Regulatory Considerations For Continuous Manufacturing Of APIs
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
API Continuous Manufacturing Background and Objectives
Continuous manufacturing of Active Pharmaceutical Ingredients (APIs) represents a paradigm shift from traditional batch processing methods that have dominated pharmaceutical production for decades. This transformative approach involves the uninterrupted production of APIs through a series of integrated unit operations, enabling a more efficient, consistent, and economical manufacturing process. The evolution of continuous manufacturing in the pharmaceutical industry began in the early 2000s, with significant acceleration in development and adoption over the past decade.
The historical context of API manufacturing reveals a predominant reliance on batch processing, characterized by discrete production steps with intermediate quality testing and storage. However, this traditional approach presents inherent limitations including scalability challenges, batch-to-batch variability, and substantial resource requirements. Continuous manufacturing emerged as a response to these limitations, offering potential solutions to long-standing industry challenges.
Regulatory agencies worldwide have recognized the potential benefits of continuous manufacturing, with the FDA's 2004 Process Analytical Technology (PAT) initiative and the 2011 Quality by Design (QbD) framework serving as catalysts for industry transformation. These regulatory developments have established a foundation for the implementation of innovative manufacturing technologies while maintaining stringent quality standards.
The primary objectives of continuous API manufacturing include enhancing process efficiency, improving product quality consistency, reducing manufacturing footprint, minimizing waste generation, and enabling real-time quality assurance. Additionally, continuous manufacturing aims to facilitate more responsive supply chains capable of adapting to market demands with greater agility.
Current technological trends in continuous API manufacturing focus on the development of integrated flow chemistry systems, advanced process analytical technologies, automated control strategies, and digital twins for process simulation. The convergence of these technologies with artificial intelligence and machine learning applications represents the frontier of innovation in this domain.
The global landscape of continuous manufacturing adoption reveals varying degrees of implementation across different regions, with North America and Europe leading in both regulatory framework development and industrial application. Emerging markets are increasingly recognizing the strategic importance of this technology for pharmaceutical sovereignty and cost-effective healthcare solutions.
Looking forward, the trajectory of continuous API manufacturing points toward increased integration with downstream processing, expanded application across diverse API classes, and enhanced regulatory harmonization to facilitate global implementation. The ultimate goal remains the establishment of end-to-end continuous manufacturing platforms that revolutionize pharmaceutical production paradigms.
The historical context of API manufacturing reveals a predominant reliance on batch processing, characterized by discrete production steps with intermediate quality testing and storage. However, this traditional approach presents inherent limitations including scalability challenges, batch-to-batch variability, and substantial resource requirements. Continuous manufacturing emerged as a response to these limitations, offering potential solutions to long-standing industry challenges.
Regulatory agencies worldwide have recognized the potential benefits of continuous manufacturing, with the FDA's 2004 Process Analytical Technology (PAT) initiative and the 2011 Quality by Design (QbD) framework serving as catalysts for industry transformation. These regulatory developments have established a foundation for the implementation of innovative manufacturing technologies while maintaining stringent quality standards.
The primary objectives of continuous API manufacturing include enhancing process efficiency, improving product quality consistency, reducing manufacturing footprint, minimizing waste generation, and enabling real-time quality assurance. Additionally, continuous manufacturing aims to facilitate more responsive supply chains capable of adapting to market demands with greater agility.
Current technological trends in continuous API manufacturing focus on the development of integrated flow chemistry systems, advanced process analytical technologies, automated control strategies, and digital twins for process simulation. The convergence of these technologies with artificial intelligence and machine learning applications represents the frontier of innovation in this domain.
The global landscape of continuous manufacturing adoption reveals varying degrees of implementation across different regions, with North America and Europe leading in both regulatory framework development and industrial application. Emerging markets are increasingly recognizing the strategic importance of this technology for pharmaceutical sovereignty and cost-effective healthcare solutions.
Looking forward, the trajectory of continuous API manufacturing points toward increased integration with downstream processing, expanded application across diverse API classes, and enhanced regulatory harmonization to facilitate global implementation. The ultimate goal remains the establishment of end-to-end continuous manufacturing platforms that revolutionize pharmaceutical production paradigms.
Market Demand Analysis for Continuous API Manufacturing
The pharmaceutical industry is witnessing a significant shift from traditional batch manufacturing to continuous manufacturing processes for Active Pharmaceutical Ingredients (APIs). Market analysis indicates that this transition is driven by multiple factors, with regulatory considerations playing a pivotal role in accelerating adoption rates.
Current market assessments value the continuous API manufacturing sector at approximately $2.3 billion globally, with projections suggesting growth to reach $3.7 billion by 2027, representing a compound annual growth rate of 10.2%. This growth trajectory reflects the increasing recognition of continuous manufacturing's advantages in meeting stringent regulatory requirements while delivering operational efficiencies.
Demand analysis reveals that pharmaceutical companies are primarily motivated by regulatory incentives when considering continuous manufacturing implementation. The FDA's Emerging Technology Program and similar initiatives by the EMA have created a favorable regulatory environment that rewards innovation in manufacturing processes. Companies adopting continuous manufacturing report 15-30% reductions in regulatory review times for new drug applications, creating a compelling business case beyond operational benefits.
Market segmentation shows varying adoption rates across different pharmaceutical sectors. Generic manufacturers demonstrate the highest growth rate at 13.5% annually, driven by competitive pressures and the need for cost-efficient regulatory compliance. Innovator companies, while showing more measured adoption at 8.7% growth, are investing more substantially in comprehensive continuous manufacturing platforms that address regulatory considerations across multiple product lines.
Regional analysis indicates that North America currently dominates the market with 42% share, followed by Europe at 35%. However, the Asia-Pacific region is experiencing the fastest growth at 14.3% annually, as regulatory harmonization efforts make continuous manufacturing an attractive option for companies serving global markets.
Customer surveys indicate that 78% of pharmaceutical executives cite "regulatory compliance advantages" as a primary driver for continuous manufacturing investments, ranking above cost savings (65%) and quality improvements (61%). This underscores the critical importance of regulatory considerations in market demand formation.
The market demonstrates increasing demand for integrated solutions that address both manufacturing efficiency and regulatory compliance simultaneously. Vendors offering comprehensive packages that include regulatory documentation support, validation protocols, and compliance monitoring systems are capturing premium market positions, commanding 25-40% price premiums compared to basic equipment providers.
Current market assessments value the continuous API manufacturing sector at approximately $2.3 billion globally, with projections suggesting growth to reach $3.7 billion by 2027, representing a compound annual growth rate of 10.2%. This growth trajectory reflects the increasing recognition of continuous manufacturing's advantages in meeting stringent regulatory requirements while delivering operational efficiencies.
Demand analysis reveals that pharmaceutical companies are primarily motivated by regulatory incentives when considering continuous manufacturing implementation. The FDA's Emerging Technology Program and similar initiatives by the EMA have created a favorable regulatory environment that rewards innovation in manufacturing processes. Companies adopting continuous manufacturing report 15-30% reductions in regulatory review times for new drug applications, creating a compelling business case beyond operational benefits.
Market segmentation shows varying adoption rates across different pharmaceutical sectors. Generic manufacturers demonstrate the highest growth rate at 13.5% annually, driven by competitive pressures and the need for cost-efficient regulatory compliance. Innovator companies, while showing more measured adoption at 8.7% growth, are investing more substantially in comprehensive continuous manufacturing platforms that address regulatory considerations across multiple product lines.
Regional analysis indicates that North America currently dominates the market with 42% share, followed by Europe at 35%. However, the Asia-Pacific region is experiencing the fastest growth at 14.3% annually, as regulatory harmonization efforts make continuous manufacturing an attractive option for companies serving global markets.
Customer surveys indicate that 78% of pharmaceutical executives cite "regulatory compliance advantages" as a primary driver for continuous manufacturing investments, ranking above cost savings (65%) and quality improvements (61%). This underscores the critical importance of regulatory considerations in market demand formation.
The market demonstrates increasing demand for integrated solutions that address both manufacturing efficiency and regulatory compliance simultaneously. Vendors offering comprehensive packages that include regulatory documentation support, validation protocols, and compliance monitoring systems are capturing premium market positions, commanding 25-40% price premiums compared to basic equipment providers.
Current Regulatory Landscape and Technical Challenges
The continuous manufacturing of Active Pharmaceutical Ingredients (APIs) faces a complex regulatory landscape globally. Currently, the U.S. Food and Drug Administration (FDA) leads regulatory advancement through its Quality by Design (QbD) initiative, which encourages manufacturers to build quality into products from development stages. The FDA's 2019 guidance document specifically addresses continuous manufacturing, providing recommendations on process validation, control strategies, and regulatory submissions.
The European Medicines Agency (EMA) has also developed guidelines supporting continuous manufacturing adoption, though these are less prescriptive than FDA's approach. EMA emphasizes risk-based approaches and real-time release testing (RTRT) as critical components for regulatory compliance. Meanwhile, regulatory frameworks in emerging markets like China and India are still evolving, creating potential barriers for global implementation.
Technical challenges in continuous manufacturing regulatory compliance center around process analytical technology (PAT) implementation. Regulatory bodies expect robust PAT systems for real-time monitoring, but standardization of these technologies remains incomplete. This creates uncertainty for manufacturers regarding which PAT solutions will satisfy regulatory requirements across different jurisdictions.
Material traceability presents another significant challenge. Regulators require manufacturers to demonstrate complete material tracking throughout continuous processes, including the ability to identify and isolate potential quality deviations. Current technologies for material tracking in continuous flow systems often fall short of regulatory expectations, particularly for complex multi-phase reactions.
Process validation methodologies for continuous manufacturing differ substantially from traditional batch approaches. Regulatory agencies have not fully harmonized expectations for continuous process validation, creating compliance uncertainties. The industry lacks consensus on appropriate sampling strategies, statistical methods for continuous data evaluation, and criteria for demonstrating process stability.
Regulatory requirements for handling process disturbances and state transitions (startup, shutdown, rate changes) remain ambiguous. Manufacturers struggle to develop control strategies that adequately address these dynamic conditions while maintaining compliance with current Good Manufacturing Practices (cGMP).
Cross-border regulatory differences create additional complications for global API manufacturers. Divergent requirements for data integrity, equipment qualification, and process monitoring between major regulatory bodies necessitate complex compliance strategies for companies operating internationally. These regulatory inconsistencies significantly increase the cost and complexity of implementing continuous manufacturing technologies.
The European Medicines Agency (EMA) has also developed guidelines supporting continuous manufacturing adoption, though these are less prescriptive than FDA's approach. EMA emphasizes risk-based approaches and real-time release testing (RTRT) as critical components for regulatory compliance. Meanwhile, regulatory frameworks in emerging markets like China and India are still evolving, creating potential barriers for global implementation.
Technical challenges in continuous manufacturing regulatory compliance center around process analytical technology (PAT) implementation. Regulatory bodies expect robust PAT systems for real-time monitoring, but standardization of these technologies remains incomplete. This creates uncertainty for manufacturers regarding which PAT solutions will satisfy regulatory requirements across different jurisdictions.
Material traceability presents another significant challenge. Regulators require manufacturers to demonstrate complete material tracking throughout continuous processes, including the ability to identify and isolate potential quality deviations. Current technologies for material tracking in continuous flow systems often fall short of regulatory expectations, particularly for complex multi-phase reactions.
Process validation methodologies for continuous manufacturing differ substantially from traditional batch approaches. Regulatory agencies have not fully harmonized expectations for continuous process validation, creating compliance uncertainties. The industry lacks consensus on appropriate sampling strategies, statistical methods for continuous data evaluation, and criteria for demonstrating process stability.
Regulatory requirements for handling process disturbances and state transitions (startup, shutdown, rate changes) remain ambiguous. Manufacturers struggle to develop control strategies that adequately address these dynamic conditions while maintaining compliance with current Good Manufacturing Practices (cGMP).
Cross-border regulatory differences create additional complications for global API manufacturers. Divergent requirements for data integrity, equipment qualification, and process monitoring between major regulatory bodies necessitate complex compliance strategies for companies operating internationally. These regulatory inconsistencies significantly increase the cost and complexity of implementing continuous manufacturing technologies.
Current Regulatory Compliance Strategies
01 Regulatory compliance frameworks for continuous manufacturing
Regulatory agencies have established specific frameworks for continuous manufacturing of Active Pharmaceutical Ingredients (APIs). These frameworks include guidelines for process validation, quality control, and documentation requirements that differ from traditional batch manufacturing. Continuous manufacturing requires real-time monitoring and control strategies to ensure consistent product quality, which must be demonstrated to regulatory authorities. Manufacturers must develop robust control strategies that align with regulatory expectations for process analytical technology (PAT) implementation.- Regulatory compliance frameworks for continuous manufacturing: Regulatory frameworks have been established to guide the implementation of continuous manufacturing processes for Active Pharmaceutical Ingredients (APIs). These frameworks address quality control requirements, validation protocols, and compliance with Good Manufacturing Practices (GMP). They provide guidelines for manufacturers to ensure that continuously produced APIs meet the same quality standards as those produced through traditional batch processes, while also streamlining the approval process for continuous manufacturing technologies.
- Real-time monitoring and process analytical technology: Continuous manufacturing of APIs requires robust real-time monitoring systems and process analytical technology (PAT) to ensure consistent quality. These technologies enable continuous assessment of critical quality attributes during production, allowing for immediate adjustments to process parameters when deviations occur. Implementation of PAT supports regulatory compliance by providing comprehensive data for quality assurance and facilitating a more thorough understanding of the manufacturing process.
- Quality by Design approach in continuous manufacturing: Quality by Design (QbD) principles are essential for regulatory approval of continuous manufacturing processes for APIs. This approach involves identifying critical quality attributes, establishing design spaces, and implementing control strategies to ensure consistent product quality. QbD facilitates regulatory acceptance by demonstrating a thorough understanding of how process parameters affect product quality, enabling more flexible regulatory approaches that focus on risk management rather than fixed process parameters.
- Validation and qualification requirements: Specific validation and qualification requirements exist for continuous manufacturing equipment and processes in pharmaceutical production. These include establishing process validation protocols that demonstrate consistent production of APIs meeting predetermined quality attributes. Regulatory considerations include validation of equipment cleaning procedures, process control systems, and the demonstration of state control during extended continuous operations. Validation strategies must address the unique aspects of continuous processing, such as steady-state operations and potential disturbances.
- Regulatory submission strategies and case studies: Successful regulatory submission strategies for continuous manufacturing processes involve comprehensive documentation of process development, validation data, and control strategies. Case studies of approved continuous manufacturing processes provide valuable insights into regulatory expectations and successful approaches. These include demonstrations of process robustness, strategies for handling deviations, and approaches to scale-up that have gained regulatory acceptance. Understanding these precedents helps manufacturers navigate the regulatory landscape when implementing continuous manufacturing for APIs.
02 Process monitoring and quality control systems
Continuous manufacturing of APIs requires sophisticated monitoring and quality control systems to ensure consistent product quality. These systems include real-time process analytical technology (PAT) tools that monitor critical process parameters and material attributes throughout the manufacturing process. Advanced control algorithms can automatically adjust process parameters to maintain quality within specified limits. Implementation of these monitoring systems is essential for regulatory approval as they provide continuous verification of product quality rather than relying on end-product testing.Expand Specific Solutions03 Risk assessment and mitigation strategies
Regulatory considerations for continuous manufacturing of APIs include comprehensive risk assessment and mitigation strategies. These strategies involve identifying potential failure modes, establishing control measures, and implementing contingency plans. Risk assessments must address specific challenges of continuous processing, such as material traceability, process disturbances, and equipment reliability. Manufacturers must demonstrate to regulatory authorities that they have robust risk management systems in place to ensure product quality and patient safety throughout the continuous manufacturing process.Expand Specific Solutions04 Validation approaches for continuous processes
Validation approaches for continuous manufacturing processes differ significantly from traditional batch validation. Continuous process validation requires demonstrating that the process remains in a state of control during extended operation periods. This includes validation of start-up and shutdown procedures, steady-state operation, and disturbance handling. Regulatory authorities expect manufacturers to establish appropriate validation protocols that address the unique aspects of continuous processing, including material residence time distribution, process dynamics, and control system performance.Expand Specific Solutions05 Digital infrastructure and data management requirements
Continuous manufacturing of APIs requires robust digital infrastructure and data management systems to meet regulatory requirements. These systems must handle large volumes of process data, ensure data integrity, and provide appropriate audit trails. Regulatory authorities expect manufacturers to implement validated computerized systems that comply with relevant data integrity guidelines. Advanced data analytics capabilities are needed to extract meaningful information from continuous process data, enabling real-time quality assurance and facilitating regulatory review of manufacturing records.Expand Specific Solutions
Key Regulatory Agencies and Industry Players
The continuous manufacturing of APIs is currently in a transitional phase, moving from early adoption to broader implementation across the pharmaceutical industry. The global market for continuous API manufacturing is expanding rapidly, projected to reach significant growth as regulatory bodies increasingly support this more efficient production method. From a technical maturity perspective, companies like IBM, Microsoft, and Salesforce are developing advanced digital monitoring and control systems essential for continuous manufacturing compliance, while pharmaceutical entities such as Insmed and Kowa are implementing practical applications. Regulatory frameworks are evolving, with agencies working closely with technology providers like Accenture and pharmaceutical manufacturers to establish standardized approaches for validation, real-time release testing, and process analytical technology integration in continuous manufacturing environments.
International Business Machines Corp.
Technical Solution: IBM has developed an AI-powered regulatory compliance platform specifically designed for continuous manufacturing of APIs. Their system leverages machine learning algorithms to analyze real-time process data and predict potential quality issues before they occur. IBM's approach integrates blockchain technology to create immutable records of manufacturing parameters and quality attributes, providing regulators with transparent, tamper-proof documentation. Their platform includes natural language processing capabilities that continuously monitor regulatory changes across global markets and automatically flag potential compliance issues for pharmaceutical manufacturers. IBM's system features a digital twin architecture that simulates process behavior under various conditions, enabling manufacturers to demonstrate process understanding and control to regulatory authorities without extensive physical testing. Their regulatory submission tools automatically generate required documentation from manufacturing data, ensuring consistency between actual operations and regulatory filings.
Strengths: Advanced AI and predictive analytics capabilities provide superior process understanding and control. Their blockchain-based documentation creates unparalleled data integrity for regulatory submissions. Weaknesses: Limited pharmaceutical-specific expertise may require partnerships with industry specialists for complete implementation in API manufacturing environments.
Kowa Co., Ltd.
Technical Solution: Kowa has developed an innovative regulatory framework for continuous API manufacturing that focuses on integration with Japan's PMDA requirements while maintaining compliance with global standards. Their approach combines continuous flow reactors with advanced in-line analytical technologies that enable real-time quality monitoring. Kowa's system features a hierarchical control architecture that maintains process parameters within a validated design space, automatically responding to deviations before they impact product quality. Their regulatory strategy includes comprehensive process modeling that demonstrates how their continuous manufacturing system maintains consistent API quality across production scales. Kowa has implemented a digital twin approach that simulates process behavior under various conditions, providing regulatory authorities with extensive evidence of process understanding and control. Their submission package includes detailed comparability protocols showing equivalence between products manufactured using continuous versus traditional batch methods.
Strengths: Extensive experience with Japanese regulatory requirements while maintaining global compliance capabilities. Their digital twin approach provides superior process understanding and predictive capabilities. Weaknesses: System complexity may create challenges for regulatory inspectors unfamiliar with continuous manufacturing technologies, potentially extending approval timelines.
Critical Quality Attributes and Process Analytical Technology
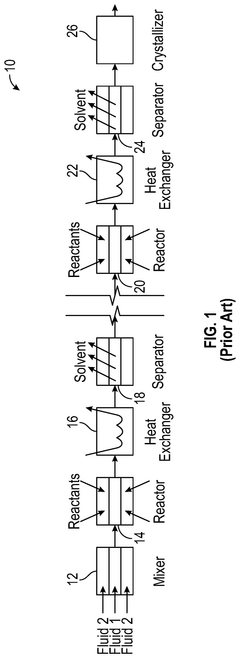
Continuous production of active pharmaceutical ingredients
PatentActiveUS12239943B2
Innovation
- The implementation of membrane-based devices and processes throughout the API manufacturing process, including membrane reactors, separators, crystallizers, and heat exchangers, to enable continuous, efficient, and controlled production of APIs.
A continuous manufacturing system for the production of active pharmaceutical ingredients including albuterol sulfate
PatentWO2025179088A1
Innovation
- A continuous manufacturing system with integrated process analytical technologies (PATs) and digital control systems for inline monitoring, enabling a streamlined synthesis of albuterol sulfate through tubular reactors, catalytic packed beds, and stirred-tank reactors, with real-time quality assessment.
Environmental Impact and Sustainability Considerations
Continuous manufacturing of Active Pharmaceutical Ingredients (APIs) offers significant environmental advantages over traditional batch processing methods. The shift towards continuous manufacturing aligns with global sustainability goals by substantially reducing resource consumption. Studies indicate that continuous processes can decrease solvent usage by 30-50% compared to batch methods, directly lowering the carbon footprint of pharmaceutical manufacturing operations. This reduction stems from more efficient reaction conditions, improved mixing capabilities, and real-time process adjustments that optimize material utilization.
Energy efficiency represents another critical environmental benefit of continuous manufacturing systems. These systems typically require smaller equipment footprints and operate at steady state conditions, resulting in energy consumption reductions of up to 40% in some documented implementations. The consistent processing parameters eliminate the energy-intensive heating and cooling cycles characteristic of batch operations, contributing to lower greenhouse gas emissions throughout the production lifecycle.
Waste generation, a significant environmental concern in pharmaceutical manufacturing, is also addressed through continuous processing technologies. The enhanced process control capabilities enable manufacturers to minimize off-specification products and reduce waste streams. Real-time monitoring and feedback systems allow for immediate process corrections, preventing the production of material that would otherwise require disposal. Industry case studies demonstrate waste reduction rates of 20-60% following implementation of continuous manufacturing platforms.
Water conservation presents another sustainability advantage, with continuous systems typically requiring 25-45% less water than comparable batch processes. This reduction becomes particularly significant considering that pharmaceutical manufacturing traditionally ranks among the most water-intensive industrial sectors. The decreased water demand not only conserves this vital resource but also reduces the energy required for water treatment and handling.
Regulatory frameworks increasingly recognize these environmental benefits, with agencies like the FDA and EMA incorporating sustainability considerations into their guidance for continuous manufacturing adoption. The ICH Q13 draft guideline specifically acknowledges the potential environmental advantages of continuous processing. Manufacturers implementing continuous API production can leverage these environmental improvements to support sustainability claims in regulatory submissions, potentially accelerating approval processes in jurisdictions with green chemistry initiatives.
Life cycle assessment (LCA) methodologies are becoming essential tools for quantifying the environmental impacts of continuous manufacturing implementations. These comprehensive analyses evaluate environmental effects across the entire production chain, from raw material extraction through manufacturing to disposal. Regulatory bodies increasingly expect pharmaceutical companies to provide LCA data demonstrating the environmental benefits of their manufacturing approach, particularly when seeking expedited reviews under sustainability-focused regulatory pathways.
Energy efficiency represents another critical environmental benefit of continuous manufacturing systems. These systems typically require smaller equipment footprints and operate at steady state conditions, resulting in energy consumption reductions of up to 40% in some documented implementations. The consistent processing parameters eliminate the energy-intensive heating and cooling cycles characteristic of batch operations, contributing to lower greenhouse gas emissions throughout the production lifecycle.
Waste generation, a significant environmental concern in pharmaceutical manufacturing, is also addressed through continuous processing technologies. The enhanced process control capabilities enable manufacturers to minimize off-specification products and reduce waste streams. Real-time monitoring and feedback systems allow for immediate process corrections, preventing the production of material that would otherwise require disposal. Industry case studies demonstrate waste reduction rates of 20-60% following implementation of continuous manufacturing platforms.
Water conservation presents another sustainability advantage, with continuous systems typically requiring 25-45% less water than comparable batch processes. This reduction becomes particularly significant considering that pharmaceutical manufacturing traditionally ranks among the most water-intensive industrial sectors. The decreased water demand not only conserves this vital resource but also reduces the energy required for water treatment and handling.
Regulatory frameworks increasingly recognize these environmental benefits, with agencies like the FDA and EMA incorporating sustainability considerations into their guidance for continuous manufacturing adoption. The ICH Q13 draft guideline specifically acknowledges the potential environmental advantages of continuous processing. Manufacturers implementing continuous API production can leverage these environmental improvements to support sustainability claims in regulatory submissions, potentially accelerating approval processes in jurisdictions with green chemistry initiatives.
Life cycle assessment (LCA) methodologies are becoming essential tools for quantifying the environmental impacts of continuous manufacturing implementations. These comprehensive analyses evaluate environmental effects across the entire production chain, from raw material extraction through manufacturing to disposal. Regulatory bodies increasingly expect pharmaceutical companies to provide LCA data demonstrating the environmental benefits of their manufacturing approach, particularly when seeking expedited reviews under sustainability-focused regulatory pathways.
Risk Management Approaches for Continuous Manufacturing
Continuous manufacturing of Active Pharmaceutical Ingredients (APIs) requires robust risk management approaches to ensure product quality and regulatory compliance. The implementation of Quality by Design (QbD) principles forms the foundation of risk management in continuous manufacturing, emphasizing thorough understanding of process parameters and their impact on product quality.
Risk assessment methodologies specifically adapted for continuous processes are essential. These include Failure Mode and Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Process Hazard Analysis (PHA). These tools help identify potential failure points in the continuous manufacturing line and establish appropriate control strategies.
Real-time monitoring and control systems represent a critical component of risk management in continuous manufacturing. Process Analytical Technology (PAT) tools enable continuous verification of critical quality attributes, allowing for immediate detection of deviations and implementation of corrective actions. This approach significantly reduces the risk of producing out-of-specification material compared to traditional batch manufacturing.
Control strategy development must address the unique challenges of continuous manufacturing, including residence time distribution, material traceability, and state of control maintenance. Manufacturers should implement strategies for material diversion when process parameters deviate beyond acceptable ranges, ensuring that potentially non-conforming product is isolated from the main production stream.
Validation approaches for continuous processes differ from traditional batch validation. Continuous process validation emphasizes ongoing verification rather than the traditional three-batch validation paradigm. This includes establishing appropriate sampling strategies that account for the dynamic nature of continuous processes and statistical approaches for evaluating process capability.
Regulatory agencies have recognized the need for specific risk management frameworks for continuous manufacturing. The FDA's guidance on Process Validation and the ICH Q8-Q12 guidelines provide relevant frameworks that can be adapted to continuous manufacturing scenarios. These frameworks emphasize the importance of process understanding, control strategy implementation, and continuous improvement.
Knowledge management systems play a vital role in risk mitigation by capturing process understanding and facilitating continuous improvement. These systems should document the relationship between process parameters and quality attributes, enabling informed decision-making during process deviations or when implementing changes.
Cross-functional collaboration between process engineers, quality assurance personnel, and regulatory affairs specialists is essential for comprehensive risk management in continuous manufacturing environments. This collaborative approach ensures that technical, quality, and regulatory considerations are integrated into a cohesive risk management strategy.
Risk assessment methodologies specifically adapted for continuous processes are essential. These include Failure Mode and Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Process Hazard Analysis (PHA). These tools help identify potential failure points in the continuous manufacturing line and establish appropriate control strategies.
Real-time monitoring and control systems represent a critical component of risk management in continuous manufacturing. Process Analytical Technology (PAT) tools enable continuous verification of critical quality attributes, allowing for immediate detection of deviations and implementation of corrective actions. This approach significantly reduces the risk of producing out-of-specification material compared to traditional batch manufacturing.
Control strategy development must address the unique challenges of continuous manufacturing, including residence time distribution, material traceability, and state of control maintenance. Manufacturers should implement strategies for material diversion when process parameters deviate beyond acceptable ranges, ensuring that potentially non-conforming product is isolated from the main production stream.
Validation approaches for continuous processes differ from traditional batch validation. Continuous process validation emphasizes ongoing verification rather than the traditional three-batch validation paradigm. This includes establishing appropriate sampling strategies that account for the dynamic nature of continuous processes and statistical approaches for evaluating process capability.
Regulatory agencies have recognized the need for specific risk management frameworks for continuous manufacturing. The FDA's guidance on Process Validation and the ICH Q8-Q12 guidelines provide relevant frameworks that can be adapted to continuous manufacturing scenarios. These frameworks emphasize the importance of process understanding, control strategy implementation, and continuous improvement.
Knowledge management systems play a vital role in risk mitigation by capturing process understanding and facilitating continuous improvement. These systems should document the relationship between process parameters and quality attributes, enabling informed decision-making during process deviations or when implementing changes.
Cross-functional collaboration between process engineers, quality assurance personnel, and regulatory affairs specialists is essential for comprehensive risk management in continuous manufacturing environments. This collaborative approach ensures that technical, quality, and regulatory considerations are integrated into a cohesive risk management strategy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!