Ammonium hydroxide as a mineralizing agent in nanochemistry
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH in Nanochemistry
Ammonium hydroxide (NH4OH) has emerged as a crucial mineralizing agent in the field of nanochemistry, playing a pivotal role in the synthesis and modification of various nanostructures. This compound, also known as aqueous ammonia, has gained significant attention due to its unique properties and versatile applications in nanomaterial fabrication.
The use of NH4OH in nanochemistry can be traced back to the early 2000s when researchers began exploring its potential as a structure-directing agent and pH regulator in nanoparticle synthesis. Since then, its applications have expanded rapidly, encompassing a wide range of nanomaterials, including metal oxides, quantum dots, and complex nanocomposites.
One of the primary functions of NH4OH in nanochemistry is its role as a mineralizing agent. It facilitates the controlled growth and crystallization of nanostructures by providing a suitable chemical environment. The ammonia molecules in NH4OH can form complexes with metal ions, influencing their solubility and reactivity. This property allows for precise control over nucleation and growth processes, leading to the formation of nanoparticles with desired sizes, shapes, and compositions.
NH4OH also serves as an effective pH regulator in nanomaterial synthesis. Its ability to maintain a stable alkaline environment is crucial for many nanoparticle formation reactions. By adjusting the concentration of NH4OH, researchers can fine-tune the pH of the reaction medium, which in turn affects the kinetics of nanoparticle formation and their resulting properties.
Furthermore, NH4OH has been extensively utilized in the synthesis of hollow nanostructures. Its etching capabilities enable the selective dissolution of certain components in core-shell nanoparticles, resulting in the formation of hollow interiors. This approach has led to the development of various hollow nanomaterials with enhanced surface areas and unique optical and catalytic properties.
In recent years, the role of NH4OH has expanded beyond traditional nanoparticle synthesis. It has found applications in the preparation of two-dimensional nanomaterials, such as graphene oxide and transition metal dichalcogenides. In these processes, NH4OH aids in the exfoliation and functionalization of layered materials, leading to the production of high-quality nanosheets.
The versatility of NH4OH in nanochemistry extends to its use in surface modification and functionalization of nanoparticles. By altering the surface chemistry of nanomaterials, researchers can enhance their stability, dispersibility, and compatibility with various matrices, opening up new avenues for applications in areas such as biomedicine, catalysis, and environmental remediation.
The use of NH4OH in nanochemistry can be traced back to the early 2000s when researchers began exploring its potential as a structure-directing agent and pH regulator in nanoparticle synthesis. Since then, its applications have expanded rapidly, encompassing a wide range of nanomaterials, including metal oxides, quantum dots, and complex nanocomposites.
One of the primary functions of NH4OH in nanochemistry is its role as a mineralizing agent. It facilitates the controlled growth and crystallization of nanostructures by providing a suitable chemical environment. The ammonia molecules in NH4OH can form complexes with metal ions, influencing their solubility and reactivity. This property allows for precise control over nucleation and growth processes, leading to the formation of nanoparticles with desired sizes, shapes, and compositions.
NH4OH also serves as an effective pH regulator in nanomaterial synthesis. Its ability to maintain a stable alkaline environment is crucial for many nanoparticle formation reactions. By adjusting the concentration of NH4OH, researchers can fine-tune the pH of the reaction medium, which in turn affects the kinetics of nanoparticle formation and their resulting properties.
Furthermore, NH4OH has been extensively utilized in the synthesis of hollow nanostructures. Its etching capabilities enable the selective dissolution of certain components in core-shell nanoparticles, resulting in the formation of hollow interiors. This approach has led to the development of various hollow nanomaterials with enhanced surface areas and unique optical and catalytic properties.
In recent years, the role of NH4OH has expanded beyond traditional nanoparticle synthesis. It has found applications in the preparation of two-dimensional nanomaterials, such as graphene oxide and transition metal dichalcogenides. In these processes, NH4OH aids in the exfoliation and functionalization of layered materials, leading to the production of high-quality nanosheets.
The versatility of NH4OH in nanochemistry extends to its use in surface modification and functionalization of nanoparticles. By altering the surface chemistry of nanomaterials, researchers can enhance their stability, dispersibility, and compatibility with various matrices, opening up new avenues for applications in areas such as biomedicine, catalysis, and environmental remediation.
Market Demand Analysis
The market demand for ammonium hydroxide as a mineralizing agent in nanochemistry has been steadily growing, driven by the increasing applications of nanoparticles across various industries. The global nanoparticle market, which heavily relies on efficient synthesis methods, is projected to reach $100 billion by 2025, with a compound annual growth rate (CAGR) of 12.5%. This growth directly impacts the demand for mineralizing agents like ammonium hydroxide.
In the semiconductor industry, the use of nanoparticles for advanced chip manufacturing has created a significant market for ammonium hydroxide-based synthesis processes. The semiconductor market, valued at $555 billion in 2021, is expected to grow at a CAGR of 7.7% until 2028, fueling the demand for high-quality nanoparticles and their precursors.
The healthcare and pharmaceutical sectors are also major contributors to the increasing demand for ammonium hydroxide in nanochemistry. Nanoparticles synthesized using this mineralizing agent find applications in drug delivery systems, diagnostic tools, and medical imaging. The global nanomedicine market, estimated at $180 billion in 2020, is forecasted to reach $350 billion by 2025, indicating a substantial growth potential for ammonium hydroxide-based nanoparticle synthesis.
Environmental applications, particularly in water treatment and air purification, represent another significant market segment. The global water treatment chemicals market, valued at $30 billion in 2020, is expected to grow at a CAGR of 6% until 2025. Nanoparticles synthesized using ammonium hydroxide play a crucial role in developing advanced filtration and purification technologies.
The energy sector, especially in the development of more efficient solar cells and energy storage solutions, is driving demand for specialized nanoparticles. The global solar energy market, worth $52 billion in 2018, is projected to reach $223 billion by 2026, with a CAGR of 20.5%. This growth directly translates to increased demand for ammonium hydroxide as a mineralizing agent in the synthesis of photovoltaic nanoparticles.
In the automotive industry, the shift towards electric vehicles and the need for lightweight materials are creating new opportunities for nanoparticle applications. The global automotive nanomaterials market is expected to grow from $4 billion in 2020 to $9 billion by 2025, with a CAGR of 17.5%. This growth will likely boost the demand for ammonium hydroxide in the synthesis of automotive-grade nanoparticles.
The cosmetics and personal care industry is another significant consumer of nanoparticles, particularly in sunscreens and anti-aging products. The global cosmetic chemicals market, valued at $21 billion in 2020, is expected to reach $27 billion by 2025, with nanoparticles playing an increasingly important role in product formulations.
In the semiconductor industry, the use of nanoparticles for advanced chip manufacturing has created a significant market for ammonium hydroxide-based synthesis processes. The semiconductor market, valued at $555 billion in 2021, is expected to grow at a CAGR of 7.7% until 2028, fueling the demand for high-quality nanoparticles and their precursors.
The healthcare and pharmaceutical sectors are also major contributors to the increasing demand for ammonium hydroxide in nanochemistry. Nanoparticles synthesized using this mineralizing agent find applications in drug delivery systems, diagnostic tools, and medical imaging. The global nanomedicine market, estimated at $180 billion in 2020, is forecasted to reach $350 billion by 2025, indicating a substantial growth potential for ammonium hydroxide-based nanoparticle synthesis.
Environmental applications, particularly in water treatment and air purification, represent another significant market segment. The global water treatment chemicals market, valued at $30 billion in 2020, is expected to grow at a CAGR of 6% until 2025. Nanoparticles synthesized using ammonium hydroxide play a crucial role in developing advanced filtration and purification technologies.
The energy sector, especially in the development of more efficient solar cells and energy storage solutions, is driving demand for specialized nanoparticles. The global solar energy market, worth $52 billion in 2018, is projected to reach $223 billion by 2026, with a CAGR of 20.5%. This growth directly translates to increased demand for ammonium hydroxide as a mineralizing agent in the synthesis of photovoltaic nanoparticles.
In the automotive industry, the shift towards electric vehicles and the need for lightweight materials are creating new opportunities for nanoparticle applications. The global automotive nanomaterials market is expected to grow from $4 billion in 2020 to $9 billion by 2025, with a CAGR of 17.5%. This growth will likely boost the demand for ammonium hydroxide in the synthesis of automotive-grade nanoparticles.
The cosmetics and personal care industry is another significant consumer of nanoparticles, particularly in sunscreens and anti-aging products. The global cosmetic chemicals market, valued at $21 billion in 2020, is expected to reach $27 billion by 2025, with nanoparticles playing an increasingly important role in product formulations.
Current Challenges
The use of ammonium hydroxide as a mineralizing agent in nanochemistry faces several significant challenges that researchers and industry professionals are actively working to overcome. One of the primary issues is the precise control of reaction conditions. Ammonium hydroxide's high volatility and sensitivity to temperature fluctuations make it difficult to maintain consistent concentration levels throughout the synthesis process. This variability can lead to inconsistent nanoparticle sizes and morphologies, affecting the reproducibility of experiments and the scalability of production processes.
Another challenge lies in the potential environmental and health impacts associated with the use of ammonium hydroxide. As a strong base, it poses risks to both human health and the environment if not handled properly. This necessitates stringent safety protocols and specialized equipment, which can increase the complexity and cost of nanomaterial synthesis processes. Furthermore, the disposal of waste products containing ammonium hydroxide requires careful consideration and treatment to minimize ecological impact.
The interaction between ammonium hydroxide and other precursor materials in nanochemistry is not fully understood in all systems. This knowledge gap can lead to unexpected side reactions or the formation of undesired byproducts, potentially compromising the purity and quality of the final nanomaterials. Researchers are still working to elucidate the exact mechanisms by which ammonium hydroxide influences nanoparticle growth and crystallization in various chemical environments.
Scalability remains a significant hurdle in the industrial application of ammonium hydroxide-based nanomaterial synthesis. While laboratory-scale processes may yield promising results, translating these methods to large-scale production often encounters difficulties in maintaining uniform reaction conditions and product quality. The challenges of heat and mass transfer in larger reactors can lead to inhomogeneous growth and aggregation of nanoparticles, diminishing the desired properties of the final product.
Additionally, the long-term stability of nanomaterials synthesized using ammonium hydroxide as a mineralizing agent is an area of ongoing research. Some nanostructures may undergo gradual changes in morphology or composition over time, potentially altering their functional properties. This instability can limit the shelf life and reliability of nanomaterials in various applications, from electronics to biomedical devices.
Lastly, the optimization of reaction parameters when using ammonium hydroxide in nanochemistry often requires extensive experimentation and characterization. The interdependence of factors such as concentration, temperature, pH, and reaction time creates a complex parameter space that can be time-consuming and resource-intensive to navigate. This challenge is compounded by the need for sophisticated analytical techniques to accurately assess the properties and quality of the resulting nanomaterials.
Another challenge lies in the potential environmental and health impacts associated with the use of ammonium hydroxide. As a strong base, it poses risks to both human health and the environment if not handled properly. This necessitates stringent safety protocols and specialized equipment, which can increase the complexity and cost of nanomaterial synthesis processes. Furthermore, the disposal of waste products containing ammonium hydroxide requires careful consideration and treatment to minimize ecological impact.
The interaction between ammonium hydroxide and other precursor materials in nanochemistry is not fully understood in all systems. This knowledge gap can lead to unexpected side reactions or the formation of undesired byproducts, potentially compromising the purity and quality of the final nanomaterials. Researchers are still working to elucidate the exact mechanisms by which ammonium hydroxide influences nanoparticle growth and crystallization in various chemical environments.
Scalability remains a significant hurdle in the industrial application of ammonium hydroxide-based nanomaterial synthesis. While laboratory-scale processes may yield promising results, translating these methods to large-scale production often encounters difficulties in maintaining uniform reaction conditions and product quality. The challenges of heat and mass transfer in larger reactors can lead to inhomogeneous growth and aggregation of nanoparticles, diminishing the desired properties of the final product.
Additionally, the long-term stability of nanomaterials synthesized using ammonium hydroxide as a mineralizing agent is an area of ongoing research. Some nanostructures may undergo gradual changes in morphology or composition over time, potentially altering their functional properties. This instability can limit the shelf life and reliability of nanomaterials in various applications, from electronics to biomedical devices.
Lastly, the optimization of reaction parameters when using ammonium hydroxide in nanochemistry often requires extensive experimentation and characterization. The interdependence of factors such as concentration, temperature, pH, and reaction time creates a complex parameter space that can be time-consuming and resource-intensive to navigate. This challenge is compounded by the need for sophisticated analytical techniques to accurately assess the properties and quality of the resulting nanomaterials.
Existing Applications
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use of ammonium hydroxide in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.
- Application in hair coloring and bleaching: Ammonium hydroxide is commonly used in hair coloring and bleaching products. It helps to open the hair cuticle, allowing the dye or bleaching agent to penetrate the hair shaft more effectively. The alkaline nature of ammonium hydroxide also assists in the oxidation process of hair dyes, resulting in longer-lasting color.
- Role in cleaning and household products: Ammonium hydroxide is a key ingredient in many cleaning and household products. Its strong alkaline properties make it effective in removing grease, grime, and stubborn stains. It is commonly found in glass cleaners, floor cleaners, and all-purpose household cleaners. The compound's ability to break down organic matter also makes it useful in certain laundry detergents.
- Use in textile processing: Ammonium hydroxide plays a significant role in textile processing. It is used in the mercerization of cotton fabrics, helping to increase the fabric's luster, strength, and dye affinity. The compound is also employed in the production of certain synthetic fibers and in the treatment of wool to improve its properties.
- Application in water treatment: Ammonium hydroxide is utilized in water treatment processes for various purposes. It can be used to adjust pH levels in water systems, control corrosion in pipes and equipment, and remove certain contaminants. In some cases, it is employed in the treatment of drinking water and in the maintenance of swimming pools to regulate chlorine levels.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental remediation
Ammonium hydroxide is employed in environmental remediation processes, particularly in air pollution control. It is used in flue gas treatment systems to neutralize acidic components and remove sulfur dioxide. In water treatment, it helps in adjusting pH levels and removing heavy metals. Its ability to react with and neutralize various pollutants makes it valuable in environmental protection applications.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, shampoos, and other hair care products. In some cosmetic formulations, it helps to stabilize emulsions and adjust the pH of the final product. Its alkaline nature also makes it useful in certain depilatory creams and hair relaxers.Expand Specific Solutions05 Application in food processing
Ammonium hydroxide has limited use in food processing as a leavening agent and pH control agent. It is used in the production of certain types of caramel coloring and in some baking processes. However, its use in food applications is strictly regulated and limited due to safety concerns. In some countries, it is approved as a food additive in specific applications and concentrations.Expand Specific Solutions
Key Industry Players
The research on ammonium hydroxide as a mineralizing agent in nanochemistry is in a developing stage, with growing market potential due to increasing applications in materials science and nanotechnology. The technology's maturity is moderate, with ongoing advancements from both academic institutions and industry players. Key companies like Mitsubishi Kasei Corp., Sumitomo Chemical Co., Ltd., and BASF Corp. are actively involved in this field, leveraging their expertise in chemical engineering and nanomaterials. Universities such as Tohoku University and Zhejiang University are contributing significant research efforts, fostering innovation and potential breakthroughs. The competitive landscape is diverse, with collaborations between academia and industry driving progress in this niche but promising area of nanochemistry.
Zhejiang University
Technical Solution: Researchers at Zhejiang University have made significant advancements in using ammonium hydroxide for the synthesis of metal oxide nanostructures. Their approach focuses on a hydrothermal method where ammonium hydroxide acts as a structure-directing agent and pH regulator[7]. This technique has been successfully applied to create various nanostructures such as nanorods, nanoflowers, and hierarchical structures of zinc oxide and titanium dioxide[9]. The university's team has also investigated the influence of ammonium hydroxide concentration on the morphology and properties of the resulting nanomaterials, providing valuable insights for tailored nanostructure design[11].
Strengths: Diverse nanostructure morphologies, fundamental understanding of growth mechanisms, potential for multifunctional materials. Weaknesses: May require specialized equipment for hydrothermal synthesis, scalability challenges for some complex nanostructures.
Northwestern University
Technical Solution: Northwestern University has conducted extensive research on the role of ammonium hydroxide in the synthesis of quantum dots and other semiconductor nanocrystals. Their approach utilizes ammonium hydroxide as a crucial component in hot-injection synthesis methods, where it serves as both a ligand and a source of hydroxide ions[8]. This technique has enabled the production of high-quality, monodisperse quantum dots with tunable optical properties. The university's team has also explored the use of ammonium hydroxide in the synthesis of doped semiconductor nanocrystals, demonstrating enhanced photoluminescence and stability[10]. Their research has provided valuable insights into the mechanisms of nucleation and growth in the presence of ammonium hydroxide, contributing to the broader understanding of nanocrystal formation[12].
Strengths: Expertise in quantum dot synthesis, advanced characterization techniques, fundamental research with potential for optoelectronic applications. Weaknesses: May be more focused on fundamental research rather than immediate industrial applications, potential challenges in scaling up synthesis methods.
Core Innovations
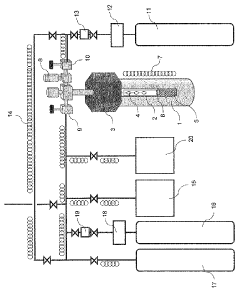
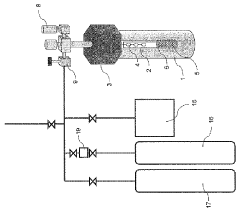
Nitride crystal manufacturing method, nitride crystal, and device for manufacturing same
PatentWO2010079814A1
Innovation
- The method involves generating a mineralizer by reacting a hydrogen halide gas, like hydrogen chloride, with ammonia to produce a nitride crystal, ensuring low oxygen and water content, and using a filtered reactive gas to grow nitride crystals in a closed system with controlled pressure and temperature, reducing oxygen impurities.
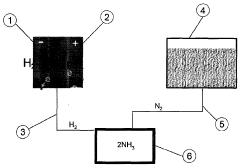
Synthesis of amonia by NANO catalytic process
PatentInactiveIN1226CHE2014A
Innovation
- The method involves using zeolites to separate nitrogen from air and manganese dioxide catalysts for hydrogen production from water, followed by purification and subsequent reaction with nano catalysts like osmium, ruthenium, and nickel at reduced temperatures and pressures to synthesize ammonia with high efficiency.
Environmental Impact
The use of ammonium hydroxide as a mineralizing agent in nanochemistry has significant environmental implications that warrant careful consideration. While this compound offers advantages in nanoparticle synthesis, its potential environmental impact must be thoroughly assessed.
Ammonium hydroxide, when released into the environment, can contribute to eutrophication in aquatic ecosystems. The excess nitrogen introduced can lead to algal blooms, disrupting the balance of aquatic life and potentially causing oxygen depletion. This effect is particularly concerning in freshwater systems, where even small changes in nutrient levels can have far-reaching consequences.
Furthermore, the alkaline nature of ammonium hydroxide can alter the pH of soil and water bodies if not properly managed. Such pH changes can affect the bioavailability of nutrients and potentially impact the survival of various organisms in the affected ecosystems. It is crucial to implement proper waste management protocols to mitigate these risks.
The volatility of ammonia, a component of ammonium hydroxide, presents additional environmental challenges. Atmospheric emissions of ammonia can contribute to the formation of particulate matter, impacting air quality and potentially affecting human health. Moreover, ammonia can react with other air pollutants, forming secondary aerosols that contribute to smog formation.
From a lifecycle perspective, the production and transportation of ammonium hydroxide also carry environmental implications. The energy-intensive manufacturing process and the associated carbon footprint must be factored into the overall environmental assessment of its use in nanochemistry.
However, it is important to note that when used in controlled laboratory settings for nanomaterial synthesis, the environmental impact of ammonium hydroxide can be significantly minimized. Proper handling, storage, and disposal practices can greatly reduce the risk of environmental contamination. Additionally, the small quantities typically used in nanochemistry research may limit the overall environmental footprint compared to industrial-scale applications.
In the context of green chemistry principles, researchers are exploring alternatives to ammonium hydroxide that may offer similar mineralizing properties with reduced environmental impact. These efforts align with the growing emphasis on sustainable practices in scientific research and industrial applications.
As the field of nanochemistry continues to evolve, it is imperative to balance the benefits of using ammonium hydroxide as a mineralizing agent with its potential environmental consequences. Ongoing research and development should focus on optimizing synthesis processes to minimize waste and exploring eco-friendly alternatives that can achieve comparable results in nanoparticle formation.
Ammonium hydroxide, when released into the environment, can contribute to eutrophication in aquatic ecosystems. The excess nitrogen introduced can lead to algal blooms, disrupting the balance of aquatic life and potentially causing oxygen depletion. This effect is particularly concerning in freshwater systems, where even small changes in nutrient levels can have far-reaching consequences.
Furthermore, the alkaline nature of ammonium hydroxide can alter the pH of soil and water bodies if not properly managed. Such pH changes can affect the bioavailability of nutrients and potentially impact the survival of various organisms in the affected ecosystems. It is crucial to implement proper waste management protocols to mitigate these risks.
The volatility of ammonia, a component of ammonium hydroxide, presents additional environmental challenges. Atmospheric emissions of ammonia can contribute to the formation of particulate matter, impacting air quality and potentially affecting human health. Moreover, ammonia can react with other air pollutants, forming secondary aerosols that contribute to smog formation.
From a lifecycle perspective, the production and transportation of ammonium hydroxide also carry environmental implications. The energy-intensive manufacturing process and the associated carbon footprint must be factored into the overall environmental assessment of its use in nanochemistry.
However, it is important to note that when used in controlled laboratory settings for nanomaterial synthesis, the environmental impact of ammonium hydroxide can be significantly minimized. Proper handling, storage, and disposal practices can greatly reduce the risk of environmental contamination. Additionally, the small quantities typically used in nanochemistry research may limit the overall environmental footprint compared to industrial-scale applications.
In the context of green chemistry principles, researchers are exploring alternatives to ammonium hydroxide that may offer similar mineralizing properties with reduced environmental impact. These efforts align with the growing emphasis on sustainable practices in scientific research and industrial applications.
As the field of nanochemistry continues to evolve, it is imperative to balance the benefits of using ammonium hydroxide as a mineralizing agent with its potential environmental consequences. Ongoing research and development should focus on optimizing synthesis processes to minimize waste and exploring eco-friendly alternatives that can achieve comparable results in nanoparticle formation.
Regulatory Framework
The regulatory framework surrounding the use of ammonium hydroxide as a mineralizing agent in nanochemistry is complex and multifaceted, involving various governmental agencies and international bodies. At the national level, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use and disposal of ammonium hydroxide under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) sets standards for workplace safety and exposure limits for this chemical compound.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of ammonium hydroxide in nanochemistry applications. REACH requires manufacturers and importers to register substances and provide safety data, ensuring a high level of protection for human health and the environment.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication for chemicals, including ammonium hydroxide. This system is widely adopted internationally, facilitating consistent safety information across borders.
Specific to nanomaterials, regulatory bodies are increasingly focusing on the unique properties and potential risks associated with nanoscale substances. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing nanomaterials, which include considerations for the use of mineralizing agents like ammonium hydroxide.
In the context of research and development, many countries have implemented regulations requiring the registration and reporting of nanomaterial usage. For instance, France has established a mandatory reporting scheme for nanomaterials, which could impact the use of ammonium hydroxide in nanochemistry research conducted within its borders.
The Food and Drug Administration (FDA) in the United States has specific guidelines for nanomaterials used in food and cosmetic applications, which may extend to the use of ammonium hydroxide as a mineralizing agent in these contexts. Similarly, the European Food Safety Authority (EFSA) provides guidance on the risk assessment of nanomaterials in food and feed applications.
As the field of nanochemistry continues to evolve, regulatory frameworks are likely to adapt and become more specific to address the unique challenges posed by nanomaterials and their production processes. Researchers and industry professionals working with ammonium hydroxide in nanochemistry must stay informed about these evolving regulations to ensure compliance and responsible development of nanotechnology applications.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of ammonium hydroxide in nanochemistry applications. REACH requires manufacturers and importers to register substances and provide safety data, ensuring a high level of protection for human health and the environment.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication for chemicals, including ammonium hydroxide. This system is widely adopted internationally, facilitating consistent safety information across borders.
Specific to nanomaterials, regulatory bodies are increasingly focusing on the unique properties and potential risks associated with nanoscale substances. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing nanomaterials, which include considerations for the use of mineralizing agents like ammonium hydroxide.
In the context of research and development, many countries have implemented regulations requiring the registration and reporting of nanomaterial usage. For instance, France has established a mandatory reporting scheme for nanomaterials, which could impact the use of ammonium hydroxide in nanochemistry research conducted within its borders.
The Food and Drug Administration (FDA) in the United States has specific guidelines for nanomaterials used in food and cosmetic applications, which may extend to the use of ammonium hydroxide as a mineralizing agent in these contexts. Similarly, the European Food Safety Authority (EFSA) provides guidance on the risk assessment of nanomaterials in food and feed applications.
As the field of nanochemistry continues to evolve, regulatory frameworks are likely to adapt and become more specific to address the unique challenges posed by nanomaterials and their production processes. Researchers and industry professionals working with ammonium hydroxide in nanochemistry must stay informed about these evolving regulations to ensure compliance and responsible development of nanotechnology applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!