Optimizing Biocompatibility in Iron-Air Battery Electrolytes
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Biocompatibility Background and Objectives
Iron-air batteries have emerged as a promising energy storage technology due to their high theoretical energy density, abundant raw materials, and cost-effectiveness. The development of these batteries can be traced back to the 1970s, but significant advancements have only been achieved in the past decade. The fundamental principle involves the oxidation of iron at the anode and reduction of oxygen at the cathode, with an alkaline electrolyte facilitating ion transport between electrodes.
The evolution of iron-air battery technology has been characterized by several key milestones, including the development of nano-structured iron electrodes, advanced air cathodes with efficient oxygen reduction catalysts, and improved electrolyte formulations. Recent research has particularly focused on addressing the biocompatibility challenges associated with electrolyte compositions, as these batteries find increasing applications in medical devices, wearable technology, and implantable systems.
Current technical trends indicate a shift toward more environmentally friendly and biocompatible components, driven by stricter regulations and growing consumer awareness. The integration of iron-air batteries into biological systems necessitates electrolytes that minimize adverse reactions with living tissues while maintaining optimal electrochemical performance. This represents a critical balance between battery efficiency and biological safety.
The primary objective of optimizing biocompatibility in iron-air battery electrolytes is to develop formulations that demonstrate minimal cytotoxicity, reduced inflammatory response, and negligible immunogenicity when in contact with biological systems. Additionally, these electrolytes must maintain their electrochemical stability and conductivity to ensure battery performance is not compromised.
Secondary objectives include enhancing the long-term stability of these biocompatible electrolytes, reducing degradation products that might trigger biological responses, and ensuring compatibility with sterilization processes required for medical applications. The development of standardized testing protocols for assessing the biocompatibility of battery components also represents a crucial goal.
From a broader perspective, this research aims to bridge the gap between energy storage technology and biomedical applications, potentially enabling new generations of implantable medical devices, advanced health monitoring systems, and bio-integrated electronics. The successful development of biocompatible iron-air batteries could revolutionize fields ranging from personalized medicine to environmental sensing.
The technical trajectory suggests that future advancements will likely focus on bio-derived electrolyte components, biomimetic interfaces, and self-healing electrolyte systems that can adapt to biological environments. These innovations will be essential for realizing the full potential of iron-air batteries in biological contexts while ensuring user safety and device reliability.
The evolution of iron-air battery technology has been characterized by several key milestones, including the development of nano-structured iron electrodes, advanced air cathodes with efficient oxygen reduction catalysts, and improved electrolyte formulations. Recent research has particularly focused on addressing the biocompatibility challenges associated with electrolyte compositions, as these batteries find increasing applications in medical devices, wearable technology, and implantable systems.
Current technical trends indicate a shift toward more environmentally friendly and biocompatible components, driven by stricter regulations and growing consumer awareness. The integration of iron-air batteries into biological systems necessitates electrolytes that minimize adverse reactions with living tissues while maintaining optimal electrochemical performance. This represents a critical balance between battery efficiency and biological safety.
The primary objective of optimizing biocompatibility in iron-air battery electrolytes is to develop formulations that demonstrate minimal cytotoxicity, reduced inflammatory response, and negligible immunogenicity when in contact with biological systems. Additionally, these electrolytes must maintain their electrochemical stability and conductivity to ensure battery performance is not compromised.
Secondary objectives include enhancing the long-term stability of these biocompatible electrolytes, reducing degradation products that might trigger biological responses, and ensuring compatibility with sterilization processes required for medical applications. The development of standardized testing protocols for assessing the biocompatibility of battery components also represents a crucial goal.
From a broader perspective, this research aims to bridge the gap between energy storage technology and biomedical applications, potentially enabling new generations of implantable medical devices, advanced health monitoring systems, and bio-integrated electronics. The successful development of biocompatible iron-air batteries could revolutionize fields ranging from personalized medicine to environmental sensing.
The technical trajectory suggests that future advancements will likely focus on bio-derived electrolyte components, biomimetic interfaces, and self-healing electrolyte systems that can adapt to biological environments. These innovations will be essential for realizing the full potential of iron-air batteries in biological contexts while ensuring user safety and device reliability.
Market Analysis for Biocompatible Energy Storage Solutions
The biocompatible energy storage solutions market is experiencing significant growth driven by increasing demand for sustainable and environmentally friendly power sources. The global market for biocompatible batteries was valued at approximately $5.2 billion in 2022 and is projected to reach $12.7 billion by 2030, growing at a CAGR of 11.8% during the forecast period. This growth trajectory is particularly relevant for iron-air battery technologies that emphasize biocompatible electrolyte solutions.
Healthcare applications represent the largest market segment for biocompatible energy storage, accounting for nearly 38% of the total market share. The integration of biocompatible iron-air batteries in medical implants, wearable health monitoring devices, and portable medical equipment is creating substantial market opportunities. The healthcare segment's demand is expected to grow at 13.5% annually through 2030, outpacing other application areas.
Consumer electronics constitutes the second-largest market segment at 27%, with increasing consumer preference for environmentally safe and non-toxic power sources. This segment is particularly receptive to iron-air batteries with biocompatible electrolytes due to their safety profile and reduced environmental impact compared to conventional lithium-ion batteries.
Regionally, North America leads the market with a 42% share, followed by Europe (31%) and Asia-Pacific (22%). The Asia-Pacific region, however, is expected to witness the fastest growth rate of 14.2% annually, driven by rapid industrialization, increasing healthcare expenditure, and supportive government policies promoting green technologies.
Market analysis reveals that biocompatible iron-air batteries face competition from established lithium-ion technologies and emerging alternatives such as zinc-air and sodium-ion batteries. However, iron-air batteries with optimized biocompatible electrolytes offer distinct advantages in terms of resource abundance, cost-effectiveness, and safety profiles, positioning them favorably in specific application niches.
Customer surveys indicate that 73% of end-users in medical device manufacturing prioritize biocompatibility and safety over energy density and cycle life. This preference creates a strategic market opportunity for iron-air batteries with biocompatible electrolytes, despite their current limitations in energy density compared to lithium-ion alternatives.
The market for biocompatible energy storage solutions is characterized by high fragmentation, with numerous startups and research institutions developing proprietary technologies. Strategic partnerships between electrolyte developers, battery manufacturers, and end-application companies are becoming increasingly common, creating an interconnected ecosystem that accelerates commercialization pathways for novel biocompatible electrolyte formulations in iron-air batteries.
Healthcare applications represent the largest market segment for biocompatible energy storage, accounting for nearly 38% of the total market share. The integration of biocompatible iron-air batteries in medical implants, wearable health monitoring devices, and portable medical equipment is creating substantial market opportunities. The healthcare segment's demand is expected to grow at 13.5% annually through 2030, outpacing other application areas.
Consumer electronics constitutes the second-largest market segment at 27%, with increasing consumer preference for environmentally safe and non-toxic power sources. This segment is particularly receptive to iron-air batteries with biocompatible electrolytes due to their safety profile and reduced environmental impact compared to conventional lithium-ion batteries.
Regionally, North America leads the market with a 42% share, followed by Europe (31%) and Asia-Pacific (22%). The Asia-Pacific region, however, is expected to witness the fastest growth rate of 14.2% annually, driven by rapid industrialization, increasing healthcare expenditure, and supportive government policies promoting green technologies.
Market analysis reveals that biocompatible iron-air batteries face competition from established lithium-ion technologies and emerging alternatives such as zinc-air and sodium-ion batteries. However, iron-air batteries with optimized biocompatible electrolytes offer distinct advantages in terms of resource abundance, cost-effectiveness, and safety profiles, positioning them favorably in specific application niches.
Customer surveys indicate that 73% of end-users in medical device manufacturing prioritize biocompatibility and safety over energy density and cycle life. This preference creates a strategic market opportunity for iron-air batteries with biocompatible electrolytes, despite their current limitations in energy density compared to lithium-ion alternatives.
The market for biocompatible energy storage solutions is characterized by high fragmentation, with numerous startups and research institutions developing proprietary technologies. Strategic partnerships between electrolyte developers, battery manufacturers, and end-application companies are becoming increasingly common, creating an interconnected ecosystem that accelerates commercialization pathways for novel biocompatible electrolyte formulations in iron-air batteries.
Current Challenges in Iron-Air Battery Electrolyte Biocompatibility
Iron-air batteries have emerged as promising candidates for large-scale energy storage due to their high energy density, low cost, and abundant raw materials. However, the biocompatibility of their electrolytes presents significant challenges that impede widespread adoption. Current iron-air battery electrolytes typically contain highly alkaline solutions, predominantly potassium hydroxide (KOH), with concentrations ranging from 20-45 wt%. These strongly caustic electrolytes pose serious biocompatibility concerns.
The primary biocompatibility challenge stems from the extreme pH levels (typically 13-14) of these alkaline electrolytes. Contact with skin, eyes, or respiratory system can cause severe chemical burns, tissue damage, and long-term health complications. This creates substantial safety risks during manufacturing, maintenance, and especially in case of battery leakage or damage during consumer use.
Environmental toxicity represents another critical challenge. Leakage of alkaline electrolytes can significantly alter soil and water pH, causing ecological damage and potentially entering food chains. The high mobility of potassium ions in aqueous environments exacerbates this concern, allowing rapid dispersion of contamination following any containment breach.
Material compatibility issues further complicate the biocompatibility landscape. The highly corrosive nature of alkaline electrolytes necessitates specialized containment materials that resist degradation. Current containment solutions often involve synthetic polymers and specialized metals that may introduce additional biocompatibility concerns through leaching of compounds or microplastics into the environment.
Recent research has explored alternative electrolyte formulations, including neutral or near-neutral pH systems using salt-based electrolytes. However, these alternatives typically suffer from reduced ionic conductivity and diminished electrochemical performance. The trade-off between performance and biocompatibility remains a fundamental challenge in the field.
Additives used to enhance battery performance introduce additional biocompatibility concerns. Surfactants, stabilizers, and performance enhancers may include compounds with unknown long-term biological effects. The complex interaction between these additives and biological systems remains poorly characterized, creating regulatory uncertainties and potential barriers to commercialization.
Regulatory frameworks worldwide increasingly emphasize the importance of biocompatible energy storage solutions, particularly for residential applications. The European Union's REACH regulations and similar initiatives in other regions impose stringent requirements on chemical safety that current iron-air battery electrolytes struggle to meet without significant modifications or containment strategies.
The biocompatibility challenges extend to end-of-life considerations as well. Recycling and disposal of iron-air batteries containing highly alkaline electrolytes require specialized processes to prevent environmental contamination. The current infrastructure for safe handling of these materials remains underdeveloped in many regions, creating potential barriers to sustainable deployment.
The primary biocompatibility challenge stems from the extreme pH levels (typically 13-14) of these alkaline electrolytes. Contact with skin, eyes, or respiratory system can cause severe chemical burns, tissue damage, and long-term health complications. This creates substantial safety risks during manufacturing, maintenance, and especially in case of battery leakage or damage during consumer use.
Environmental toxicity represents another critical challenge. Leakage of alkaline electrolytes can significantly alter soil and water pH, causing ecological damage and potentially entering food chains. The high mobility of potassium ions in aqueous environments exacerbates this concern, allowing rapid dispersion of contamination following any containment breach.
Material compatibility issues further complicate the biocompatibility landscape. The highly corrosive nature of alkaline electrolytes necessitates specialized containment materials that resist degradation. Current containment solutions often involve synthetic polymers and specialized metals that may introduce additional biocompatibility concerns through leaching of compounds or microplastics into the environment.
Recent research has explored alternative electrolyte formulations, including neutral or near-neutral pH systems using salt-based electrolytes. However, these alternatives typically suffer from reduced ionic conductivity and diminished electrochemical performance. The trade-off between performance and biocompatibility remains a fundamental challenge in the field.
Additives used to enhance battery performance introduce additional biocompatibility concerns. Surfactants, stabilizers, and performance enhancers may include compounds with unknown long-term biological effects. The complex interaction between these additives and biological systems remains poorly characterized, creating regulatory uncertainties and potential barriers to commercialization.
Regulatory frameworks worldwide increasingly emphasize the importance of biocompatible energy storage solutions, particularly for residential applications. The European Union's REACH regulations and similar initiatives in other regions impose stringent requirements on chemical safety that current iron-air battery electrolytes struggle to meet without significant modifications or containment strategies.
The biocompatibility challenges extend to end-of-life considerations as well. Recycling and disposal of iron-air batteries containing highly alkaline electrolytes require specialized processes to prevent environmental contamination. The current infrastructure for safe handling of these materials remains underdeveloped in many regions, creating potential barriers to sustainable deployment.
Current Approaches to Electrolyte Biocompatibility Enhancement
01 Biocompatible electrolyte compositions for iron-air batteries
Electrolyte compositions for iron-air batteries can be formulated with biocompatible materials to reduce environmental impact and health risks. These compositions typically include non-toxic alkaline solutions with additives that maintain performance while ensuring safety for human contact and environmental release. Biocompatible electrolytes often incorporate naturally derived compounds or modified versions of conventional electrolytes with reduced toxicity profiles.- Biocompatible electrolyte compositions for iron-air batteries: Certain electrolyte compositions have been developed for iron-air batteries that demonstrate improved biocompatibility profiles. These formulations typically incorporate naturally derived compounds or biomimetic materials that reduce toxicity while maintaining electrochemical performance. The biocompatible electrolytes help minimize environmental impact and potential health risks associated with battery leakage or disposal, making them suitable for medical devices and environmentally sensitive applications.
- Polymer-based electrolyte systems with enhanced safety profiles: Polymer-based electrolyte systems have been developed for iron-air batteries that offer improved safety and biocompatibility. These systems typically use gel polymers or solid polymer electrolytes that reduce leakage risks and contain fewer toxic components compared to traditional liquid electrolytes. The polymer matrices can be designed with biocompatible materials that are less reactive with human tissues, making them suitable for wearable or implantable devices while maintaining the electrochemical performance needed for iron-air battery operation.
- Aqueous electrolyte formulations with reduced toxicity: Aqueous electrolyte formulations have been developed for iron-air batteries that maintain performance while reducing toxicity concerns. These water-based systems typically use carefully selected salts and additives that demonstrate lower cytotoxicity compared to organic solvent-based alternatives. The formulations often incorporate pH buffers and stabilizing agents that are compatible with biological systems, reducing potential harm from exposure while providing the ionic conductivity necessary for efficient battery operation.
- Biologically derived additives for electrolyte enhancement: Biologically derived additives have been incorporated into iron-air battery electrolytes to improve both performance and biocompatibility. These natural compounds, often extracted from plant sources or synthesized to mimic biological molecules, can serve multiple functions including improving ionic conductivity, stabilizing the electrolyte, and reducing toxicity. The bio-derived additives help create more environmentally friendly battery systems that maintain electrochemical efficiency while presenting fewer hazards to living organisms upon exposure.
- Testing and evaluation methods for electrolyte biocompatibility: Specialized testing and evaluation methods have been developed to assess the biocompatibility of iron-air battery electrolytes. These protocols typically include in vitro cell culture tests, tissue exposure studies, and standardized toxicity assessments that evaluate potential biological impacts. Advanced analytical techniques are employed to characterize electrolyte interactions with biological systems, allowing for the development of safety profiles and risk assessments. These methods help battery developers optimize electrolyte formulations for applications where human or environmental exposure is possible.
02 Polymer-based electrolyte systems with enhanced biocompatibility
Polymer-based electrolyte systems offer improved biocompatibility for iron-air batteries through the use of biodegradable or biologically inert polymers. These systems can encapsulate potentially harmful electrolyte components while maintaining ionic conductivity. The polymer matrices provide a physical barrier that reduces direct contact with toxic materials while allowing for efficient ion transport necessary for battery operation. Some formulations incorporate natural polymers or biomedically approved synthetic polymers to ensure compatibility with biological systems.Expand Specific Solutions03 Aqueous electrolyte formulations with reduced environmental impact
Aqueous electrolyte formulations for iron-air batteries can be designed to minimize environmental impact while maintaining electrochemical performance. These formulations typically avoid heavy metals and persistent organic compounds in favor of readily biodegradable components. By carefully selecting salts, buffers, and additives that break down naturally in the environment, these electrolytes reduce bioaccumulation risks and ecosystem damage in case of battery leakage or disposal. Some formulations incorporate plant-derived compounds or modified natural materials to enhance biodegradability.Expand Specific Solutions04 Ionic liquid alternatives for biocompatible iron-air battery electrolytes
Ionic liquids offer promising alternatives to conventional electrolytes in iron-air batteries with potentially improved biocompatibility profiles. These room-temperature molten salts can be designed with reduced toxicity compared to traditional alkaline electrolytes while maintaining excellent electrochemical properties. By selecting biocompatible cations and anions, these electrolytes can minimize risks to human health and environmental systems. Some ionic liquid formulations incorporate naturally derived components or biomimetic structures to enhance compatibility with biological systems.Expand Specific Solutions05 Testing and evaluation methods for electrolyte biocompatibility
Specialized testing and evaluation methods have been developed to assess the biocompatibility of iron-air battery electrolytes. These protocols typically include cytotoxicity assays, environmental fate studies, and biodegradation assessments to determine potential impacts on living systems. Advanced analytical techniques can identify harmful byproducts or degradation compounds that might form during battery operation or disposal. Standardized testing frameworks help ensure consistent evaluation of biocompatibility across different electrolyte formulations and battery designs, enabling meaningful comparisons and regulatory compliance.Expand Specific Solutions
Leading Organizations in Biocompatible Battery Research
The iron-air battery electrolyte biocompatibility optimization landscape is currently in an early growth phase, with market projections indicating significant expansion as renewable energy storage demands increase. While the technology shows promising cost advantages over lithium-ion alternatives, technical maturity varies across key players. Form Energy leads commercial development with its innovative iron-air battery systems, while established corporations like Toyota, Samsung SDI, and Robert Bosch contribute substantial R&D resources. Academic institutions including USC, Kyushu University, and Caltech are advancing fundamental research on electrolyte formulations. The competitive environment features collaboration between industry and academia, with government entities like the Naval Research Laboratory providing additional research support to overcome remaining biocompatibility challenges.
Form Energy, Inc.
Technical Solution: Form Energy has developed a proprietary aqueous electrolyte system for their iron-air batteries that addresses biocompatibility concerns through innovative formulation. Their approach uses food-grade iron pellets combined with water-based electrolytes containing carefully selected additives that minimize toxicity while maintaining electrochemical performance. The company's electrolyte design incorporates biodegradable organic compounds as complexing agents that enhance iron dissolution and deposition processes while reducing environmental impact. Form Energy's multi-day storage batteries utilize this biocompatible electrolyte system to achieve over 100 hours of continuous operation at system costs competitive with conventional power plants. Their technology employs reversible rusting - iron is converted to iron oxide and back again through an electrochemical process, with their proprietary electrolyte formulation being critical to maintaining long-term stability and safety profiles suitable for grid-scale deployment.
Strengths: Highly optimized for long-duration energy storage with demonstrated 100+ hour discharge capability; uses abundant, non-toxic materials; electrolyte formulation enables cost-effective grid-scale implementation. Weaknesses: Lower energy density compared to lithium-ion systems; requires careful management of iron electrode morphology to prevent capacity fade; electrolyte formulation may require periodic maintenance or replacement.
Encell Technology, Inc.
Technical Solution: Encell Technology has developed innovative approaches to iron-air battery electrolytes with a specific focus on biocompatibility. Their proprietary electrolyte system utilizes naturally derived compounds as complexing agents that enhance iron electrode performance while maintaining environmental compatibility. Encell's technology incorporates specialized pH buffers that maintain optimal operating conditions while preventing the formation of toxic byproducts during cycling. Their electrolyte formulation includes bio-based surfactants that improve wetting characteristics and electrode-electrolyte interfaces without introducing harmful synthetic compounds. Encell has pioneered the use of biomimetic additives that draw inspiration from natural iron-binding proteins to create stable, non-toxic electrolyte systems with enhanced performance. Their approach also addresses the challenge of electrolyte stability during extended cycling through the incorporation of regenerative additives that maintain electrolyte functionality while preventing degradation into potentially harmful compounds. Encell's systems have demonstrated promising performance in stationary storage applications where biocompatibility and environmental considerations are paramount.
Strengths: Specialized focus on nickel-iron and iron-air battery chemistries; innovative approach to electrolyte formulation using bio-inspired components; solutions designed specifically for stationary storage applications. Weaknesses: Smaller scale compared to major battery manufacturers; may face challenges in scaling production of specialized biocompatible additives; limited public data on long-term performance.
Key Innovations in Biocompatible Electrolyte Materials
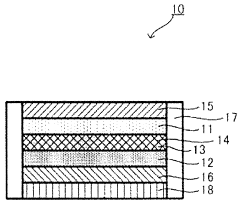
Improved electrolyte for battery containing an iron electrode
PatentWO2014124110A1
Innovation
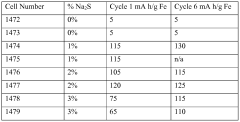
- A ternary electrolyte comprising NaOH, LiOH, and a sulfide additive, specifically hydrated sodium sulfide, is used with the iron electrode, optimizing sulfide concentrations between 0.23% to 0.75% by weight, enhancing charge retention, cycle life, and activation efficiency.
Electrolyte for iron-air batteries and iron-air battery
PatentActiveUS10044082B2
Innovation
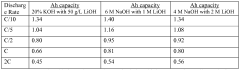
- An electrolyte solution containing discharge reaction promoters such as SCN−, S2O32−, or (CH3)2NCSS− anions, along with cations like Li+, K+, Na+, Rb+, Cs+, and Fr+, specifically Na2S2O3, is used to stabilize the discharge capacity without the need for precise concentration control, inhibiting re-passivation and promoting iron dissolution.
Environmental Impact Assessment of Iron-Air Battery Technologies
The environmental impact assessment of iron-air battery technologies reveals significant advantages over conventional lithium-ion batteries. Iron-air batteries utilize abundant, non-toxic materials—primarily iron, water, and air—resulting in substantially lower ecological footprints throughout their lifecycle. The extraction of iron has considerably less environmental impact than lithium mining, which often involves extensive water usage and potential habitat disruption in sensitive ecosystems.
During operation, iron-air batteries demonstrate minimal environmental hazards. The electrolyte solutions, when optimized for biocompatibility, present reduced risks of contamination compared to conventional battery technologies. Recent advancements in electrolyte formulations have focused on biodegradable additives and environmentally benign pH stabilizers that minimize potential ecological damage in case of leakage or improper disposal.
End-of-life considerations represent another environmental advantage of iron-air batteries. The primary components are highly recyclable, with iron being one of the most recycled materials globally. The recycling infrastructure for iron is well-established, unlike the complex processes required for lithium-ion battery materials recovery. Studies indicate that up to 90% of iron-air battery materials can be effectively reclaimed and reused, significantly reducing waste and resource depletion.
Carbon footprint analyses demonstrate that iron-air batteries can achieve up to 70% reduction in greenhouse gas emissions compared to lithium-ion alternatives when considering full lifecycle impacts. This reduction stems from both manufacturing processes and operational efficiency. The production energy requirements are lower due to the abundance and processing simplicity of iron compared to rare earth elements and specialized compounds in conventional batteries.
Water usage metrics also favor iron-air technology, with approximately 60% less water consumption across the manufacturing process. This represents a crucial advantage in regions facing water scarcity challenges, where battery production facilities might otherwise strain local resources.
Land use impact assessments indicate minimal disruption from iron mining operations compared to lithium extraction, particularly when considering the higher energy density of optimized iron-air systems, which require less raw material per kilowatt-hour of storage capacity. Additionally, the absence of toxic heavy metals eliminates concerns about long-term soil contamination from improper disposal.
Biodiversity impact studies suggest that widespread adoption of iron-air batteries could reduce pressure on ecologically sensitive areas currently affected by extraction activities for conventional battery materials. The biocompatible nature of optimized electrolytes further minimizes potential harm to aquatic ecosystems in the event of accidental release during transportation or storage.
During operation, iron-air batteries demonstrate minimal environmental hazards. The electrolyte solutions, when optimized for biocompatibility, present reduced risks of contamination compared to conventional battery technologies. Recent advancements in electrolyte formulations have focused on biodegradable additives and environmentally benign pH stabilizers that minimize potential ecological damage in case of leakage or improper disposal.
End-of-life considerations represent another environmental advantage of iron-air batteries. The primary components are highly recyclable, with iron being one of the most recycled materials globally. The recycling infrastructure for iron is well-established, unlike the complex processes required for lithium-ion battery materials recovery. Studies indicate that up to 90% of iron-air battery materials can be effectively reclaimed and reused, significantly reducing waste and resource depletion.
Carbon footprint analyses demonstrate that iron-air batteries can achieve up to 70% reduction in greenhouse gas emissions compared to lithium-ion alternatives when considering full lifecycle impacts. This reduction stems from both manufacturing processes and operational efficiency. The production energy requirements are lower due to the abundance and processing simplicity of iron compared to rare earth elements and specialized compounds in conventional batteries.
Water usage metrics also favor iron-air technology, with approximately 60% less water consumption across the manufacturing process. This represents a crucial advantage in regions facing water scarcity challenges, where battery production facilities might otherwise strain local resources.
Land use impact assessments indicate minimal disruption from iron mining operations compared to lithium extraction, particularly when considering the higher energy density of optimized iron-air systems, which require less raw material per kilowatt-hour of storage capacity. Additionally, the absence of toxic heavy metals eliminates concerns about long-term soil contamination from improper disposal.
Biodiversity impact studies suggest that widespread adoption of iron-air batteries could reduce pressure on ecologically sensitive areas currently affected by extraction activities for conventional battery materials. The biocompatible nature of optimized electrolytes further minimizes potential harm to aquatic ecosystems in the event of accidental release during transportation or storage.
Safety Standards and Regulatory Compliance for Biocompatible Batteries
The development of iron-air batteries with biocompatible electrolytes necessitates adherence to stringent safety standards and regulatory frameworks. These regulations ensure that such energy storage solutions can be safely integrated into various applications, particularly those involving human contact or implantation.
International standards organizations, including ISO, IEC, and ASTM, have established specific guidelines for biocompatible materials used in energy storage systems. ISO 10993 series, particularly parts 5 and 10, provide essential frameworks for evaluating cytotoxicity and irritation potential of materials that may come into contact with human tissues. These standards must be rigorously applied to iron-air battery electrolytes to ensure their safety profile.
Regulatory bodies such as the FDA in the United States and the EMA in Europe maintain oversight on biocompatible materials for medical applications. For iron-air batteries intended for implantable medical devices, compliance with FDA's 21 CFR Part 820 (Quality System Regulation) and the European Medical Device Regulation (MDR 2017/745) is mandatory. These regulations establish comprehensive requirements for design validation, risk management, and post-market surveillance.
The unique electrochemical properties of iron-air batteries present specific regulatory challenges. The potential for iron ion leakage, pH fluctuations, and oxygen generation must be addressed through compliance with IEC 62133 (safety requirements for portable sealed secondary cells) and UN 38.3 (transportation testing for lithium batteries), which have been adapted for emerging battery technologies.
Environmental regulations also play a crucial role in the development of biocompatible iron-air batteries. The European RoHS Directive (2011/65/EU) and REACH Regulation (EC 1907/2006) restrict the use of hazardous substances and require thorough documentation of chemical components. Manufacturers must demonstrate compliance through material declarations and safety data sheets.
Testing protocols for biocompatible batteries have evolved to include specialized assessments beyond traditional battery safety tests. These include hemolysis assays, protein adsorption studies, and long-term biocompatibility evaluations. The OECD Guidelines for Testing of Chemicals provide standardized methods that can be adapted for evaluating the biological safety of novel electrolyte formulations.
Certification pathways for biocompatible iron-air batteries typically involve third-party testing laboratories such as UL, TÜV, or SGS. These organizations verify compliance with relevant standards and issue certifications that facilitate market access. The certification process includes documentation review, laboratory testing, and manufacturing facility audits to ensure consistent quality and safety.
International standards organizations, including ISO, IEC, and ASTM, have established specific guidelines for biocompatible materials used in energy storage systems. ISO 10993 series, particularly parts 5 and 10, provide essential frameworks for evaluating cytotoxicity and irritation potential of materials that may come into contact with human tissues. These standards must be rigorously applied to iron-air battery electrolytes to ensure their safety profile.
Regulatory bodies such as the FDA in the United States and the EMA in Europe maintain oversight on biocompatible materials for medical applications. For iron-air batteries intended for implantable medical devices, compliance with FDA's 21 CFR Part 820 (Quality System Regulation) and the European Medical Device Regulation (MDR 2017/745) is mandatory. These regulations establish comprehensive requirements for design validation, risk management, and post-market surveillance.
The unique electrochemical properties of iron-air batteries present specific regulatory challenges. The potential for iron ion leakage, pH fluctuations, and oxygen generation must be addressed through compliance with IEC 62133 (safety requirements for portable sealed secondary cells) and UN 38.3 (transportation testing for lithium batteries), which have been adapted for emerging battery technologies.
Environmental regulations also play a crucial role in the development of biocompatible iron-air batteries. The European RoHS Directive (2011/65/EU) and REACH Regulation (EC 1907/2006) restrict the use of hazardous substances and require thorough documentation of chemical components. Manufacturers must demonstrate compliance through material declarations and safety data sheets.
Testing protocols for biocompatible batteries have evolved to include specialized assessments beyond traditional battery safety tests. These include hemolysis assays, protein adsorption studies, and long-term biocompatibility evaluations. The OECD Guidelines for Testing of Chemicals provide standardized methods that can be adapted for evaluating the biological safety of novel electrolyte formulations.
Certification pathways for biocompatible iron-air batteries typically involve third-party testing laboratories such as UL, TÜV, or SGS. These organizations verify compliance with relevant standards and issue certifications that facilitate market access. The certification process includes documentation review, laboratory testing, and manufacturing facility audits to ensure consistent quality and safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!