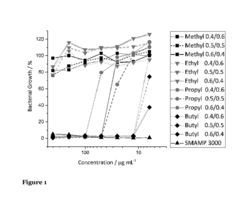
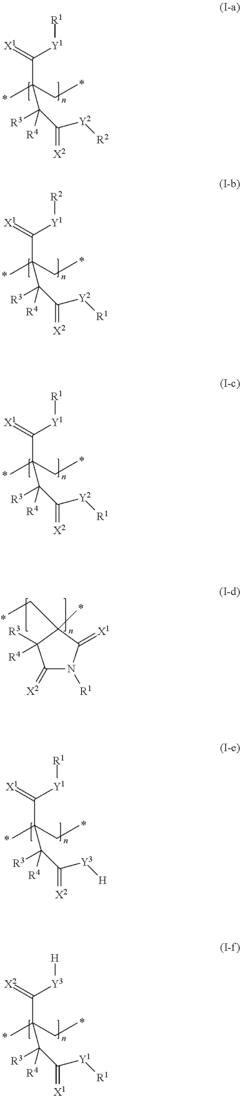
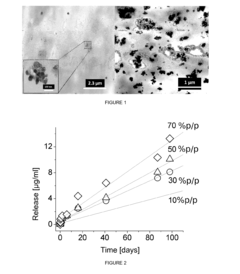
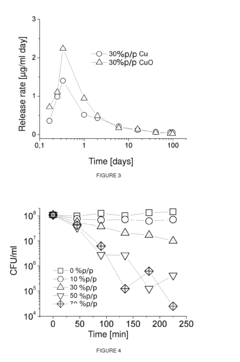
Research on Antimicrobial Properties of Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Antimicrobial Technology Background and Objectives
The field of antimicrobial biomedical polymers has evolved significantly over the past several decades, driven by the increasing prevalence of healthcare-associated infections and the growing threat of antimicrobial resistance. Initially developed in the 1960s as simple coatings for medical devices, these materials have transformed into sophisticated systems with controlled release mechanisms and targeted antimicrobial activity.
The evolution of this technology has been marked by several key milestones, including the development of silver-impregnated polymers in the 1980s, quaternary ammonium compound integration in the 1990s, and more recently, the emergence of nanoparticle-enhanced polymeric systems. The progression from passive antimicrobial surfaces to active, responsive materials represents a fundamental shift in approach to combating microbial colonization on biomedical materials.
Current technological trends indicate a movement toward multifunctional polymers that combine antimicrobial properties with other desirable characteristics such as biocompatibility, biodegradability, and mechanical strength. Additionally, there is growing interest in sustainable and environmentally friendly antimicrobial agents to replace traditional biocides that may contribute to resistance development or environmental concerns.
The global healthcare burden of antimicrobial resistance, estimated to cause 700,000 deaths annually and projected to reach 10 million by 2050, underscores the critical importance of this research area. Medical device-associated infections alone account for approximately 25% of all healthcare-associated infections, representing a significant clinical challenge and economic burden.
The primary technical objectives of this research include developing novel polymer systems with enhanced antimicrobial efficacy against a broad spectrum of pathogens, including drug-resistant strains. These systems should demonstrate long-term stability and sustained antimicrobial activity under physiological conditions while maintaining biocompatibility with host tissues.
Additional goals include creating polymers with "smart" or stimuli-responsive antimicrobial release mechanisms that can be triggered by specific environmental cues such as pH changes, enzyme activity, or the presence of bacterial metabolites. This approach would allow for targeted antimicrobial delivery only when needed, potentially reducing the risk of resistance development.
The ultimate aim is to translate these advanced materials into clinically viable products that can significantly reduce infection rates in medical devices, wound dressings, and tissue engineering scaffolds. This requires not only technical innovation but also consideration of scalability, cost-effectiveness, and regulatory pathways to ensure successful commercialization and widespread clinical adoption.
The evolution of this technology has been marked by several key milestones, including the development of silver-impregnated polymers in the 1980s, quaternary ammonium compound integration in the 1990s, and more recently, the emergence of nanoparticle-enhanced polymeric systems. The progression from passive antimicrobial surfaces to active, responsive materials represents a fundamental shift in approach to combating microbial colonization on biomedical materials.
Current technological trends indicate a movement toward multifunctional polymers that combine antimicrobial properties with other desirable characteristics such as biocompatibility, biodegradability, and mechanical strength. Additionally, there is growing interest in sustainable and environmentally friendly antimicrobial agents to replace traditional biocides that may contribute to resistance development or environmental concerns.
The global healthcare burden of antimicrobial resistance, estimated to cause 700,000 deaths annually and projected to reach 10 million by 2050, underscores the critical importance of this research area. Medical device-associated infections alone account for approximately 25% of all healthcare-associated infections, representing a significant clinical challenge and economic burden.
The primary technical objectives of this research include developing novel polymer systems with enhanced antimicrobial efficacy against a broad spectrum of pathogens, including drug-resistant strains. These systems should demonstrate long-term stability and sustained antimicrobial activity under physiological conditions while maintaining biocompatibility with host tissues.
Additional goals include creating polymers with "smart" or stimuli-responsive antimicrobial release mechanisms that can be triggered by specific environmental cues such as pH changes, enzyme activity, or the presence of bacterial metabolites. This approach would allow for targeted antimicrobial delivery only when needed, potentially reducing the risk of resistance development.
The ultimate aim is to translate these advanced materials into clinically viable products that can significantly reduce infection rates in medical devices, wound dressings, and tissue engineering scaffolds. This requires not only technical innovation but also consideration of scalability, cost-effectiveness, and regulatory pathways to ensure successful commercialization and widespread clinical adoption.
Market Analysis for Antimicrobial Biomedical Polymers
The global market for antimicrobial biomedical polymers has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections and the growing demand for implantable medical devices. The market value reached approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate of 8.7% through 2028, potentially reaching $2.1 billion by the end of the forecast period.
North America currently dominates the market with a share of roughly 42%, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure and higher healthcare expenditure in developed economies.
By application segment, the market can be categorized into implantable devices, surgical tools, wound dressings, dental materials, and others. Implantable devices represent the largest segment, accounting for approximately 35% of the market, followed by wound dressings at 25%. This distribution highlights the critical importance of infection prevention in long-term implants and chronic wound management.
The demand drivers for antimicrobial biomedical polymers are multifaceted. The rising prevalence of chronic diseases requiring long-term implants, increasing surgical procedures, and growing awareness about healthcare-associated infections are primary factors fueling market growth. Additionally, the aging global population, with its higher susceptibility to infections and greater need for medical interventions, is creating sustained demand.
Regulatory trends are significantly influencing market dynamics. Stringent regulations regarding hospital-acquired infections in developed countries are pushing healthcare facilities to adopt antimicrobial materials. Simultaneously, regulatory bodies are increasingly scrutinizing the environmental impact and potential for antimicrobial resistance development associated with these materials.
Customer preferences are evolving toward materials that offer broad-spectrum antimicrobial properties without compromising biocompatibility or mechanical properties. There is also growing demand for sustainable and biodegradable antimicrobial polymers, reflecting broader environmental concerns in healthcare procurement.
Pricing trends indicate a gradual decrease in the cost of basic antimicrobial polymers due to increased production volumes and competition. However, advanced formulations with targeted release mechanisms or combined functionalities command premium prices. The market is witnessing increased price sensitivity in public healthcare systems, while private healthcare providers often prioritize performance over cost considerations.
Future market growth is expected to be particularly strong in emerging economies, where healthcare infrastructure is rapidly developing and awareness of infection control is increasing. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of infection prevention in healthcare settings.
North America currently dominates the market with a share of roughly 42%, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure and higher healthcare expenditure in developed economies.
By application segment, the market can be categorized into implantable devices, surgical tools, wound dressings, dental materials, and others. Implantable devices represent the largest segment, accounting for approximately 35% of the market, followed by wound dressings at 25%. This distribution highlights the critical importance of infection prevention in long-term implants and chronic wound management.
The demand drivers for antimicrobial biomedical polymers are multifaceted. The rising prevalence of chronic diseases requiring long-term implants, increasing surgical procedures, and growing awareness about healthcare-associated infections are primary factors fueling market growth. Additionally, the aging global population, with its higher susceptibility to infections and greater need for medical interventions, is creating sustained demand.
Regulatory trends are significantly influencing market dynamics. Stringent regulations regarding hospital-acquired infections in developed countries are pushing healthcare facilities to adopt antimicrobial materials. Simultaneously, regulatory bodies are increasingly scrutinizing the environmental impact and potential for antimicrobial resistance development associated with these materials.
Customer preferences are evolving toward materials that offer broad-spectrum antimicrobial properties without compromising biocompatibility or mechanical properties. There is also growing demand for sustainable and biodegradable antimicrobial polymers, reflecting broader environmental concerns in healthcare procurement.
Pricing trends indicate a gradual decrease in the cost of basic antimicrobial polymers due to increased production volumes and competition. However, advanced formulations with targeted release mechanisms or combined functionalities command premium prices. The market is witnessing increased price sensitivity in public healthcare systems, while private healthcare providers often prioritize performance over cost considerations.
Future market growth is expected to be particularly strong in emerging economies, where healthcare infrastructure is rapidly developing and awareness of infection control is increasing. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of infection prevention in healthcare settings.
Current Status and Challenges in Antimicrobial Polymer Development
The global landscape of antimicrobial polymer development presents a complex picture of significant progress alongside persistent challenges. Currently, several classes of antimicrobial polymers have demonstrated efficacy in laboratory settings, including quaternary ammonium-containing polymers, phosphonium-based materials, and peptide-mimicking structures. These polymers exhibit varying mechanisms of action, from membrane disruption to intracellular component targeting, with efficacy against both Gram-positive and Gram-negative bacteria.
Despite these advances, the field faces substantial technical hurdles. One primary challenge is achieving selective toxicity—developing polymers that effectively eliminate pathogenic microorganisms while remaining biocompatible with human tissues. Current antimicrobial polymers often demonstrate cytotoxicity at concentrations only marginally higher than their effective antimicrobial doses, limiting their clinical applicability.
Another significant obstacle is the emergence of antimicrobial resistance. Bacteria have demonstrated remarkable adaptability against conventional antibiotics, and early evidence suggests similar adaptation mechanisms may develop against polymer-based antimicrobials. This necessitates continuous innovation in polymer design to stay ahead of resistance development.
The translation from laboratory success to clinical application represents perhaps the most formidable barrier. Many promising antimicrobial polymers exhibit reduced efficacy in complex biological environments due to protein adsorption, enzymatic degradation, or unfavorable interactions with biological fluids. Additionally, manufacturing scalability and cost-effectiveness remain problematic for many advanced polymer systems.
Geographically, research leadership in this field is distributed across North America, Europe, and increasingly Asia, particularly China and Singapore. The United States maintains prominence through institutions like MIT and Harvard, while European research clusters in Germany and the UK focus on sustainable antimicrobial solutions. Asian contributions have grown substantially, with particular emphasis on novel synthesis methods and hybrid materials.
Regulatory hurdles further complicate advancement, as antimicrobial polymers occupy an ambiguous position between medical devices and pharmaceuticals in many regulatory frameworks. The lengthy approval processes and substantial testing requirements have slowed commercialization efforts, particularly for implantable or long-term contact applications.
The environmental impact of antimicrobial polymers has emerged as a recent concern, with questions about biodegradability and potential ecosystem effects of these materials after disposal. This has prompted increased research into environmentally responsible antimicrobial polymer designs that maintain efficacy while minimizing ecological footprint.
Despite these advances, the field faces substantial technical hurdles. One primary challenge is achieving selective toxicity—developing polymers that effectively eliminate pathogenic microorganisms while remaining biocompatible with human tissues. Current antimicrobial polymers often demonstrate cytotoxicity at concentrations only marginally higher than their effective antimicrobial doses, limiting their clinical applicability.
Another significant obstacle is the emergence of antimicrobial resistance. Bacteria have demonstrated remarkable adaptability against conventional antibiotics, and early evidence suggests similar adaptation mechanisms may develop against polymer-based antimicrobials. This necessitates continuous innovation in polymer design to stay ahead of resistance development.
The translation from laboratory success to clinical application represents perhaps the most formidable barrier. Many promising antimicrobial polymers exhibit reduced efficacy in complex biological environments due to protein adsorption, enzymatic degradation, or unfavorable interactions with biological fluids. Additionally, manufacturing scalability and cost-effectiveness remain problematic for many advanced polymer systems.
Geographically, research leadership in this field is distributed across North America, Europe, and increasingly Asia, particularly China and Singapore. The United States maintains prominence through institutions like MIT and Harvard, while European research clusters in Germany and the UK focus on sustainable antimicrobial solutions. Asian contributions have grown substantially, with particular emphasis on novel synthesis methods and hybrid materials.
Regulatory hurdles further complicate advancement, as antimicrobial polymers occupy an ambiguous position between medical devices and pharmaceuticals in many regulatory frameworks. The lengthy approval processes and substantial testing requirements have slowed commercialization efforts, particularly for implantable or long-term contact applications.
The environmental impact of antimicrobial polymers has emerged as a recent concern, with questions about biodegradability and potential ecosystem effects of these materials after disposal. This has prompted increased research into environmentally responsible antimicrobial polymer designs that maintain efficacy while minimizing ecological footprint.
Current Antimicrobial Polymer Formulation Approaches
01 Antimicrobial polymers for medical devices
Biomedical polymers can be formulated with antimicrobial properties specifically for medical devices such as catheters, implants, and wound dressings. These polymers are designed to prevent bacterial colonization and biofilm formation on the surface of medical devices, reducing the risk of device-associated infections. The antimicrobial activity can be achieved through the incorporation of active agents into the polymer matrix or through surface modification techniques.- Antimicrobial polymers for medical devices: Biomedical polymers can be formulated with antimicrobial properties specifically for medical devices such as catheters, implants, and wound dressings. These polymers are designed to prevent bacterial colonization and biofilm formation on the surface of medical devices, reducing the risk of device-associated infections. The antimicrobial activity can be achieved through the incorporation of active agents into the polymer matrix or through surface modification techniques.
- Silver-based antimicrobial polymer composites: Silver nanoparticles or silver compounds can be incorporated into biomedical polymers to impart antimicrobial properties. These silver-based polymer composites exhibit broad-spectrum antimicrobial activity against bacteria, fungi, and viruses. The silver ions are released gradually from the polymer matrix, providing sustained antimicrobial protection. These materials are particularly useful in wound care applications, medical implants, and other healthcare-related products where infection control is critical.
- Quaternary ammonium-functionalized polymers: Biomedical polymers can be functionalized with quaternary ammonium compounds to create materials with inherent antimicrobial properties. These positively charged functional groups disrupt bacterial cell membranes, leading to cell death. Quaternary ammonium-functionalized polymers provide contact-killing antimicrobial surfaces that do not rely on the release of biocides. These materials are effective against a wide range of microorganisms and can maintain their antimicrobial activity for extended periods.
- Biodegradable antimicrobial polymers: Biodegradable polymers with antimicrobial properties are being developed for temporary medical applications such as sutures, tissue scaffolds, and drug delivery systems. These polymers combine the benefits of biodegradability with antimicrobial activity, reducing the risk of infection during the healing process while eventually being metabolized or excreted from the body. The antimicrobial activity can be intrinsic to the polymer structure or achieved through the incorporation of antimicrobial agents that are released during polymer degradation.
- Stimuli-responsive antimicrobial polymers: Smart biomedical polymers that can respond to environmental stimuli such as pH, temperature, or specific biological markers are being developed with antimicrobial properties. These stimuli-responsive systems can activate their antimicrobial function only when needed, such as in the presence of bacteria or during an infection. This targeted approach minimizes unnecessary exposure to antimicrobial agents and can help prevent the development of antimicrobial resistance. Applications include smart wound dressings, controlled drug delivery systems, and responsive surface coatings for medical devices.
02 Metal-containing antimicrobial polymers
Biomedical polymers can be infused with metal ions or nanoparticles, such as silver, copper, and zinc, to impart antimicrobial properties. These metal-containing polymers exhibit broad-spectrum antimicrobial activity against bacteria, fungi, and viruses. The metal ions are slowly released from the polymer matrix, providing sustained antimicrobial action. This approach is particularly effective for creating infection-resistant surfaces in medical applications while maintaining biocompatibility.Expand Specific Solutions03 Quaternary ammonium-based antimicrobial polymers
Quaternary ammonium compounds (QACs) can be incorporated into or covalently bound to polymer chains to create antimicrobial biomedical materials. These positively charged polymers disrupt bacterial cell membranes, leading to cell death. QAC-based antimicrobial polymers offer advantages such as non-leaching antimicrobial activity, long-term effectiveness, and reduced potential for developing antimicrobial resistance. They are particularly useful in applications requiring durable antimicrobial properties.Expand Specific Solutions04 Biodegradable antimicrobial polymers
Biodegradable polymers with inherent or incorporated antimicrobial properties are being developed for temporary medical applications such as sutures, tissue scaffolds, and drug delivery systems. These polymers provide antimicrobial protection during the critical healing period and then safely degrade into non-toxic byproducts. The antimicrobial activity can be achieved through the incorporation of antibiotics, antimicrobial peptides, or other bioactive compounds that are released as the polymer degrades.Expand Specific Solutions05 Surface-modified antimicrobial polymers
Surface modification techniques can be used to impart antimicrobial properties to biomedical polymers without altering their bulk properties. These techniques include plasma treatment, UV grafting, layer-by-layer deposition, and chemical functionalization. Surface-modified antimicrobial polymers can prevent bacterial adhesion and biofilm formation while maintaining the mechanical properties and biocompatibility of the base material. This approach is particularly useful for complex medical devices where bulk modification might compromise performance.Expand Specific Solutions
Key Industry Players in Antimicrobial Polymer Research
The antimicrobial biomedical polymers market is in a growth phase, driven by increasing healthcare-associated infections and demand for advanced medical devices. The global market is projected to reach significant value as healthcare facilities prioritize infection control. Technologically, the field shows varying maturity levels with established players like Becton, Dickinson & Co. and DSM IP Assets BV leading commercial applications, while academic institutions (University of California, Southeast University, South China University of Technology) drive fundamental research. Research collaboration between industry and academia is accelerating innovation, with specialized companies like PolyMedix and Livinguard AG developing proprietary antimicrobial technologies. The competitive landscape features both multinational corporations and specialized startups focusing on novel polymer formulations with enhanced antimicrobial properties.
DSM IP Assets BV
Technical Solution: DSM has developed advanced antimicrobial biomedical polymers utilizing their proprietary BioPM technology platform. Their approach incorporates quaternary ammonium compounds (QACs) into polymer matrices to create contact-killing surfaces that disrupt bacterial cell membranes. The company has successfully engineered polymers with controlled release mechanisms for antimicrobial agents, allowing for sustained efficacy over extended periods. Their research has demonstrated effectiveness against both Gram-positive and Gram-negative bacteria, with particular success against MRSA and E. coli strains. DSM's biomedical polymers feature tunable degradation profiles, making them suitable for various medical applications including implantable devices, wound dressings, and drug delivery systems. Their materials show antimicrobial efficacy while maintaining biocompatibility with human tissues.
Strengths: Extensive polymer science expertise, established manufacturing infrastructure, and strong IP portfolio. Their materials demonstrate excellent biocompatibility while maintaining antimicrobial properties. Weaknesses: Some formulations may have limited effectiveness against certain fungal species and require specific processing conditions that increase production costs.
PolyMedix, Inc.
Technical Solution: PolyMedix has pioneered the development of biomimetic antimicrobial polymers that mimic the structure and function of natural host defense peptides. Their flagship technology involves synthetic mimics of antimicrobial peptides (SMAMPs) that selectively target bacterial membranes while sparing mammalian cells. These polymers contain precisely balanced hydrophobic and cationic components that enable them to disrupt bacterial cell membranes through a biophysical mechanism rather than a biochemical one, significantly reducing the likelihood of resistance development. Their polymers have demonstrated broad-spectrum activity against drug-resistant pathogens including MRSA, VRE, and multidrug-resistant Gram-negative bacteria. PolyMedix's materials can be incorporated into various medical devices, coatings, and wound care products to provide protection against biofilm formation.
Strengths: Novel biomimetic approach that addresses antimicrobial resistance concerns, broad-spectrum activity against multiple pathogens, and reduced likelihood of resistance development. Weaknesses: Higher production costs compared to conventional antimicrobials, potential regulatory hurdles due to novel mechanism of action, and limited long-term clinical data on sustained efficacy.
Critical Patents and Research in Antimicrobial Polymer Science
Polymer having antimicrobial and/or antifouling properties
PatentInactiveUS20180327607A1
Innovation
- Development of a polymer with antimicrobial and antifouling properties, comprising repeat units with cationic moieties and facially amphiphilic structures, polymerizable by standard methods without toxic initiators, sourced from renewable resources, and capable of coating surfaces to prevent microbial growth and biofouling.
Polymeric materials with antifouling, biocidal, antiviral and antimicrobial properties; elaboration method and its uses
PatentActiveUS9481800B2
Innovation
- Incorporating pre-treated nanoparticles of metallic copper or cupric oxide into thermoplastic resins, forming secondary structures that enable a controlled, high initial release of ions, maintaining antimicrobial activity over extended periods without surface exposure, thus avoiding microorganism growth and adherence.
Regulatory Framework for Medical-Grade Antimicrobial Materials
The regulatory landscape governing antimicrobial biomedical polymers is complex and multifaceted, requiring manufacturers to navigate various approval pathways depending on the intended use and jurisdiction. In the United States, the Food and Drug Administration (FDA) classifies antimicrobial medical devices based on risk levels, with Class I devices facing minimal regulatory requirements while Class III devices undergo rigorous premarket approval processes. The FDA's guidance document "Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents" specifically addresses requirements for polymers with antimicrobial properties.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence and post-market surveillance. Antimicrobial polymers must comply with these regulations, with particular attention to the unique identification system and risk classification rules. The EU also emphasizes the importance of biocompatibility testing according to ISO 10993 standards.
International standards play a crucial role in regulatory compliance, with ISO 22196 specifically designed for measuring antimicrobial activity on plastic surfaces. Additionally, ASTM E2180 provides standard test methods for determining the activity of incorporated antimicrobial agents in polymeric materials. These standardized testing protocols ensure consistency in evaluating antimicrobial efficacy across different materials and applications.
Environmental regulations also impact antimicrobial polymer development, with increasing scrutiny on potentially harmful substances. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation restricts certain antimicrobial compounds, pushing manufacturers toward more environmentally friendly alternatives. Similarly, the EPA in the United States regulates antimicrobial substances under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).
Recent regulatory trends indicate a shift toward more sustainable antimicrobial solutions, with authorities encouraging the development of non-leaching antimicrobial polymers that maintain long-term efficacy without releasing potentially harmful substances into the environment. This has prompted innovation in covalently bound antimicrobial agents and naturally derived compounds.
Regulatory bodies are also addressing the growing concern of antimicrobial resistance, implementing stricter guidelines for claims related to antimicrobial effectiveness. Manufacturers must now provide substantial scientific evidence supporting specific antimicrobial claims, particularly those related to clinical outcomes rather than merely demonstrating in vitro antimicrobial activity.
The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence and post-market surveillance. Antimicrobial polymers must comply with these regulations, with particular attention to the unique identification system and risk classification rules. The EU also emphasizes the importance of biocompatibility testing according to ISO 10993 standards.
International standards play a crucial role in regulatory compliance, with ISO 22196 specifically designed for measuring antimicrobial activity on plastic surfaces. Additionally, ASTM E2180 provides standard test methods for determining the activity of incorporated antimicrobial agents in polymeric materials. These standardized testing protocols ensure consistency in evaluating antimicrobial efficacy across different materials and applications.
Environmental regulations also impact antimicrobial polymer development, with increasing scrutiny on potentially harmful substances. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation restricts certain antimicrobial compounds, pushing manufacturers toward more environmentally friendly alternatives. Similarly, the EPA in the United States regulates antimicrobial substances under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).
Recent regulatory trends indicate a shift toward more sustainable antimicrobial solutions, with authorities encouraging the development of non-leaching antimicrobial polymers that maintain long-term efficacy without releasing potentially harmful substances into the environment. This has prompted innovation in covalently bound antimicrobial agents and naturally derived compounds.
Regulatory bodies are also addressing the growing concern of antimicrobial resistance, implementing stricter guidelines for claims related to antimicrobial effectiveness. Manufacturers must now provide substantial scientific evidence supporting specific antimicrobial claims, particularly those related to clinical outcomes rather than merely demonstrating in vitro antimicrobial activity.
Biocompatibility and Safety Assessment Methods
The assessment of biocompatibility and safety represents a critical component in the development of antimicrobial biomedical polymers. These evaluations must be comprehensive and systematic to ensure that materials designed to combat microbial colonization do not themselves introduce new risks to patients or healthcare environments.
Standard in vitro cytotoxicity assays form the foundation of biocompatibility testing, typically beginning with direct contact tests, extract tests, and agar diffusion methods according to ISO 10993 guidelines. For antimicrobial polymers specifically, these tests must be modified to distinguish between toxicity to mammalian cells versus microbial targets, ensuring selective antimicrobial action without host cell damage.
Hemocompatibility testing holds particular importance for blood-contacting devices, requiring specialized protocols to evaluate hemolysis potential, thrombogenicity, and complement activation. The antimicrobial agents incorporated into polymers may alter surface properties that influence blood interactions, necessitating careful examination of these parameters through standardized methods such as ASTM F756 for hemolysis assessment.
Inflammatory response evaluation typically involves both in vitro assessment of pro-inflammatory cytokine production and in vivo implantation studies. The latter examines tissue response over time, with histopathological analysis to quantify inflammatory cell infiltration, fibrous encapsulation, and tissue integration. For antimicrobial polymers, this assessment must determine whether the antimicrobial components trigger excessive inflammatory reactions that could compromise healing.
Genotoxicity and mutagenicity testing has become increasingly sophisticated, employing assays such as the Ames test, chromosomal aberration studies, and micronucleus tests. These evaluations are particularly relevant for antimicrobial polymers that may release bioactive compounds over time, requiring careful assessment of potential DNA damage or mutagenic effects.
Long-term implantation studies represent the gold standard for comprehensive safety assessment, typically conducted in appropriate animal models over periods ranging from weeks to months. These studies evaluate local tissue response, systemic toxicity, and the potential for antimicrobial resistance development—a unique concern for antimicrobial materials that may create selective pressure on microbial populations.
Leachable and extractable testing has gained prominence in recent regulatory frameworks, requiring sophisticated analytical techniques such as HPLC-MS and GC-MS to identify and quantify compounds that might migrate from the polymer matrix. For antimicrobial polymers, this analysis must carefully track the release kinetics of antimicrobial agents to ensure they remain within therapeutic windows without reaching toxic thresholds.
Standardized testing protocols continue to evolve, with regulatory bodies increasingly requiring customized testing approaches that address the specific mechanisms and potential risks of novel antimicrobial technologies in biomedical polymers.
Standard in vitro cytotoxicity assays form the foundation of biocompatibility testing, typically beginning with direct contact tests, extract tests, and agar diffusion methods according to ISO 10993 guidelines. For antimicrobial polymers specifically, these tests must be modified to distinguish between toxicity to mammalian cells versus microbial targets, ensuring selective antimicrobial action without host cell damage.
Hemocompatibility testing holds particular importance for blood-contacting devices, requiring specialized protocols to evaluate hemolysis potential, thrombogenicity, and complement activation. The antimicrobial agents incorporated into polymers may alter surface properties that influence blood interactions, necessitating careful examination of these parameters through standardized methods such as ASTM F756 for hemolysis assessment.
Inflammatory response evaluation typically involves both in vitro assessment of pro-inflammatory cytokine production and in vivo implantation studies. The latter examines tissue response over time, with histopathological analysis to quantify inflammatory cell infiltration, fibrous encapsulation, and tissue integration. For antimicrobial polymers, this assessment must determine whether the antimicrobial components trigger excessive inflammatory reactions that could compromise healing.
Genotoxicity and mutagenicity testing has become increasingly sophisticated, employing assays such as the Ames test, chromosomal aberration studies, and micronucleus tests. These evaluations are particularly relevant for antimicrobial polymers that may release bioactive compounds over time, requiring careful assessment of potential DNA damage or mutagenic effects.
Long-term implantation studies represent the gold standard for comprehensive safety assessment, typically conducted in appropriate animal models over periods ranging from weeks to months. These studies evaluate local tissue response, systemic toxicity, and the potential for antimicrobial resistance development—a unique concern for antimicrobial materials that may create selective pressure on microbial populations.
Leachable and extractable testing has gained prominence in recent regulatory frameworks, requiring sophisticated analytical techniques such as HPLC-MS and GC-MS to identify and quantify compounds that might migrate from the polymer matrix. For antimicrobial polymers, this analysis must carefully track the release kinetics of antimicrobial agents to ensure they remain within therapeutic windows without reaching toxic thresholds.
Standardized testing protocols continue to evolve, with regulatory bodies increasingly requiring customized testing approaches that address the specific mechanisms and potential risks of novel antimicrobial technologies in biomedical polymers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!