Microreactor Designs For Gas-Liquid-Solid Reactions In Pharmaceuticals
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactor technology has evolved significantly over the past three decades, transforming from laboratory curiosities to industrially viable solutions for pharmaceutical manufacturing. The initial development in the 1990s focused primarily on simple single-phase reactions, with pioneering work conducted at Massachusetts Institute of Technology and ETH Zurich establishing fundamental principles of microfluidic control and heat transfer advantages.
The early 2000s marked a critical transition period when researchers began exploring biphasic liquid-liquid systems, addressing challenges of phase separation and control. By 2005-2010, the technology expanded to include gas-liquid reactions, with significant breakthroughs in catalyst immobilization techniques and channel design optimization to enhance mass transfer across phase boundaries.
The most recent decade has witnessed accelerated development in gas-liquid-solid microreactor systems specifically tailored for pharmaceutical applications. This evolution has been driven by increasing regulatory pressure for continuous manufacturing adoption and pharmaceutical companies seeking more sustainable, efficient production methods with improved quality control.
Current microreactor designs for multiphase reactions incorporate sophisticated features including structured catalyst supports, advanced mixing elements, and integrated analytical monitoring capabilities. These developments have enabled precise control over reaction parameters that was previously unattainable in conventional batch reactors, resulting in higher yields, improved selectivity, and reduced waste generation.
The primary technical objectives for modern microreactor designs in pharmaceutical multiphase reactions include: achieving uniform distribution of all three phases throughout the reaction channel; preventing channel clogging from solid formation or catalyst particles; maintaining consistent catalyst activity over extended operation periods; and ensuring scalability while preserving the inherent advantages of microscale processing.
Looking forward, the field aims to develop fully integrated continuous manufacturing platforms where microreactors for gas-liquid-solid reactions serve as critical components in end-to-end pharmaceutical production. This includes seamless integration with downstream separation processes, real-time quality control systems, and automated control architectures capable of self-optimization.
Another key objective is enhancing the sustainability profile of pharmaceutical manufacturing through microreactor technology, with specific targets for reducing solvent usage, minimizing energy consumption, and enabling the use of greener reaction pathways that were previously impractical in conventional equipment due to safety or selectivity limitations.
The early 2000s marked a critical transition period when researchers began exploring biphasic liquid-liquid systems, addressing challenges of phase separation and control. By 2005-2010, the technology expanded to include gas-liquid reactions, with significant breakthroughs in catalyst immobilization techniques and channel design optimization to enhance mass transfer across phase boundaries.
The most recent decade has witnessed accelerated development in gas-liquid-solid microreactor systems specifically tailored for pharmaceutical applications. This evolution has been driven by increasing regulatory pressure for continuous manufacturing adoption and pharmaceutical companies seeking more sustainable, efficient production methods with improved quality control.
Current microreactor designs for multiphase reactions incorporate sophisticated features including structured catalyst supports, advanced mixing elements, and integrated analytical monitoring capabilities. These developments have enabled precise control over reaction parameters that was previously unattainable in conventional batch reactors, resulting in higher yields, improved selectivity, and reduced waste generation.
The primary technical objectives for modern microreactor designs in pharmaceutical multiphase reactions include: achieving uniform distribution of all three phases throughout the reaction channel; preventing channel clogging from solid formation or catalyst particles; maintaining consistent catalyst activity over extended operation periods; and ensuring scalability while preserving the inherent advantages of microscale processing.
Looking forward, the field aims to develop fully integrated continuous manufacturing platforms where microreactors for gas-liquid-solid reactions serve as critical components in end-to-end pharmaceutical production. This includes seamless integration with downstream separation processes, real-time quality control systems, and automated control architectures capable of self-optimization.
Another key objective is enhancing the sustainability profile of pharmaceutical manufacturing through microreactor technology, with specific targets for reducing solvent usage, minimizing energy consumption, and enabling the use of greener reaction pathways that were previously impractical in conventional equipment due to safety or selectivity limitations.
Pharmaceutical Industry Demand for Microreactor Solutions
The pharmaceutical industry is experiencing a paradigm shift in manufacturing processes, with a growing demand for more efficient, sustainable, and precise production methods. Microreactor technology has emerged as a promising solution to address these evolving needs, particularly for complex gas-liquid-solid reactions that are common in pharmaceutical synthesis. Market analysis indicates that pharmaceutical companies are increasingly seeking microreactor solutions to overcome limitations of traditional batch processing.
Recent market surveys reveal that approximately 65% of pharmaceutical manufacturers are exploring continuous flow technologies, with microreactors being a key component of this transition. This demand is driven by several factors, including regulatory pressures for quality-by-design approaches, cost reduction imperatives, and sustainability goals. The FDA's encouragement of continuous manufacturing adoption has further accelerated interest in microreactor technologies.
The global pharmaceutical microreactor market is experiencing robust growth, with particular demand coming from specialty and high-potency drug manufacturers. These segments require precise reaction control and enhanced safety measures that microreactors can provide. Additionally, the biologics sector is showing increased interest in microreactor technology for certain process steps, expanding the potential market beyond traditional small molecule applications.
Pharmaceutical companies are specifically demanding microreactor designs that can efficiently handle multiphase reactions. Gas-liquid-solid reactions present unique challenges in terms of mass transfer, mixing efficiency, and catalyst integration. Market feedback indicates that manufacturers require solutions that can maintain consistent reaction conditions, prevent catalyst deactivation, and ensure reproducible product quality.
The demand for customizable and modular microreactor systems is particularly strong. Pharmaceutical companies seek flexible platforms that can be adapted to different reaction types and scaled according to production needs. This reflects the industry's move toward more agile manufacturing capabilities that can respond quickly to market demands and accommodate diverse product portfolios.
Economic drivers are also significant in shaping market demand. The potential for reduced capital expenditure through smaller equipment footprints, lower energy consumption, and decreased waste generation makes microreactors attractive from a financial perspective. Companies report seeking ROI not only through direct production cost savings but also through faster time-to-market and reduced regulatory compliance costs.
Geographically, demand is strongest in North America and Europe, where regulatory frameworks are more supportive of innovative manufacturing technologies. However, emerging markets in Asia are showing accelerated adoption rates as pharmaceutical manufacturing capacity expands in these regions and companies seek to implement state-of-the-art technologies from the outset.
Recent market surveys reveal that approximately 65% of pharmaceutical manufacturers are exploring continuous flow technologies, with microreactors being a key component of this transition. This demand is driven by several factors, including regulatory pressures for quality-by-design approaches, cost reduction imperatives, and sustainability goals. The FDA's encouragement of continuous manufacturing adoption has further accelerated interest in microreactor technologies.
The global pharmaceutical microreactor market is experiencing robust growth, with particular demand coming from specialty and high-potency drug manufacturers. These segments require precise reaction control and enhanced safety measures that microreactors can provide. Additionally, the biologics sector is showing increased interest in microreactor technology for certain process steps, expanding the potential market beyond traditional small molecule applications.
Pharmaceutical companies are specifically demanding microreactor designs that can efficiently handle multiphase reactions. Gas-liquid-solid reactions present unique challenges in terms of mass transfer, mixing efficiency, and catalyst integration. Market feedback indicates that manufacturers require solutions that can maintain consistent reaction conditions, prevent catalyst deactivation, and ensure reproducible product quality.
The demand for customizable and modular microreactor systems is particularly strong. Pharmaceutical companies seek flexible platforms that can be adapted to different reaction types and scaled according to production needs. This reflects the industry's move toward more agile manufacturing capabilities that can respond quickly to market demands and accommodate diverse product portfolios.
Economic drivers are also significant in shaping market demand. The potential for reduced capital expenditure through smaller equipment footprints, lower energy consumption, and decreased waste generation makes microreactors attractive from a financial perspective. Companies report seeking ROI not only through direct production cost savings but also through faster time-to-market and reduced regulatory compliance costs.
Geographically, demand is strongest in North America and Europe, where regulatory frameworks are more supportive of innovative manufacturing technologies. However, emerging markets in Asia are showing accelerated adoption rates as pharmaceutical manufacturing capacity expands in these regions and companies seek to implement state-of-the-art technologies from the outset.
Current Challenges in Gas-Liquid-Solid Reaction Systems
Despite significant advancements in microreactor technology, several critical challenges persist in gas-liquid-solid (G-L-S) reaction systems for pharmaceutical applications. The fundamental complexity arises from the simultaneous management of three phases within confined microchannels, creating unique mass transfer, mixing, and operational hurdles that conventional reactor designs struggle to overcome.
Mass transfer limitations represent one of the most significant barriers in G-L-S microreactors. The interfacial area between phases directly impacts reaction efficiency, yet maintaining optimal contact between gas, liquid, and solid phases remains difficult. Current designs often suffer from uneven distribution of solid catalysts and inconsistent gas bubble formation, leading to reaction hotspots and reduced yield. Additionally, the presence of solid particles frequently causes clogging in microchannels, disrupting flow patterns and creating unpredictable pressure drops across the system.
Flow regime control presents another substantial challenge. The transition between different flow patterns (slug flow, annular flow, bubbly flow) significantly affects reaction kinetics and mass transfer coefficients. Current microreactor designs lack robust mechanisms to maintain consistent flow regimes across varying reaction conditions and scaling parameters. This inconsistency introduces variability in reaction outcomes, particularly problematic for pharmaceutical processes where product purity and consistency are paramount.
Catalyst integration and recovery pose unique difficulties in G-L-S microreactors. While wall-coated catalysts offer simplified designs, they provide limited catalytic surface area and suffer from deactivation issues. Alternatively, packed-bed configurations increase catalyst loading but exacerbate pressure drop and clogging problems. Suspended catalyst particles improve mass transfer but complicate downstream separation and recovery processes, adding cost and complexity to pharmaceutical manufacturing operations.
Scale-up challenges remain particularly problematic for pharmaceutical applications. Laboratory-scale successes often fail to translate to production environments due to changing heat and mass transfer dynamics at larger scales. The "numbering-up" approach (adding parallel microreactor units) introduces flow distribution problems across multiple channels, while traditional scale-up methods compromise the inherent advantages of microreactor technology.
Monitoring and control systems for G-L-S microreactors remain underdeveloped compared to conventional reactors. Real-time analysis of multiphase reactions within microchannels requires specialized sensors and analytical techniques that can operate at microscale dimensions without disrupting flow patterns. The pharmaceutical industry's stringent quality requirements necessitate advanced process analytical technology (PAT) solutions specifically designed for microreactor environments, an area still in its infancy.
Mass transfer limitations represent one of the most significant barriers in G-L-S microreactors. The interfacial area between phases directly impacts reaction efficiency, yet maintaining optimal contact between gas, liquid, and solid phases remains difficult. Current designs often suffer from uneven distribution of solid catalysts and inconsistent gas bubble formation, leading to reaction hotspots and reduced yield. Additionally, the presence of solid particles frequently causes clogging in microchannels, disrupting flow patterns and creating unpredictable pressure drops across the system.
Flow regime control presents another substantial challenge. The transition between different flow patterns (slug flow, annular flow, bubbly flow) significantly affects reaction kinetics and mass transfer coefficients. Current microreactor designs lack robust mechanisms to maintain consistent flow regimes across varying reaction conditions and scaling parameters. This inconsistency introduces variability in reaction outcomes, particularly problematic for pharmaceutical processes where product purity and consistency are paramount.
Catalyst integration and recovery pose unique difficulties in G-L-S microreactors. While wall-coated catalysts offer simplified designs, they provide limited catalytic surface area and suffer from deactivation issues. Alternatively, packed-bed configurations increase catalyst loading but exacerbate pressure drop and clogging problems. Suspended catalyst particles improve mass transfer but complicate downstream separation and recovery processes, adding cost and complexity to pharmaceutical manufacturing operations.
Scale-up challenges remain particularly problematic for pharmaceutical applications. Laboratory-scale successes often fail to translate to production environments due to changing heat and mass transfer dynamics at larger scales. The "numbering-up" approach (adding parallel microreactor units) introduces flow distribution problems across multiple channels, while traditional scale-up methods compromise the inherent advantages of microreactor technology.
Monitoring and control systems for G-L-S microreactors remain underdeveloped compared to conventional reactors. Real-time analysis of multiphase reactions within microchannels requires specialized sensors and analytical techniques that can operate at microscale dimensions without disrupting flow patterns. The pharmaceutical industry's stringent quality requirements necessitate advanced process analytical technology (PAT) solutions specifically designed for microreactor environments, an area still in its infancy.
Contemporary Microreactor Designs for Multiphase Pharmaceutical Reactions
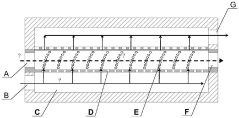
01 Microreactor channel design and fabrication
Microreactors can be designed with specific channel geometries and configurations to enhance mixing, heat transfer, and reaction efficiency. Various fabrication techniques are employed to create these microchannels, including etching, micromachining, and 3D printing. The channel design can include features such as baffles, zigzag patterns, or spiral configurations to promote turbulence and improve mass transfer within the confined space of the microreactor.- Microreactor channel design and fabrication: Microreactors can be designed with specific channel geometries and configurations to enhance mixing, heat transfer, and reaction efficiency. Various fabrication techniques are employed to create these channels, including etching, micromachining, and 3D printing. The channel design can include features such as baffles, zigzag patterns, or spiral configurations to promote turbulence and improve mass transfer. Materials selection for channel construction is critical for chemical compatibility and durability.
- Integration of catalysts in microreactors: Catalysts can be integrated into microreactors through various methods including wall coating, packed bed configurations, or as suspended particles. The integration of catalysts enhances reaction rates and selectivity while reducing energy requirements. Specialized designs allow for efficient catalyst utilization, easy replacement, and improved contact between reactants and catalytic surfaces. These approaches enable intensified processes and can significantly reduce the amount of catalyst required compared to conventional reactors.
- Heat management systems in microreactors: Effective heat management is crucial in microreactor design to control reaction temperatures, prevent hotspots, and optimize energy efficiency. Microreactors can incorporate various heat exchange mechanisms such as integrated cooling channels, thermoelectric elements, or phase-change materials. The high surface-to-volume ratio of microreactors facilitates rapid heat transfer, allowing for precise temperature control even in highly exothermic or endothermic reactions. Advanced designs may include temperature sensors and feedback control systems for dynamic thermal management.
- Modular and scalable microreactor systems: Modular microreactor designs allow for flexible configuration and easy scaling of chemical processes. These systems can be arranged in parallel or series to increase throughput while maintaining the advantages of microscale processing. Modular approaches facilitate maintenance, replacement of components, and adaptation to different reaction requirements. Standardized interfaces between modules enable plug-and-play functionality and integration with various analytical instruments or downstream processing equipment.
- Microfluidic control and monitoring systems: Advanced microreactors incorporate sophisticated control systems for precise regulation of fluid flow, mixing, and reaction conditions. These systems may include micropumps, microvalves, flow sensors, and pressure regulators integrated directly into the microreactor platform. Real-time monitoring capabilities using spectroscopic or electrochemical sensors allow for continuous analysis of reaction progress and product quality. Digital control interfaces enable automation of complex reaction sequences and integration with laboratory information management systems.
02 Integration of catalysts in microreactors
Catalysts can be integrated into microreactors through various methods to enhance reaction rates and selectivity. These methods include coating the channel walls with catalytic materials, incorporating catalyst particles within the microchannels, or using catalyst-impregnated membranes. The integration of catalysts in microreactors allows for more efficient use of catalytic materials and can significantly improve reaction performance while reducing the amount of catalyst required.Expand Specific Solutions03 Heat management systems in microreactors
Effective heat management is crucial in microreactor design to control reaction temperatures and prevent hot spots. Microreactors can incorporate various heat exchange mechanisms, such as integrated cooling channels, thermoelectric elements, or phase-change materials. The high surface-to-volume ratio of microreactors facilitates efficient heat transfer, allowing for precise temperature control during exothermic or endothermic reactions.Expand Specific Solutions04 Flow control and mixing enhancement
Microreactors can be designed with specialized structures to control flow patterns and enhance mixing of reactants. These designs may include micromixers, static mixers, or flow distributors that create controlled turbulence or laminar flow depending on the reaction requirements. Advanced flow control mechanisms can be incorporated to ensure uniform residence time distribution and prevent back-mixing, which is essential for achieving consistent product quality.Expand Specific Solutions05 Modular and scalable microreactor systems
Modular microreactor designs allow for flexibility in scaling up processes from laboratory to industrial production. These systems can be designed as stackable units or parallel arrays that maintain the advantages of microreactors while increasing throughput. The modular approach enables easy maintenance, replacement of individual components, and the ability to reconfigure the system for different reactions or process conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Microreactor Technology
Microreactor technology for gas-liquid-solid reactions in pharmaceuticals is currently in a growth phase, with increasing market adoption driven by efficiency and sustainability demands. The market is expanding rapidly, projected to reach significant scale as pharmaceutical companies seek process intensification solutions. Technologically, the field shows varying maturity levels across players. Corning, Inc. and YMC Co. demonstrate advanced capabilities with commercial microreactor systems, while Hitachi, FUJIFILM, and BASF are leveraging their materials expertise to develop innovative reactor designs. Academic institutions like National University of Singapore and Stevens Institute of Technology are contributing fundamental research, creating a competitive ecosystem where industrial-academic partnerships are accelerating innovation. Japanese and European companies currently lead commercialization efforts, with Chinese institutions rapidly advancing their technological capabilities.
Corning, Inc.
Technical Solution: Corning has developed the Advanced-Flow™ Reactor (AFR) technology specifically designed for gas-liquid-solid pharmaceutical reactions. Their microreactor design features a heart-shaped mixing chamber that creates uniform mixing patterns and controlled residence time distribution. The AFR system incorporates specially designed fluidic modules with internal volumes ranging from 0.45mL to 9.7L, allowing for seamless scale-up from lab to production[1]. Corning's microreactors utilize glass as the primary construction material, providing superior chemical resistance, transparency for process monitoring, and excellent heat transfer capabilities. Their systems can handle multiphase reactions with solid formation through specialized channel geometries that prevent clogging while maintaining high mass transfer rates between gas, liquid, and solid phases[2][3]. The technology enables continuous processing with precise temperature control (±1°C) and residence times, critical for pharmaceutical applications requiring strict quality control.
Strengths: Superior chemical compatibility with glass construction; excellent visibility for process monitoring; proven scalability from lab to production; exceptional heat transfer capabilities enabling highly exothermic reactions. Weaknesses: Higher initial capital investment compared to batch systems; requires specialized expertise for implementation; potential challenges with very high solid loadings or highly viscous reaction mixtures.
FUJIFILM Corp.
Technical Solution: FUJIFILM has developed the "Flow-Through Synthesis System" (FTSS) specifically engineered for pharmaceutical gas-liquid-solid reactions. Their microreactor design incorporates a proprietary micromesh structure that creates highly uniform flow patterns while preventing channel clogging during solid-forming reactions. The system features specialized gas introduction modules that generate microbubbles with controlled size distribution, dramatically increasing gas-liquid interfacial area and mass transfer efficiency[1]. FUJIFILM's microreactors utilize advanced fluoropolymer materials providing exceptional chemical resistance across a wide pH range while minimizing unwanted surface interactions. Their technology incorporates precise temperature control systems capable of maintaining isothermal conditions (±0.5°C) even during highly exothermic reactions, critical for pharmaceutical synthesis requiring strict control of impurity profiles[2]. The FTSS platform includes integrated real-time monitoring capabilities using FUJIFILM's imaging expertise, allowing visualization of multiphase flow patterns and solid formation dynamics. Their modular design enables flexible configuration of reaction pathways and seamless scale-up through numbering-up approaches rather than traditional scale-up, maintaining consistent reaction performance from development to production scale.
Strengths: Exceptional control of multiphase flow patterns; superior chemical compatibility through advanced materials; integrated imaging capabilities for process visualization; proven performance in solid-forming reactions. Weaknesses: Relatively new technology with limited long-term operational data; higher complexity requiring specialized training; potential challenges with very high gas flow rates or reactions producing large solid particles.
Key Patents and Innovations in Pharmaceutical Microreactor Technology
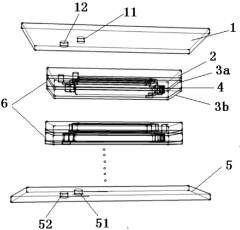
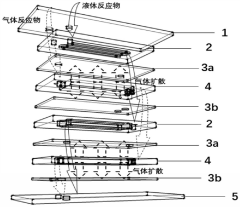
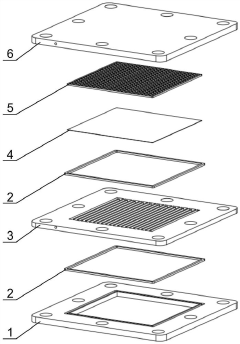
Gas-liquid-solid three-phase membrane microreactor with stacked structure
PatentActiveCN110813208B
Innovation
- A gas-liquid-solid three-phase membrane microreactor with a stacked structure is designed. Through the three-phase microreaction units stacked up and down, each unit is composed of a liquid phase microchannel layer, a first breathable membrane and a second breathable membrane. The breathable membrane is loaded with a solid catalyst, and the gas phase reactants diffuse in two directions, while the liquid phase reactants increase the residence time and increase the reaction conversion rate.
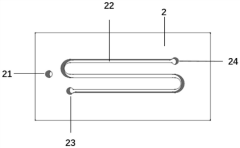
Gas-liquid and gas-liquid-solid multiphase micro-channel reactor and reaction system
PatentInactiveCN112206728A
Innovation
- By changing the flow direction of gas and liquid, the flow rate interference phenomenon between the gas phase and the liquid phase in the microreactor is greatly reduced. Using the design of the air inlet chamber, reaction chamber and air outlet chamber, the gas phase enters the liquid phase through the microhole array, forming a micro-reactor. The bubbles react with the liquid phase and float up through the liquid-blocking breathable membrane, prolonging the residence time of the liquid phase and achieving full contact between the gas and liquid phases.
Scale-up Strategies for Industrial Implementation
Scaling up microreactor technology from laboratory to industrial scale presents unique challenges and opportunities for pharmaceutical manufacturing. The transition requires systematic approaches that maintain the inherent advantages of microreactors while addressing production volume requirements. A comprehensive scale-up strategy typically follows three primary pathways: numbering-up (parallel operation of multiple identical units), smart scale-out (modular designs with integrated control systems), and dimensional scale-up (increasing channel dimensions while preserving key performance parameters).
Numbering-up represents the most straightforward approach, where multiple identical microreactor units operate in parallel to increase throughput. This method preserves the reaction conditions and performance of the laboratory-scale prototype but requires sophisticated flow distribution systems to ensure uniform conditions across all units. Companies like Corning and Ehrfeld Mikrotechnik have successfully implemented this approach for pharmaceutical intermediates production, achieving throughputs of several tons per year while maintaining the high selectivity and yield of lab-scale operations.
Smart scale-out extends the numbering-up concept by incorporating advanced process control systems that monitor and adjust conditions across multiple reactor modules. This approach enables real-time quality control and process optimization, critical for pharmaceutical GMP compliance. The integration of PAT (Process Analytical Technology) tools with microreactor arrays allows continuous monitoring of reaction parameters and product quality, facilitating rapid intervention when deviations occur.
Dimensional scale-up involves increasing the channel dimensions while carefully preserving key performance metrics such as mixing efficiency, heat transfer, and mass transfer coefficients. This approach requires sophisticated computational fluid dynamics modeling to ensure that the increased dimensions do not compromise the advantages of microreactor technology. Recent advances in 3D printing and advanced manufacturing techniques have enabled the production of meso-scale reactors that bridge the gap between micro and conventional reactors.
Industrial implementation also necessitates addressing practical considerations such as materials compatibility, fouling prevention, and maintenance protocols. Pharmaceutical applications often involve corrosive reagents or solid-forming reactions that can lead to channel blockage over time. Innovative surface treatments and self-cleaning mechanisms have been developed to extend operational lifetimes and reduce maintenance requirements.
Economic considerations play a crucial role in scale-up decisions. While microreactor technology typically requires higher initial capital investment compared to conventional batch reactors, the improved yield, reduced waste, and enhanced process safety often result in favorable long-term economics. Several pharmaceutical companies have reported payback periods of less than two years for microreactor implementations in high-value API production.
Numbering-up represents the most straightforward approach, where multiple identical microreactor units operate in parallel to increase throughput. This method preserves the reaction conditions and performance of the laboratory-scale prototype but requires sophisticated flow distribution systems to ensure uniform conditions across all units. Companies like Corning and Ehrfeld Mikrotechnik have successfully implemented this approach for pharmaceutical intermediates production, achieving throughputs of several tons per year while maintaining the high selectivity and yield of lab-scale operations.
Smart scale-out extends the numbering-up concept by incorporating advanced process control systems that monitor and adjust conditions across multiple reactor modules. This approach enables real-time quality control and process optimization, critical for pharmaceutical GMP compliance. The integration of PAT (Process Analytical Technology) tools with microreactor arrays allows continuous monitoring of reaction parameters and product quality, facilitating rapid intervention when deviations occur.
Dimensional scale-up involves increasing the channel dimensions while carefully preserving key performance metrics such as mixing efficiency, heat transfer, and mass transfer coefficients. This approach requires sophisticated computational fluid dynamics modeling to ensure that the increased dimensions do not compromise the advantages of microreactor technology. Recent advances in 3D printing and advanced manufacturing techniques have enabled the production of meso-scale reactors that bridge the gap between micro and conventional reactors.
Industrial implementation also necessitates addressing practical considerations such as materials compatibility, fouling prevention, and maintenance protocols. Pharmaceutical applications often involve corrosive reagents or solid-forming reactions that can lead to channel blockage over time. Innovative surface treatments and self-cleaning mechanisms have been developed to extend operational lifetimes and reduce maintenance requirements.
Economic considerations play a crucial role in scale-up decisions. While microreactor technology typically requires higher initial capital investment compared to conventional batch reactors, the improved yield, reduced waste, and enhanced process safety often result in favorable long-term economics. Several pharmaceutical companies have reported payback periods of less than two years for microreactor implementations in high-value API production.
Sustainability and Green Chemistry Applications
Microreactor technology represents a significant advancement in sustainable pharmaceutical manufacturing, offering numerous environmental benefits compared to traditional batch processes. The implementation of microreactors for gas-liquid-solid reactions aligns perfectly with the principles of green chemistry by minimizing waste generation, reducing energy consumption, and enhancing resource efficiency.
The continuous flow nature of microreactors enables precise control over reaction parameters, resulting in higher selectivity and fewer unwanted by-products. This directly addresses the first principle of green chemistry: waste prevention. Studies have demonstrated that pharmaceutical processes converted from batch to microreactor systems can reduce waste generation by 30-50%, significantly decreasing the environmental footprint of drug manufacturing.
Solvent usage, a major environmental concern in pharmaceutical production, can be substantially reduced through microreactor implementation. The enhanced mass transfer efficiency in microreactors allows for lower solvent-to-reactant ratios while maintaining or improving reaction performance. Some microreactor designs have achieved solvent reductions of up to 80% compared to conventional methods, addressing the critical green chemistry principle of safer solvent selection and minimization.
Energy efficiency represents another key sustainability advantage of microreactors. Their high surface-to-volume ratio facilitates exceptional heat transfer capabilities, reducing energy requirements for heating and cooling. This characteristic is particularly valuable for gas-liquid-solid reactions that often involve exothermic processes requiring careful temperature management. Quantitative assessments indicate energy savings of 20-60% when implementing microreactor technology.
The intensification of processes through microreactor technology also contributes to greener manufacturing through reduced equipment footprint. Smaller production facilities require fewer construction materials and consume less energy for facility operations such as lighting, heating, and ventilation. This space efficiency translates to lower environmental impact throughout the facility lifecycle.
From a life cycle assessment perspective, microreactors enable on-demand, decentralized production models that can significantly reduce transportation-related emissions in pharmaceutical supply chains. This distributed manufacturing approach aligns with sustainability goals by minimizing the carbon footprint associated with global pharmaceutical distribution networks.
Recent innovations in catalyst immobilization techniques for microreactors have further enhanced their green chemistry credentials. Fixed-bed and wall-coated catalytic microreactors facilitate catalyst recovery and reuse, addressing the ninth principle of green chemistry regarding catalysis. These designs have demonstrated catalyst longevity improvements of 200-300% compared to conventional slurry reactors, reducing the environmental impact of catalyst production and disposal.
The continuous flow nature of microreactors enables precise control over reaction parameters, resulting in higher selectivity and fewer unwanted by-products. This directly addresses the first principle of green chemistry: waste prevention. Studies have demonstrated that pharmaceutical processes converted from batch to microreactor systems can reduce waste generation by 30-50%, significantly decreasing the environmental footprint of drug manufacturing.
Solvent usage, a major environmental concern in pharmaceutical production, can be substantially reduced through microreactor implementation. The enhanced mass transfer efficiency in microreactors allows for lower solvent-to-reactant ratios while maintaining or improving reaction performance. Some microreactor designs have achieved solvent reductions of up to 80% compared to conventional methods, addressing the critical green chemistry principle of safer solvent selection and minimization.
Energy efficiency represents another key sustainability advantage of microreactors. Their high surface-to-volume ratio facilitates exceptional heat transfer capabilities, reducing energy requirements for heating and cooling. This characteristic is particularly valuable for gas-liquid-solid reactions that often involve exothermic processes requiring careful temperature management. Quantitative assessments indicate energy savings of 20-60% when implementing microreactor technology.
The intensification of processes through microreactor technology also contributes to greener manufacturing through reduced equipment footprint. Smaller production facilities require fewer construction materials and consume less energy for facility operations such as lighting, heating, and ventilation. This space efficiency translates to lower environmental impact throughout the facility lifecycle.
From a life cycle assessment perspective, microreactors enable on-demand, decentralized production models that can significantly reduce transportation-related emissions in pharmaceutical supply chains. This distributed manufacturing approach aligns with sustainability goals by minimizing the carbon footprint associated with global pharmaceutical distribution networks.
Recent innovations in catalyst immobilization techniques for microreactors have further enhanced their green chemistry credentials. Fixed-bed and wall-coated catalytic microreactors facilitate catalyst recovery and reuse, addressing the ninth principle of green chemistry regarding catalysis. These designs have demonstrated catalyst longevity improvements of 200-300% compared to conventional slurry reactors, reducing the environmental impact of catalyst production and disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!