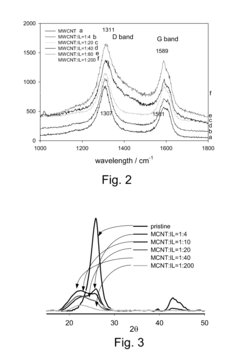
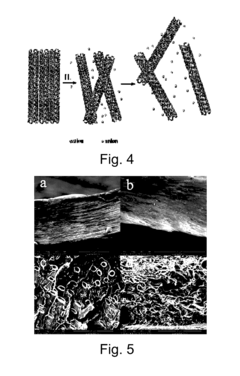
Research on Biomedical Polymer Nanocomposites
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Nanocomposites Background and Objectives
Biomedical polymer nanocomposites represent a revolutionary class of materials that have emerged at the intersection of polymer science, nanotechnology, and biomedical engineering. The evolution of these materials can be traced back to the early 1990s when researchers began exploring the incorporation of nanoscale fillers into polymer matrices to enhance their mechanical, thermal, and barrier properties. Over the past three decades, this field has witnessed remarkable growth, transitioning from basic research to advanced applications in healthcare.
The technological trajectory of biomedical polymer nanocomposites has been characterized by continuous innovation in synthesis methods, processing techniques, and characterization tools. Initially focused on structural enhancements, the field has progressively shifted toward functional properties and bioactive capabilities. Recent advances in controlled polymerization techniques, surface modification strategies, and nanofiller engineering have significantly expanded the design space for these materials.
Current research trends indicate a growing emphasis on stimuli-responsive nanocomposites, biodegradable systems, and materials with inherent therapeutic properties. The integration of smart functionalities, such as self-healing, shape memory, and controlled drug release, represents a paradigm shift from passive implants to active therapeutic platforms. Additionally, the emergence of 3D printing and other additive manufacturing techniques has opened new avenues for personalized medical devices based on nanocomposite materials.
The primary objectives of research in this field are multifaceted. First, to develop biocompatible nanocomposites with enhanced mechanical properties that can better mimic natural tissues while maintaining long-term stability in physiological environments. Second, to engineer multifunctional materials capable of simultaneous diagnostic and therapeutic functions (theranostics). Third, to establish scalable and reproducible manufacturing processes that can translate laboratory innovations into clinically viable products.
Another critical goal is to understand the complex interactions between nanocomposite materials and biological systems at multiple scales—from protein adsorption and cellular responses to tissue integration and systemic effects. This knowledge is essential for designing next-generation biomaterials with predictable in vivo performance and minimal adverse reactions.
Looking forward, the field aims to leverage advances in computational modeling, high-throughput screening, and artificial intelligence to accelerate materials discovery and optimization. The ultimate vision is to create "intelligent" nanocomposites that can dynamically respond to physiological cues, promote tissue regeneration, deliver therapeutics with spatiotemporal precision, and integrate seamlessly with the host's biological systems.
The technological trajectory of biomedical polymer nanocomposites has been characterized by continuous innovation in synthesis methods, processing techniques, and characterization tools. Initially focused on structural enhancements, the field has progressively shifted toward functional properties and bioactive capabilities. Recent advances in controlled polymerization techniques, surface modification strategies, and nanofiller engineering have significantly expanded the design space for these materials.
Current research trends indicate a growing emphasis on stimuli-responsive nanocomposites, biodegradable systems, and materials with inherent therapeutic properties. The integration of smart functionalities, such as self-healing, shape memory, and controlled drug release, represents a paradigm shift from passive implants to active therapeutic platforms. Additionally, the emergence of 3D printing and other additive manufacturing techniques has opened new avenues for personalized medical devices based on nanocomposite materials.
The primary objectives of research in this field are multifaceted. First, to develop biocompatible nanocomposites with enhanced mechanical properties that can better mimic natural tissues while maintaining long-term stability in physiological environments. Second, to engineer multifunctional materials capable of simultaneous diagnostic and therapeutic functions (theranostics). Third, to establish scalable and reproducible manufacturing processes that can translate laboratory innovations into clinically viable products.
Another critical goal is to understand the complex interactions between nanocomposite materials and biological systems at multiple scales—from protein adsorption and cellular responses to tissue integration and systemic effects. This knowledge is essential for designing next-generation biomaterials with predictable in vivo performance and minimal adverse reactions.
Looking forward, the field aims to leverage advances in computational modeling, high-throughput screening, and artificial intelligence to accelerate materials discovery and optimization. The ultimate vision is to create "intelligent" nanocomposites that can dynamically respond to physiological cues, promote tissue regeneration, deliver therapeutics with spatiotemporal precision, and integrate seamlessly with the host's biological systems.
Healthcare Market Demand Analysis
The global healthcare market for biomedical polymer nanocomposites is experiencing unprecedented growth, driven by increasing demand for advanced medical devices, drug delivery systems, and tissue engineering applications. Current market analysis indicates that the biomedical polymer nanocomposites sector is expanding at a compound annual growth rate of approximately 15%, significantly outpacing traditional medical materials.
Patient demographics are shifting dramatically worldwide, with aging populations in developed regions creating substantial demand for implantable devices and regenerative medicine solutions. This demographic trend has directly contributed to the rising need for biocompatible polymer nanocomposites that can interface with biological systems while providing enhanced mechanical and antimicrobial properties.
The COVID-19 pandemic has accelerated market demand for antimicrobial polymer nanocomposites in healthcare settings, particularly for high-touch surfaces and personal protective equipment. Healthcare facilities are increasingly adopting these materials to reduce hospital-acquired infections, creating a specialized market segment with robust growth potential.
Drug delivery applications represent the fastest-growing segment within the biomedical polymer nanocomposites market. The pharmaceutical industry's shift toward targeted therapies and controlled-release formulations has created significant demand for nanocomposite carriers that can improve drug efficacy while reducing side effects. Market research indicates that polymer-based nanomedicine products are projected to capture substantial market share from conventional drug formulations over the next decade.
Orthopedic and dental applications constitute another major market driver, with demand for bone tissue engineering scaffolds and dental restorative materials showing strong growth trajectories. The superior mechanical properties and bioactivity of polymer nanocomposites make them ideal candidates for load-bearing implants and dental reconstructions, addressing a market need valued in billions annually.
Regulatory considerations are significantly influencing market dynamics, with stricter safety standards creating barriers to entry but also opportunities for companies with advanced testing capabilities. The FDA and European regulatory bodies have established specialized pathways for nanomaterial-containing medical products, shaping market access strategies for industry participants.
Consumer preferences are evolving toward personalized healthcare solutions, creating demand for customizable polymer nanocomposite platforms that can be tailored to individual patient needs. This trend aligns with the broader movement toward precision medicine and represents a high-value market segment with substantial growth potential in both developed and emerging economies.
Patient demographics are shifting dramatically worldwide, with aging populations in developed regions creating substantial demand for implantable devices and regenerative medicine solutions. This demographic trend has directly contributed to the rising need for biocompatible polymer nanocomposites that can interface with biological systems while providing enhanced mechanical and antimicrobial properties.
The COVID-19 pandemic has accelerated market demand for antimicrobial polymer nanocomposites in healthcare settings, particularly for high-touch surfaces and personal protective equipment. Healthcare facilities are increasingly adopting these materials to reduce hospital-acquired infections, creating a specialized market segment with robust growth potential.
Drug delivery applications represent the fastest-growing segment within the biomedical polymer nanocomposites market. The pharmaceutical industry's shift toward targeted therapies and controlled-release formulations has created significant demand for nanocomposite carriers that can improve drug efficacy while reducing side effects. Market research indicates that polymer-based nanomedicine products are projected to capture substantial market share from conventional drug formulations over the next decade.
Orthopedic and dental applications constitute another major market driver, with demand for bone tissue engineering scaffolds and dental restorative materials showing strong growth trajectories. The superior mechanical properties and bioactivity of polymer nanocomposites make them ideal candidates for load-bearing implants and dental reconstructions, addressing a market need valued in billions annually.
Regulatory considerations are significantly influencing market dynamics, with stricter safety standards creating barriers to entry but also opportunities for companies with advanced testing capabilities. The FDA and European regulatory bodies have established specialized pathways for nanomaterial-containing medical products, shaping market access strategies for industry participants.
Consumer preferences are evolving toward personalized healthcare solutions, creating demand for customizable polymer nanocomposite platforms that can be tailored to individual patient needs. This trend aligns with the broader movement toward precision medicine and represents a high-value market segment with substantial growth potential in both developed and emerging economies.
Current Technological Landscape and Challenges
Biomedical polymer nanocomposites represent a rapidly evolving field at the intersection of materials science, nanotechnology, and medicine. Currently, the global landscape of this technology demonstrates significant advancements across multiple regions, with North America, Europe, and East Asia emerging as primary innovation hubs. The United States leads in research output and patent filings, followed closely by China, Germany, and Japan, reflecting the strategic importance these nations place on biomaterials development.
The current technological state features several dominant approaches, including polymer-clay nanocomposites, carbon nanotube-reinforced polymers, graphene-polymer systems, and metal nanoparticle-embedded matrices. Each system offers unique advantages for specific biomedical applications, from drug delivery to tissue engineering. Recent breakthroughs in processing techniques have enabled better dispersion of nanofillers within polymer matrices, significantly enhancing mechanical properties and biocompatibility.
Despite these advances, the field faces substantial challenges that impede broader clinical translation. Foremost among these is the persistent issue of nanomaterial dispersion and agglomeration within polymer matrices, which can compromise mechanical integrity and functional performance. Researchers continue to struggle with achieving uniform distribution of nanoparticles, particularly at higher loading percentages where agglomeration becomes more pronounced.
Biocompatibility and long-term safety concerns represent another critical challenge. The potential cytotoxicity of certain nanomaterials and their degradation products remains inadequately characterized, creating regulatory hurdles for clinical applications. Additionally, the biological fate of nanoparticles that may leach from composite matrices over time requires more comprehensive investigation to ensure patient safety.
Scale-up and manufacturing consistency present significant obstacles to commercialization. Laboratory-scale production methods often fail to translate effectively to industrial settings, resulting in batch-to-batch variability that compromises product reliability. The complex interactions between processing parameters and final material properties necessitate more robust manufacturing protocols.
Regulatory frameworks worldwide have struggled to keep pace with technological developments in this field. The unique properties of nanocomposites often fall outside traditional material classification systems, creating uncertainty in approval pathways. This regulatory ambiguity has slowed clinical translation despite promising preclinical results.
Cost factors remain a substantial barrier to widespread adoption. Many advanced nanofillers, such as functionalized graphene or specialized carbon nanotubes, carry prohibitive production costs that limit commercial viability. The complex processing requirements for achieving optimal nanocomposite properties further contribute to elevated manufacturing expenses.
The current technological state features several dominant approaches, including polymer-clay nanocomposites, carbon nanotube-reinforced polymers, graphene-polymer systems, and metal nanoparticle-embedded matrices. Each system offers unique advantages for specific biomedical applications, from drug delivery to tissue engineering. Recent breakthroughs in processing techniques have enabled better dispersion of nanofillers within polymer matrices, significantly enhancing mechanical properties and biocompatibility.
Despite these advances, the field faces substantial challenges that impede broader clinical translation. Foremost among these is the persistent issue of nanomaterial dispersion and agglomeration within polymer matrices, which can compromise mechanical integrity and functional performance. Researchers continue to struggle with achieving uniform distribution of nanoparticles, particularly at higher loading percentages where agglomeration becomes more pronounced.
Biocompatibility and long-term safety concerns represent another critical challenge. The potential cytotoxicity of certain nanomaterials and their degradation products remains inadequately characterized, creating regulatory hurdles for clinical applications. Additionally, the biological fate of nanoparticles that may leach from composite matrices over time requires more comprehensive investigation to ensure patient safety.
Scale-up and manufacturing consistency present significant obstacles to commercialization. Laboratory-scale production methods often fail to translate effectively to industrial settings, resulting in batch-to-batch variability that compromises product reliability. The complex interactions between processing parameters and final material properties necessitate more robust manufacturing protocols.
Regulatory frameworks worldwide have struggled to keep pace with technological developments in this field. The unique properties of nanocomposites often fall outside traditional material classification systems, creating uncertainty in approval pathways. This regulatory ambiguity has slowed clinical translation despite promising preclinical results.
Cost factors remain a substantial barrier to widespread adoption. Many advanced nanofillers, such as functionalized graphene or specialized carbon nanotubes, carry prohibitive production costs that limit commercial viability. The complex processing requirements for achieving optimal nanocomposite properties further contribute to elevated manufacturing expenses.
Current Synthesis and Fabrication Methodologies
01 Biodegradable polymer nanocomposites for medical applications
Biodegradable polymer nanocomposites are being developed for various biomedical applications including drug delivery, tissue engineering, and implantable devices. These materials combine biodegradable polymers with nanofillers to create materials with enhanced mechanical properties, controlled degradation rates, and improved biocompatibility. The biodegradable nature of these composites eliminates the need for removal surgeries and reduces long-term complications in patients.- Polymer nanocomposites for drug delivery systems: Biomedical polymer nanocomposites can be formulated as drug delivery systems to enhance therapeutic efficacy. These nanocomposites provide controlled release of active pharmaceutical ingredients, improved bioavailability, and targeted delivery to specific tissues. The polymer matrix can be designed to respond to specific biological triggers such as pH, temperature, or enzymatic activity, allowing for precise control over drug release kinetics. These systems can also protect sensitive drugs from degradation in the biological environment.
- Biodegradable polymer nanocomposites for tissue engineering: Biodegradable polymer nanocomposites are used in tissue engineering applications to create scaffolds that support cell growth and tissue regeneration. These materials combine the mechanical strength of nanofillers with the biocompatibility of polymers to mimic the extracellular matrix. The nanocomposites can be designed with specific porosity, surface properties, and degradation rates to match the requirements of different tissue types. As the scaffold degrades, it is gradually replaced by natural tissue, eliminating the need for removal surgery.
- Antimicrobial polymer nanocomposites for medical devices: Polymer nanocomposites with antimicrobial properties are developed for medical devices to prevent infection and biofilm formation. These materials typically incorporate nanoparticles such as silver, zinc oxide, or copper that release ions toxic to microorganisms. The polymer matrix controls the release rate of antimicrobial agents, providing long-term protection. These nanocomposites can be used in catheters, wound dressings, implants, and other medical devices where bacterial colonization poses significant risks to patients.
- Polymer nanocomposites with enhanced mechanical properties for implants: Biomedical polymer nanocomposites can be engineered to possess enhanced mechanical properties suitable for load-bearing implants. The incorporation of nanofillers such as carbon nanotubes, graphene, or ceramic nanoparticles significantly improves the strength, stiffness, and wear resistance of the polymer matrix. These materials can be tailored to match the mechanical properties of natural tissues, reducing stress shielding effects and improving long-term implant performance. The nanocomposites can be processed into complex shapes required for anatomical implants.
- Smart polymer nanocomposites for biosensing applications: Smart polymer nanocomposites are designed to respond to biological stimuli, making them ideal for biosensing applications. These materials can incorporate nanoparticles with optical, electrical, or magnetic properties that change in response to specific biomarkers. The polymer matrix provides biocompatibility and can be functionalized to enhance selectivity toward target analytes. These nanocomposites enable the development of implantable sensors for continuous monitoring of physiological parameters, point-of-care diagnostic devices, and theranostic platforms that combine diagnostic and therapeutic functions.
02 Polymer-clay nanocomposites for biomedical applications
Polymer-clay nanocomposites represent an important class of biomedical materials where nanoscale clay particles are dispersed within a polymer matrix. These nanocomposites exhibit improved barrier properties, mechanical strength, and thermal stability compared to conventional polymers. The incorporation of clay nanoparticles can also enhance drug release kinetics and provide antimicrobial properties, making these materials suitable for wound dressings, drug delivery systems, and medical device coatings.Expand Specific Solutions03 Carbon-based polymer nanocomposites for biomedical engineering
Carbon-based nanomaterials such as carbon nanotubes, graphene, and carbon nanofibers are being incorporated into polymer matrices to create nanocomposites with unique electrical, thermal, and mechanical properties for biomedical applications. These materials show promise in neural interfaces, biosensors, tissue engineering scaffolds, and drug delivery systems. The electrical conductivity of these nanocomposites makes them particularly valuable for applications requiring electrical stimulation or sensing capabilities.Expand Specific Solutions04 Antimicrobial polymer nanocomposites for medical devices
Antimicrobial polymer nanocomposites incorporate nanoparticles with inherent antimicrobial properties, such as silver, zinc oxide, or copper nanoparticles, into polymer matrices. These materials are designed to prevent bacterial colonization and biofilm formation on medical devices, implants, and wound dressings. The controlled release of antimicrobial agents from these nanocomposites provides long-term protection against infections while maintaining biocompatibility with human tissues.Expand Specific Solutions05 Processing techniques for biomedical polymer nanocomposites
Various processing techniques have been developed to fabricate polymer nanocomposites for biomedical applications, including solution blending, melt processing, in-situ polymerization, and electrospinning. These techniques aim to achieve uniform dispersion of nanoparticles within the polymer matrix, which is crucial for optimizing the mechanical, thermal, and biological properties of the resulting nanocomposites. Advanced processing methods can also enable the creation of complex structures with controlled porosity and surface characteristics for specific biomedical applications.Expand Specific Solutions
Leading Research Institutions and Industry Players
The biomedical polymer nanocomposites field is currently in a growth phase, with an estimated global market size of $5-7 billion and projected annual growth of 15-20%. The competitive landscape features established medical device companies like Boston Scientific leading commercialization efforts, while academic institutions (Sichuan University, Xiamen University, Fudan University) drive fundamental research innovations. Chemical companies such as Solvay SA provide specialized materials. The technology is approaching maturity in certain applications, with companies like NanoProteagen and Polynew developing specialized therapeutic applications. Research collaboration between industry and academia is accelerating development, particularly in drug delivery systems, tissue engineering, and implantable devices where polymer nanocomposites offer superior biocompatibility and functionality.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed proprietary biomedical polymer nanocomposites specifically designed for cardiovascular and endovascular applications. Their technology platform focuses on creating ultra-thin, high-strength materials for stents, catheters, and other minimally invasive devices. The company utilizes a combination of medical-grade polymers (including polyurethanes, polyamides, and silicones) reinforced with precisely engineered nanoparticles such as silica, hydroxyapatite, and silver nanoparticles. Their nanocomposites feature enhanced mechanical properties with up to 50% improvement in flexibility while maintaining tensile strength, critical for navigating tortuous vascular pathways. A key innovation is their drug-eluting nanocomposite technology that incorporates active pharmaceutical ingredients within the polymer matrix, allowing for controlled release profiles lasting from days to months depending on the clinical requirement. Their materials also demonstrate improved thromboresistance and reduced bacterial adhesion, addressing two critical challenges in implantable cardiovascular devices.
Strengths: Exceptional balance of flexibility and strength optimized for cardiovascular applications; advanced drug-eluting capabilities with precisely controlled release kinetics. Weaknesses: Higher manufacturing complexity leading to increased production costs; some formulations have limited shelf life requiring special storage conditions.
Solvay SA
Technical Solution: Solvay has developed high-performance biomedical polymer nanocomposites through their advanced materials division, focusing on implantable medical devices and drug delivery systems. Their technology platform utilizes specialty polymers such as PEEK (polyether ether ketone) and PVDF (polyvinylidene fluoride) reinforced with carefully selected nanofillers including hydroxyapatite, silica nanoparticles, and carbon nanotubes. Solvay's proprietary processing techniques ensure homogeneous dispersion of nanofillers within the polymer matrix, addressing one of the key challenges in nanocomposite manufacturing. Their materials exhibit exceptional mechanical properties with up to 60% improvement in tensile strength and 45% enhancement in modulus compared to neat polymers. A significant innovation is their development of radiopaque nanocomposites that incorporate bismuth compounds or barium sulfate nanoparticles, enabling improved visualization during minimally invasive procedures while maintaining mechanical integrity and biocompatibility.
Strengths: Industry-leading expertise in processing techniques ensuring homogeneous nanofiller dispersion; strong commercial infrastructure for scaling production to industrial levels. Weaknesses: Higher cost compared to conventional medical polymers; limited biodegradability options in their current portfolio.
Key Patents and Scientific Breakthroughs
Polymer nanocomposites and methods of making nanocomposites
PatentInactiveUS9650494B2
Innovation
- The development of polymer nanocomposites using a combination of polyetherimide (PEI) polymer, ionic liquids, and carbon nanotubes, where the ionic liquid is mixed with carbon nanotubes to form a gel, which is then incorporated into the PEI matrix, enhancing dispersion and alignment, and thereby improving electrical conductivity, mechanical properties, and thermal stability.
Process for preparing polymer nanocomposites and nanocomposites prepared therefrom
PatentInactiveUS20100152325A1
Innovation
- A continuous process involving the combination of polymers and nanoparticles, subjected to ultrasound energy within a specific frequency range while in a melted state under pressure, to achieve intercalation and partial exfoliation of clay layers, enhancing dispersion and mechanical properties.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations represent critical aspects in the development and application of biomedical polymer nanocomposites. These advanced materials, while offering tremendous potential for medical applications, must undergo rigorous evaluation to ensure they do not elicit adverse biological responses when interfacing with human tissues and physiological systems.
The biocompatibility assessment of polymer nanocomposites follows a multi-tiered approach, beginning with in vitro cytotoxicity testing using relevant cell lines. These tests evaluate direct cellular responses including viability, proliferation, and morphological changes upon exposure to the materials. More sophisticated in vitro models incorporating co-cultures or 3D tissue constructs provide insights into tissue-specific responses and potential inflammatory reactions.
Systemic biocompatibility requires comprehensive in vivo studies examining acute and chronic inflammatory responses, immune system activation, and potential organ toxicity. The biodistribution and clearance pathways of nanocomposites must be thoroughly characterized, as accumulation in specific organs may lead to long-term complications. Particular attention must be paid to the blood-material interactions, including hemolysis, thrombogenicity, and complement activation.
Nanoscale components present unique safety challenges beyond those of conventional biomaterials. The small size of nanoparticles enables them to cross biological barriers and potentially reach unintended tissues. Surface chemistry modifications, while enhancing functionality, may alter biological interactions in unpredictable ways. Additionally, the potential for nanoparticle leaching from the polymer matrix during degradation requires careful evaluation of long-term stability under physiological conditions.
Regulatory frameworks for biomedical polymer nanocomposites continue to evolve as scientific understanding advances. The FDA, EMA, and other regulatory bodies have established guidelines for nanomaterial safety assessment, though harmonization remains incomplete. Manufacturers must navigate these complex regulatory landscapes while demonstrating both safety and efficacy through standardized testing protocols such as ISO 10993 for biological evaluation of medical devices.
Emerging concerns in nanocomposite safety include potential genotoxicity, reproductive toxicity, and environmental impact upon disposal. Advanced analytical techniques including high-resolution imaging, proteomics, and transcriptomics are increasingly employed to detect subtle biological responses that might escape traditional toxicology assessments. The concept of "safe-by-design" has gained prominence, emphasizing the integration of safety considerations from the earliest stages of material development rather than retrospective evaluation.
The biocompatibility assessment of polymer nanocomposites follows a multi-tiered approach, beginning with in vitro cytotoxicity testing using relevant cell lines. These tests evaluate direct cellular responses including viability, proliferation, and morphological changes upon exposure to the materials. More sophisticated in vitro models incorporating co-cultures or 3D tissue constructs provide insights into tissue-specific responses and potential inflammatory reactions.
Systemic biocompatibility requires comprehensive in vivo studies examining acute and chronic inflammatory responses, immune system activation, and potential organ toxicity. The biodistribution and clearance pathways of nanocomposites must be thoroughly characterized, as accumulation in specific organs may lead to long-term complications. Particular attention must be paid to the blood-material interactions, including hemolysis, thrombogenicity, and complement activation.
Nanoscale components present unique safety challenges beyond those of conventional biomaterials. The small size of nanoparticles enables them to cross biological barriers and potentially reach unintended tissues. Surface chemistry modifications, while enhancing functionality, may alter biological interactions in unpredictable ways. Additionally, the potential for nanoparticle leaching from the polymer matrix during degradation requires careful evaluation of long-term stability under physiological conditions.
Regulatory frameworks for biomedical polymer nanocomposites continue to evolve as scientific understanding advances. The FDA, EMA, and other regulatory bodies have established guidelines for nanomaterial safety assessment, though harmonization remains incomplete. Manufacturers must navigate these complex regulatory landscapes while demonstrating both safety and efficacy through standardized testing protocols such as ISO 10993 for biological evaluation of medical devices.
Emerging concerns in nanocomposite safety include potential genotoxicity, reproductive toxicity, and environmental impact upon disposal. Advanced analytical techniques including high-resolution imaging, proteomics, and transcriptomics are increasingly employed to detect subtle biological responses that might escape traditional toxicology assessments. The concept of "safe-by-design" has gained prominence, emphasizing the integration of safety considerations from the earliest stages of material development rather than retrospective evaluation.
Regulatory Framework for Medical Materials
The regulatory landscape for biomedical polymer nanocomposites is complex and multifaceted, requiring careful navigation to ensure compliance across different jurisdictions. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for nanomaterials in medical applications, with particular emphasis on polymer-based nanocomposites. These materials are typically regulated under the medical device framework (21 CFR Part 820) or as combination products, depending on their intended use and mechanism of action.
The European Union implements more stringent requirements through the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which explicitly address nanomaterials in medical applications. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also imposes additional requirements for nanomaterials, including polymer nanocomposites intended for biomedical applications.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have developed specific regulatory pathways for novel biomaterials, including polymer nanocomposites. These frameworks often require extensive biocompatibility testing according to ISO 10993 standards, with particular attention to potential nanoparticle leaching and long-term stability.
International harmonization efforts are being led by organizations such as the International Medical Device Regulators Forum (IMDRF) and the International Organization for Standardization (ISO). The ISO/TC 194 committee has developed specific standards for biological evaluation of medical devices, while ISO/TC 229 focuses on nanotechnologies, both directly applicable to polymer nanocomposites.
A critical regulatory consideration for biomedical polymer nanocomposites is the classification of risk, which determines the extent of pre-market testing required. Materials intended for implantable devices or drug delivery systems typically face the highest regulatory scrutiny, requiring extensive toxicological profiling and clinical evaluation data.
Emerging regulatory trends include increased focus on environmental impact assessment and end-of-life considerations for nanomaterials. Several jurisdictions are implementing mandatory post-market surveillance requirements specific to nanomaterial-containing medical products, reflecting concerns about long-term safety profiles and potential unforeseen interactions in the human body.
Manufacturers developing biomedical polymer nanocomposites must establish robust quality management systems that address the unique challenges of nanomaterial characterization, process validation, and stability testing. Documentation requirements typically include detailed information on particle size distribution, surface characteristics, and potential for agglomeration under physiological conditions.
The European Union implements more stringent requirements through the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which explicitly address nanomaterials in medical applications. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also imposes additional requirements for nanomaterials, including polymer nanocomposites intended for biomedical applications.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have developed specific regulatory pathways for novel biomaterials, including polymer nanocomposites. These frameworks often require extensive biocompatibility testing according to ISO 10993 standards, with particular attention to potential nanoparticle leaching and long-term stability.
International harmonization efforts are being led by organizations such as the International Medical Device Regulators Forum (IMDRF) and the International Organization for Standardization (ISO). The ISO/TC 194 committee has developed specific standards for biological evaluation of medical devices, while ISO/TC 229 focuses on nanotechnologies, both directly applicable to polymer nanocomposites.
A critical regulatory consideration for biomedical polymer nanocomposites is the classification of risk, which determines the extent of pre-market testing required. Materials intended for implantable devices or drug delivery systems typically face the highest regulatory scrutiny, requiring extensive toxicological profiling and clinical evaluation data.
Emerging regulatory trends include increased focus on environmental impact assessment and end-of-life considerations for nanomaterials. Several jurisdictions are implementing mandatory post-market surveillance requirements specific to nanomaterial-containing medical products, reflecting concerns about long-term safety profiles and potential unforeseen interactions in the human body.
Manufacturers developing biomedical polymer nanocomposites must establish robust quality management systems that address the unique challenges of nanomaterial characterization, process validation, and stability testing. Documentation requirements typically include detailed information on particle size distribution, surface characteristics, and potential for agglomeration under physiological conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!