also present limitations when evaluating in-service performance. Non-destructive testing methods often lack the sensitivity to detect early-stage degradation patterns specific to saline exposure, while destructive testing disrupts the very protection system being evaluated.
The interaction between galvanic protection and mechanical stress represents another frontier challenge. Stress corrosion cracking becomes particularly problematic in saline environments where chloride ions concentrate at stress points, potentially leading to catastrophic failures that current protection systems cannot reliably prevent.
Additionally, environmental regulations increasingly restrict the use of certain corrosion inhibitors that have historically provided enhanced protection in saline conditions. This regulatory landscape necessitates the development of equally effective but environmentally compatible alternatives, a challenge that remains largely unresolved in current protection technologies.
The primary challenge lies in the breakdown of the passive zinc oxide layer that typically forms on galvanized coatings. In saline environments, chloride ions penetrate this protective layer, initiating localized corrosion sites. This phenomenon, known as pitting corrosion, creates microscopic anodic areas that rapidly deteriorate, undermining the structural integrity of the galvanized coating system.
Temperature fluctuations in marine and coastal environments further complicate protection efforts. Higher temperatures accelerate electrochemical reactions, while temperature cycling causes expansion and contraction that can create microcracks in protective coatings. These physical defects provide additional pathways for chloride ion penetration, exacerbating the corrosion process.
Current quantification methods for corrosion resistance present another significant challenge. Traditional testing protocols often fail to accurately simulate real-world saline exposure conditions, leading to discrepancies between laboratory performance and field results. The lack of standardized accelerated testing methods specifically designed for saline environments hampers comparative analysis between different protection systems.
The zinc consumption rate in galvanized coatings exposed to saline conditions remains difficult to predict with precision. Variables such as salinity concentration, pH fluctuations, dissolved oxygen levels, and biological factors create a multidimensional problem that current mathematical models cannot fully capture. This unpredictability complicates service life estimations for critical infrastructure.
Existing coating thickness measurement techniques also present limitations when evaluating in-service performance. Non-destructive testing methods often lack the sensitivity to detect early-stage degradation patterns specific to saline exposure, while destructive testing disrupts the very protection system being evaluated.
The interaction between galvanic protection and mechanical stress represents another frontier challenge. Stress corrosion cracking becomes particularly problematic in saline environments where chloride ions concentrate at stress points, potentially leading to catastrophic failures that current protection systems cannot reliably prevent.
Additionally, environmental regulations increasingly restrict the use of certain corrosion inhibitors that have historically provided enhanced protection in saline conditions. This regulatory landscape necessitates the development of equally effective but environmentally compatible alternatives, a challenge that remains largely unresolved in current protection technologies.
Leading Companies in Galvanized Steel Manufacturing
The galvanized steel rust inhibition market under saline conditions is in a mature growth phase with an estimated global market size of $15-20 billion annually. Leading players include NIPPON STEEL CORP. and its subsidiary Nippon Steel Nisshin, who dominate with advanced coating technologies, alongside POSCO Holdings and JFE Steel Corp. as major Asian competitors. Western market presence is maintained by ArcelorMittal France and JSW Steel. The technology landscape shows high maturity with incremental innovations focusing on environmentally friendly solutions, as evidenced by Wuxi Epec Technology's chromium-free passivation products and Ecolab USA's specialized cleaning solutions. Research collaboration between corporations and institutions like Kunming University of Science & Technology is accelerating improvements in galvanized steel performance under extreme saline environments.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL has developed advanced galvanized steel products with enhanced rust inhibition properties specifically designed for saline environments. Their technology involves a multi-layer coating approach where zinc is applied through hot-dip galvanization followed by proprietary chromium-free passivation treatments. Their quantitative testing methodology includes accelerated corrosion tests using salt spray chambers that simulate marine environments with precise control of salt concentration (typically 5% NaCl), temperature (35°C), and humidity (>95%). They measure rust inhibition performance through weight loss measurements, electrochemical impedance spectroscopy, and surface analysis techniques including SEM-EDS to quantify corrosion products. Their Super-Dyma® coating technology incorporates aluminum, magnesium, and silicon with zinc to form complex protective layers that provide up to 10 times greater corrosion resistance than conventional galvanized steel in saline conditions[1]. Their research has established quantitative correlations between coating composition and long-term performance in various saline environments.
Strengths: Industry-leading corrosion resistance in high-salinity environments; comprehensive testing protocols that accurately predict real-world performance; environmentally compliant chromium-free technologies. Weaknesses: Higher production costs compared to standard galvanized products; potential for coating damage during forming operations; requires specialized application equipment.
Nippon Steel Nisshin Co., Ltd.
Technical Solution: Nippon Steel Nisshin has developed a sophisticated quantitative approach to evaluating galvanized steel's rust inhibition under saline conditions through their ZAM® (Zinc-Aluminum-Magnesium) coating technology. Their methodology involves precisely controlled alloy compositions with zinc as the base metal enhanced with 6% aluminum and 3% magnesium. Their quantitative assessment protocol includes standardized salt spray testing (SST) with 5% NaCl solution at 35°C for periods extending to 2,000 hours, cyclic corrosion testing that alternates between salt spray, humidity, and dry conditions, and outdoor exposure testing at multiple coastal sites with varying chloride deposition rates. The company employs advanced analytical techniques including cross-sectional SEM-EDS analysis to quantify the formation and composition of protective corrosion products. Their research has demonstrated that ZAM® coated steel exhibits corrosion resistance approximately 8 times greater than conventional hot-dip galvanized steel in saline environments[5]. Nippon Steel Nisshin has established quantitative relationships between coating thickness, composition, and corrosion performance, finding that the magnesium content forms dense protective layers of simonkolleite and magnesium hydroxide that significantly reduce corrosion rates. Their testing includes electrochemical measurements to determine polarization resistance and corrosion current density as quantitative indicators of protective performance.
Strengths: Superior cut-edge protection compared to conventional galvanized products; excellent performance in highly saline environments; comprehensive quantitative data from both accelerated and natural exposure testing. Weaknesses: Higher production costs than standard galvanized steel; requires specialized processing equipment; potential for reduced coating adhesion under certain forming conditions.
Key Scientific Advances in Galvanic Protection Mechanisms
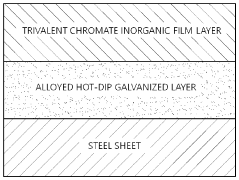
Solution composition containing trivalent chromium for surface treatment of steel sheet, galvanized steel sheet surface—treated with same, and method for manufacturing galvanized
PatentActiveUS11634818B2
Innovation
- A surface treatment solution composition containing trivalent chromium, chromium phosphate, chromium nitrate, silane compounds, vanadium-based, and cobalt-based rust-inhibiting agents, forming an inorganic film on the steel sheet to enhance corrosion resistance, blackening resistance, weldability, and alkali resistance without using hexavalent chromium or organic components.
Environmental Impact of Zinc Coating Processes
The zinc coating processes used in galvanizing steel have significant environmental implications that warrant careful consideration. Traditional hot-dip galvanizing involves immersing steel in molten zinc at temperatures around 450°C, which requires substantial energy consumption and contributes to greenhouse gas emissions. The process typically consumes between 2-3 GJ of energy per ton of galvanized steel, with associated carbon emissions ranging from 0.15 to 0.25 tons of CO2 equivalent.
Zinc mining and refining, necessary precursors to the coating process, present additional environmental challenges. These activities can lead to soil contamination, habitat disruption, and the release of heavy metals into surrounding ecosystems. The extraction process typically generates 1-2 tons of mining waste per ton of zinc produced, creating long-term environmental management issues.
During the galvanizing process itself, zinc ash and dross are generated as byproducts, containing zinc oxide and other metallic compounds that require proper handling and disposal. If improperly managed, these materials can leach into groundwater systems, potentially affecting aquatic ecosystems and drinking water sources. Studies indicate that zinc concentrations exceeding 0.12 mg/L can be toxic to certain aquatic organisms.
The pickling stage of galvanizing, which uses hydrochloric or sulfuric acid to clean steel surfaces, produces spent acid solutions containing dissolved metals. These solutions require neutralization and treatment before disposal, adding to the environmental footprint of the process. Approximately 20-30 liters of acid waste can be generated per ton of galvanized steel.
However, recent technological innovations have significantly improved the environmental profile of zinc coating processes. Closed-loop systems now recover and reuse up to 95% of process chemicals, while advanced filtration systems capture zinc-containing particulates with over 99% efficiency. Thermal recovery systems have reduced energy consumption by 15-25% compared to traditional methods.
Electrogalvanizing, an alternative to hot-dip processes, offers reduced energy requirements and lower emissions, though at higher production costs. This method typically consumes 30-40% less energy than traditional hot-dip galvanizing and produces minimal zinc waste, making it increasingly attractive from an environmental perspective despite economic considerations.
When evaluating the full lifecycle impact, it's important to note that the environmental benefits of corrosion protection often outweigh the initial environmental costs of the galvanizing process. By extending steel product lifespans by 50-100 years, galvanized coatings reduce the need for replacement and the associated resource consumption, ultimately resulting in net environmental benefits over time.
Zinc mining and refining, necessary precursors to the coating process, present additional environmental challenges. These activities can lead to soil contamination, habitat disruption, and the release of heavy metals into surrounding ecosystems. The extraction process typically generates 1-2 tons of mining waste per ton of zinc produced, creating long-term environmental management issues.
During the galvanizing process itself, zinc ash and dross are generated as byproducts, containing zinc oxide and other metallic compounds that require proper handling and disposal. If improperly managed, these materials can leach into groundwater systems, potentially affecting aquatic ecosystems and drinking water sources. Studies indicate that zinc concentrations exceeding 0.12 mg/L can be toxic to certain aquatic organisms.
The pickling stage of galvanizing, which uses hydrochloric or sulfuric acid to clean steel surfaces, produces spent acid solutions containing dissolved metals. These solutions require neutralization and treatment before disposal, adding to the environmental footprint of the process. Approximately 20-30 liters of acid waste can be generated per ton of galvanized steel.
However, recent technological innovations have significantly improved the environmental profile of zinc coating processes. Closed-loop systems now recover and reuse up to 95% of process chemicals, while advanced filtration systems capture zinc-containing particulates with over 99% efficiency. Thermal recovery systems have reduced energy consumption by 15-25% compared to traditional methods.
Electrogalvanizing, an alternative to hot-dip processes, offers reduced energy requirements and lower emissions, though at higher production costs. This method typically consumes 30-40% less energy than traditional hot-dip galvanizing and produces minimal zinc waste, making it increasingly attractive from an environmental perspective despite economic considerations.
When evaluating the full lifecycle impact, it's important to note that the environmental benefits of corrosion protection often outweigh the initial environmental costs of the galvanizing process. By extending steel product lifespans by 50-100 years, galvanized coatings reduce the need for replacement and the associated resource consumption, ultimately resulting in net environmental benefits over time.
Standardization and Testing Protocols for Corrosion Resistance
Standardization of corrosion testing protocols for galvanized steel in saline environments is essential for accurate quantification of rust inhibition performance. Currently, the industry faces significant challenges due to the lack of unified testing methodologies, making it difficult to compare results across different studies and applications. The ASTM B117 salt spray test, while widely adopted, has limitations in replicating real-world exposure conditions, particularly for coastal and marine applications where galvanized steel is frequently deployed.
Advanced testing protocols have emerged to address these limitations, including cyclic corrosion testing (CCT) methods such as ASTM G85 and ISO 14993, which better simulate the wet-dry cycles encountered in actual service conditions. These protocols incorporate varying temperature, humidity, and salt concentration parameters to provide more realistic performance assessments. Additionally, electrochemical impedance spectroscopy (EIS) has gained prominence as a quantitative method for evaluating the protective properties of zinc coatings in saline environments.
The standardization landscape is further complicated by regional variations in testing requirements. European standards (EN ISO 9227), North American standards (ASTM), and Asian standards (JIS Z 2371) each emphasize different aspects of corrosion resistance, creating challenges for global manufacturers and researchers seeking consistent evaluation metrics. Harmonization efforts are underway through international organizations like ISO, but full alignment remains a work in progress.
Field exposure testing represents another critical component of comprehensive corrosion resistance evaluation. These tests, conducted at designated exposure sites with varying atmospheric conditions, provide valuable long-term performance data but require standardized documentation and analysis protocols to ensure reproducibility. The ISO 9223 standard offers a framework for classifying atmospheric corrosivity, which can be integrated into field testing methodologies.
Recent developments in digital imaging and artificial intelligence have introduced new possibilities for standardized corrosion assessment. Computer vision algorithms can now quantify surface rust formation with greater precision and objectivity than traditional visual inspection methods. These technologies enable more consistent evaluation of zinc coating performance across different testing facilities and conditions.
For galvanized steel specifically, standardized protocols must address the unique characteristics of zinc coatings, including thickness measurement (ASTM A123), adhesion testing (ASTM A653), and evaluation of the zinc-iron alloy layers. The development of accelerated testing methods that accurately predict long-term performance remains a significant challenge, requiring correlation studies between laboratory tests and real-world exposure data.
Advanced testing protocols have emerged to address these limitations, including cyclic corrosion testing (CCT) methods such as ASTM G85 and ISO 14993, which better simulate the wet-dry cycles encountered in actual service conditions. These protocols incorporate varying temperature, humidity, and salt concentration parameters to provide more realistic performance assessments. Additionally, electrochemical impedance spectroscopy (EIS) has gained prominence as a quantitative method for evaluating the protective properties of zinc coatings in saline environments.
The standardization landscape is further complicated by regional variations in testing requirements. European standards (EN ISO 9227), North American standards (ASTM), and Asian standards (JIS Z 2371) each emphasize different aspects of corrosion resistance, creating challenges for global manufacturers and researchers seeking consistent evaluation metrics. Harmonization efforts are underway through international organizations like ISO, but full alignment remains a work in progress.
Field exposure testing represents another critical component of comprehensive corrosion resistance evaluation. These tests, conducted at designated exposure sites with varying atmospheric conditions, provide valuable long-term performance data but require standardized documentation and analysis protocols to ensure reproducibility. The ISO 9223 standard offers a framework for classifying atmospheric corrosivity, which can be integrated into field testing methodologies.
Recent developments in digital imaging and artificial intelligence have introduced new possibilities for standardized corrosion assessment. Computer vision algorithms can now quantify surface rust formation with greater precision and objectivity than traditional visual inspection methods. These technologies enable more consistent evaluation of zinc coating performance across different testing facilities and conditions.
For galvanized steel specifically, standardized protocols must address the unique characteristics of zinc coatings, including thickness measurement (ASTM A123), adhesion testing (ASTM A653), and evaluation of the zinc-iron alloy layers. The development of accelerated testing methods that accurately predict long-term performance remains a significant challenge, requiring correlation studies between laboratory tests and real-world exposure data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!