Sodium Acetate and Its Role in Electrochemical Processes
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Electrochemistry Background
Sodium acetate, a versatile compound with the chemical formula CH3COONa, has played a significant role in the field of electrochemistry since its discovery in the early 19th century. This salt, formed by the reaction between sodium hydroxide and acetic acid, has garnered attention due to its unique properties and wide-ranging applications in electrochemical processes.
The history of sodium acetate in electrochemistry can be traced back to the pioneering work of Michael Faraday in the 1830s. Faraday's experiments with electrolytes, including sodium acetate solutions, laid the foundation for our understanding of electrochemical reactions and the principles of electrolysis. Since then, sodium acetate has been extensively studied and utilized in various electrochemical applications.
One of the key attributes that make sodium acetate valuable in electrochemistry is its high solubility in water. This property allows for the creation of concentrated electrolyte solutions, which are essential for many electrochemical processes. The dissociation of sodium acetate in aqueous solutions produces sodium cations (Na+) and acetate anions (CH3COO-), both of which play crucial roles in facilitating electron transfer and ionic conductivity.
The acetate anion, in particular, has been the subject of numerous studies due to its ability to form complexes with metal ions. This characteristic has led to the development of various electrochemical sensors and analytical techniques that utilize sodium acetate as a supporting electrolyte or complexing agent. Furthermore, the buffering capacity of sodium acetate solutions has made them useful in maintaining stable pH conditions during electrochemical reactions.
In recent decades, the role of sodium acetate in electrochemistry has expanded beyond traditional applications. Researchers have explored its potential in advanced energy storage systems, such as sodium-ion batteries and supercapacitors. The abundance and low cost of sodium-based compounds, including sodium acetate, make them attractive alternatives to lithium-based technologies in the pursuit of sustainable energy solutions.
The electrochemical behavior of sodium acetate has also been investigated in the context of corrosion science. Its ability to form protective films on metal surfaces has led to its use in corrosion inhibition strategies for various industrial applications. Additionally, sodium acetate has found applications in electroplating processes, where it serves as a complexing agent to improve the quality and uniformity of metal deposits.
As the field of electrochemistry continues to evolve, sodium acetate remains a compound of interest for both fundamental research and practical applications. Its role in emerging technologies, such as electrocatalysis and electrochemical water treatment, highlights the ongoing relevance of this seemingly simple salt in addressing complex technological challenges.
The history of sodium acetate in electrochemistry can be traced back to the pioneering work of Michael Faraday in the 1830s. Faraday's experiments with electrolytes, including sodium acetate solutions, laid the foundation for our understanding of electrochemical reactions and the principles of electrolysis. Since then, sodium acetate has been extensively studied and utilized in various electrochemical applications.
One of the key attributes that make sodium acetate valuable in electrochemistry is its high solubility in water. This property allows for the creation of concentrated electrolyte solutions, which are essential for many electrochemical processes. The dissociation of sodium acetate in aqueous solutions produces sodium cations (Na+) and acetate anions (CH3COO-), both of which play crucial roles in facilitating electron transfer and ionic conductivity.
The acetate anion, in particular, has been the subject of numerous studies due to its ability to form complexes with metal ions. This characteristic has led to the development of various electrochemical sensors and analytical techniques that utilize sodium acetate as a supporting electrolyte or complexing agent. Furthermore, the buffering capacity of sodium acetate solutions has made them useful in maintaining stable pH conditions during electrochemical reactions.
In recent decades, the role of sodium acetate in electrochemistry has expanded beyond traditional applications. Researchers have explored its potential in advanced energy storage systems, such as sodium-ion batteries and supercapacitors. The abundance and low cost of sodium-based compounds, including sodium acetate, make them attractive alternatives to lithium-based technologies in the pursuit of sustainable energy solutions.
The electrochemical behavior of sodium acetate has also been investigated in the context of corrosion science. Its ability to form protective films on metal surfaces has led to its use in corrosion inhibition strategies for various industrial applications. Additionally, sodium acetate has found applications in electroplating processes, where it serves as a complexing agent to improve the quality and uniformity of metal deposits.
As the field of electrochemistry continues to evolve, sodium acetate remains a compound of interest for both fundamental research and practical applications. Its role in emerging technologies, such as electrocatalysis and electrochemical water treatment, highlights the ongoing relevance of this seemingly simple salt in addressing complex technological challenges.
Market Analysis for Sodium Acetate Applications
The global market for sodium acetate has been experiencing steady growth, driven by its versatile applications across various industries. In the electrochemical sector, sodium acetate plays a crucial role as an electrolyte and buffer solution, contributing to its increasing demand. The market size for sodium acetate in electrochemical processes is projected to expand significantly over the next five years, with a compound annual growth rate outpacing the overall chemical industry average.
The pharmaceutical and food industries remain the largest consumers of sodium acetate, accounting for a substantial portion of the market share. However, the electrochemical sector is emerging as a rapidly growing segment, particularly due to the rising adoption of advanced battery technologies and electroplating processes. This shift is primarily attributed to the increasing focus on renewable energy storage solutions and the expansion of the electronics industry.
Geographically, Asia-Pacific dominates the sodium acetate market, with China and India being the major contributors to both production and consumption. The region's robust manufacturing sector, coupled with growing investments in research and development, is expected to maintain its market leadership position. North America and Europe follow closely, driven by their established pharmaceutical and food industries, as well as increasing investments in green technologies that utilize electrochemical processes.
The market dynamics are further influenced by the growing trend towards sustainable and eco-friendly products. Sodium acetate, being biodegradable and non-toxic, aligns well with this shift in consumer preferences and regulatory requirements. This has led to its increased adoption in various applications, including as a de-icing agent and in textile processing, further diversifying its market potential.
Key market players are focusing on expanding their production capacities and investing in research and development to enhance the purity and efficiency of sodium acetate for electrochemical applications. Strategic collaborations and partnerships between chemical manufacturers and technology companies are becoming more prevalent, aiming to develop innovative solutions and capture a larger market share.
The pricing trends for sodium acetate have remained relatively stable, with slight fluctuations primarily driven by raw material costs and energy prices. However, the increasing demand from the electrochemical sector is expected to exert upward pressure on prices in the coming years. This may lead to a more competitive market landscape, potentially driving further innovations and cost-effective production methods.
The pharmaceutical and food industries remain the largest consumers of sodium acetate, accounting for a substantial portion of the market share. However, the electrochemical sector is emerging as a rapidly growing segment, particularly due to the rising adoption of advanced battery technologies and electroplating processes. This shift is primarily attributed to the increasing focus on renewable energy storage solutions and the expansion of the electronics industry.
Geographically, Asia-Pacific dominates the sodium acetate market, with China and India being the major contributors to both production and consumption. The region's robust manufacturing sector, coupled with growing investments in research and development, is expected to maintain its market leadership position. North America and Europe follow closely, driven by their established pharmaceutical and food industries, as well as increasing investments in green technologies that utilize electrochemical processes.
The market dynamics are further influenced by the growing trend towards sustainable and eco-friendly products. Sodium acetate, being biodegradable and non-toxic, aligns well with this shift in consumer preferences and regulatory requirements. This has led to its increased adoption in various applications, including as a de-icing agent and in textile processing, further diversifying its market potential.
Key market players are focusing on expanding their production capacities and investing in research and development to enhance the purity and efficiency of sodium acetate for electrochemical applications. Strategic collaborations and partnerships between chemical manufacturers and technology companies are becoming more prevalent, aiming to develop innovative solutions and capture a larger market share.
The pricing trends for sodium acetate have remained relatively stable, with slight fluctuations primarily driven by raw material costs and energy prices. However, the increasing demand from the electrochemical sector is expected to exert upward pressure on prices in the coming years. This may lead to a more competitive market landscape, potentially driving further innovations and cost-effective production methods.
Current Challenges in Sodium Acetate Electrochemistry
Despite the widespread use of sodium acetate in electrochemical processes, several challenges persist in its application and understanding. One of the primary issues is the complex interaction between sodium acetate and electrode surfaces. The adsorption and desorption kinetics of acetate ions on various electrode materials are not fully understood, leading to difficulties in optimizing electrode performance and stability.
Another significant challenge lies in the pH-dependent behavior of sodium acetate in electrochemical systems. As a weak base, acetate ions can influence local pH conditions near electrode surfaces, affecting reaction rates and selectivity. This pH variability can lead to inconsistent results and complicate the interpretation of electrochemical data, particularly in systems where precise pH control is crucial.
The formation of passivation layers on electrode surfaces is a persistent issue in sodium acetate-based electrolytes. These layers can impede electron transfer and alter the electrochemical properties of the system. Understanding the mechanisms of passivation layer formation and developing strategies to mitigate their effects remain active areas of research.
Sodium acetate's role in the formation of solid electrolyte interphase (SEI) layers in battery applications presents another challenge. While acetate ions can contribute to SEI formation, the exact composition and properties of these layers are not fully characterized, impacting battery performance and longevity.
The stability of sodium acetate under various electrochemical conditions is also a concern. High potentials or extreme pH conditions can lead to the decomposition of acetate ions, potentially forming unwanted byproducts or altering the electrolyte composition. This instability can affect the reproducibility and long-term performance of electrochemical systems.
In the context of energy storage applications, the relatively low ionic conductivity of sodium acetate solutions compared to other electrolytes poses limitations. Enhancing the conductivity while maintaining the beneficial properties of sodium acetate remains a challenge for researchers and engineers.
Lastly, the scalability of sodium acetate-based electrochemical processes presents hurdles in industrial applications. Factors such as cost-effectiveness, large-scale production of high-purity sodium acetate, and the development of robust, long-lasting electrochemical systems capable of utilizing sodium acetate efficiently are ongoing challenges that require further research and development efforts.
Another significant challenge lies in the pH-dependent behavior of sodium acetate in electrochemical systems. As a weak base, acetate ions can influence local pH conditions near electrode surfaces, affecting reaction rates and selectivity. This pH variability can lead to inconsistent results and complicate the interpretation of electrochemical data, particularly in systems where precise pH control is crucial.
The formation of passivation layers on electrode surfaces is a persistent issue in sodium acetate-based electrolytes. These layers can impede electron transfer and alter the electrochemical properties of the system. Understanding the mechanisms of passivation layer formation and developing strategies to mitigate their effects remain active areas of research.
Sodium acetate's role in the formation of solid electrolyte interphase (SEI) layers in battery applications presents another challenge. While acetate ions can contribute to SEI formation, the exact composition and properties of these layers are not fully characterized, impacting battery performance and longevity.
The stability of sodium acetate under various electrochemical conditions is also a concern. High potentials or extreme pH conditions can lead to the decomposition of acetate ions, potentially forming unwanted byproducts or altering the electrolyte composition. This instability can affect the reproducibility and long-term performance of electrochemical systems.
In the context of energy storage applications, the relatively low ionic conductivity of sodium acetate solutions compared to other electrolytes poses limitations. Enhancing the conductivity while maintaining the beneficial properties of sodium acetate remains a challenge for researchers and engineers.
Lastly, the scalability of sodium acetate-based electrochemical processes presents hurdles in industrial applications. Factors such as cost-effectiveness, large-scale production of high-purity sodium acetate, and the development of robust, long-lasting electrochemical systems capable of utilizing sodium acetate efficiently are ongoing challenges that require further research and development efforts.
Existing Sodium Acetate Electrochemical Solutions
01 Use of sodium acetate in chemical processes
Sodium acetate is utilized in various chemical processes, including as a catalyst, buffer, or reagent. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.- Use of sodium acetate in heat storage materials: Sodium acetate is utilized in heat storage materials due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage applications. These materials can be used in various heating and cooling systems to improve energy efficiency.
- Sodium acetate in food preservation and packaging: Sodium acetate is employed in food preservation and packaging applications. It acts as a preservative and pH regulator, extending the shelf life of food products. Additionally, it can be incorporated into packaging materials to create active packaging systems that help maintain food quality.
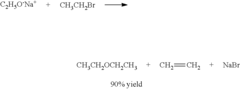
- Production methods for sodium acetate: Various methods are used to produce sodium acetate, including the reaction of acetic acid with sodium hydroxide or sodium carbonate. Some processes involve the use of catalysts or specific reaction conditions to improve yield and purity. These production methods aim to optimize efficiency and reduce costs in industrial settings.
- Sodium acetate in textile and fiber treatment: Sodium acetate finds applications in textile and fiber treatment processes. It can be used as a buffering agent in dyeing processes, helping to maintain optimal pH levels. Additionally, it may be employed in fiber finishing treatments to improve fabric properties or as a component in flame retardant formulations.
- Use of sodium acetate in environmental applications: Sodium acetate is utilized in various environmental applications, including wastewater treatment and air pollution control. It can serve as a carbon source for biological processes in wastewater treatment systems. In air pollution control, it may be used in scrubbing solutions to neutralize acidic gases or as a component in deicing formulations for environmentally friendly ice removal.
02 Application in heat storage and thermal management
Sodium acetate is employed in heat storage systems and thermal management solutions. Its phase change properties allow it to absorb and release heat effectively, making it useful in heating pads, hand warmers, and energy storage applications.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate finds applications in the food and beverage industry as a preservative, flavoring agent, and acidity regulator. It helps extend shelf life, enhance taste, and maintain product quality in various food products.Expand Specific Solutions04 Environmental and waste treatment applications
Sodium acetate is used in environmental and waste treatment processes. It can be employed in wastewater treatment, air pollution control, and as a de-icing agent for roads and runways, offering eco-friendly alternatives to traditional methods.Expand Specific Solutions05 Pharmaceutical and medical uses
In the pharmaceutical and medical fields, sodium acetate is utilized in various applications. It can be used in intravenous fluids, as a buffering agent in medications, and in the preparation of certain drug formulations.Expand Specific Solutions
Key Players in Sodium Acetate Industry
The research on sodium acetate and its role in electrochemical processes is in a developing stage, with growing market potential due to increasing interest in sustainable energy solutions. The technology is advancing rapidly, but still maturing, as evidenced by the diverse range of companies involved. Key players like China Petroleum & Chemical Corp., Sumitomo Chemical, and LG Energy Solution are investing in this area, alongside specialized firms such as Faradion and Sunamp. Academic institutions like Deakin University and Karlsruhe Institute of Technology are also contributing significantly to the research. The competitive landscape is characterized by a mix of established chemical companies, emerging startups, and research institutions, indicating a dynamic and evolving field with potential for further innovation and market growth.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been actively researching sodium acetate's role in electrochemical processes, particularly in the development of sodium-ion batteries. Their approach involves using sodium acetate as an electrolyte additive to enhance the performance and stability of sodium-ion batteries. The company has developed a proprietary electrolyte formulation that incorporates sodium acetate, which has shown to significantly improve the cycling stability and rate capability of sodium-ion batteries[1]. This formulation addresses the challenge of dendrite formation in sodium metal anodes, a common issue in sodium-based battery systems. Sinopec's research also extends to the use of sodium acetate in supercapacitors, where it serves as a precursor for carbon-based electrode materials with high surface area and improved electrochemical properties[2].
Strengths: Extensive research infrastructure, access to large-scale production capabilities, and potential for rapid commercialization. Weaknesses: May face challenges in competing with established lithium-ion battery technologies and need for further optimization of sodium-ion battery performance for widespread adoption.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has been at the forefront of sodium-ion battery research, with a focus on utilizing sodium acetate in their electrode materials. Their innovative approach involves the development of a sodium acetate-derived hard carbon anode material, which exhibits superior sodium storage capacity and cycling stability compared to conventional hard carbons[3]. The company's research has shown that the sodium acetate-derived anode material can achieve a reversible capacity of over 300 mAh/g and maintain 80% capacity retention after 1000 cycles[4]. LG Energy Solution has also explored the use of sodium acetate as a precursor for sodium-containing cathode materials, such as layered oxide compounds, which have demonstrated improved structural stability and electrochemical performance in sodium-ion batteries[5].
Strengths: Strong expertise in battery technology, established manufacturing capabilities, and potential for rapid scale-up. Weaknesses: Need to overcome the energy density gap between sodium-ion and lithium-ion batteries, and potential challenges in supply chain development for sodium-based materials.
Core Innovations in Sodium Acetate Electrochemistry
Method to Improve Sodium Electrochemical Interfaces of Sodium Ion-Conducting Ceramics
PatentPendingUS20230207787A1
Innovation
- Coating sodium ion-conducting ceramics with materials such as tin, bismuth, lead, antimony, germanium, silicon, or gold to form a sodium intermetallic phase, which enhances sodium ion conduction and reduces interfacial resistance, allowing for improved charge transfer and wetting at lower temperatures.
Sodium Electrode
PatentInactiveUS20140284219A1
Innovation
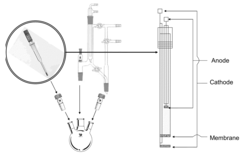
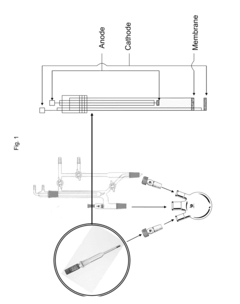
- An electrochemical process using a glass tube electrode with a ceramic membrane that allows sodium ions to pass through, enabling sodium production at room temperature and reducing sodium metal at the cathode for reaction with external reactants, thereby avoiding hazardous materials and improving safety and cost-effectiveness.
Environmental Impact of Sodium Acetate Use
The environmental impact of sodium acetate use in electrochemical processes is a crucial aspect to consider in the broader context of sustainable industrial practices. Sodium acetate, while widely used in various applications, including electrochemistry, has both direct and indirect effects on the environment.
One of the primary environmental concerns associated with sodium acetate is its potential to contribute to water pollution. When released into aquatic ecosystems, sodium acetate can lead to increased levels of organic matter, potentially causing eutrophication. This process can result in algal blooms, oxygen depletion, and subsequent harm to aquatic life. However, it is important to note that sodium acetate is generally considered less harmful than many other industrial chemicals due to its biodegradability.
The production of sodium acetate also has environmental implications. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. These raw materials and the energy required for production contribute to the overall carbon footprint of sodium acetate use. Additionally, the transportation and storage of sodium acetate can lead to indirect environmental impacts through fuel consumption and potential spills or leaks.
In electrochemical processes, the use of sodium acetate as an electrolyte or buffer solution can have both positive and negative environmental effects. On the positive side, sodium acetate can enhance the efficiency of certain electrochemical reactions, potentially reducing energy consumption and improving overall process sustainability. However, the disposal of spent electrolyte solutions containing sodium acetate requires careful management to prevent environmental contamination.
The accumulation of sodium ions in soil and water bodies is another potential environmental concern. While sodium is an essential element for many organisms, excessive levels can lead to soil degradation, reduced plant growth, and altered ecosystem dynamics. This is particularly relevant in areas where large-scale electrochemical processes using sodium acetate are conducted.
To mitigate these environmental impacts, several strategies can be employed. Implementing closed-loop systems for sodium acetate recovery and reuse can significantly reduce waste and environmental release. Advanced wastewater treatment technologies can help remove sodium acetate from effluents before discharge. Additionally, exploring alternative, more environmentally friendly electrolytes or buffer solutions could potentially reduce the reliance on sodium acetate in certain electrochemical applications.
Research into the long-term environmental fate and ecotoxicological effects of sodium acetate is ongoing. This research is crucial for developing more accurate environmental risk assessments and informing regulatory decisions regarding its use in industrial processes, including electrochemistry. As sustainability becomes an increasingly important factor in industrial operations, understanding and minimizing the environmental impact of sodium acetate use will be essential for the continued development of electrochemical technologies.
One of the primary environmental concerns associated with sodium acetate is its potential to contribute to water pollution. When released into aquatic ecosystems, sodium acetate can lead to increased levels of organic matter, potentially causing eutrophication. This process can result in algal blooms, oxygen depletion, and subsequent harm to aquatic life. However, it is important to note that sodium acetate is generally considered less harmful than many other industrial chemicals due to its biodegradability.
The production of sodium acetate also has environmental implications. The manufacturing process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. These raw materials and the energy required for production contribute to the overall carbon footprint of sodium acetate use. Additionally, the transportation and storage of sodium acetate can lead to indirect environmental impacts through fuel consumption and potential spills or leaks.
In electrochemical processes, the use of sodium acetate as an electrolyte or buffer solution can have both positive and negative environmental effects. On the positive side, sodium acetate can enhance the efficiency of certain electrochemical reactions, potentially reducing energy consumption and improving overall process sustainability. However, the disposal of spent electrolyte solutions containing sodium acetate requires careful management to prevent environmental contamination.
The accumulation of sodium ions in soil and water bodies is another potential environmental concern. While sodium is an essential element for many organisms, excessive levels can lead to soil degradation, reduced plant growth, and altered ecosystem dynamics. This is particularly relevant in areas where large-scale electrochemical processes using sodium acetate are conducted.
To mitigate these environmental impacts, several strategies can be employed. Implementing closed-loop systems for sodium acetate recovery and reuse can significantly reduce waste and environmental release. Advanced wastewater treatment technologies can help remove sodium acetate from effluents before discharge. Additionally, exploring alternative, more environmentally friendly electrolytes or buffer solutions could potentially reduce the reliance on sodium acetate in certain electrochemical applications.
Research into the long-term environmental fate and ecotoxicological effects of sodium acetate is ongoing. This research is crucial for developing more accurate environmental risk assessments and informing regulatory decisions regarding its use in industrial processes, including electrochemistry. As sustainability becomes an increasingly important factor in industrial operations, understanding and minimizing the environmental impact of sodium acetate use will be essential for the continued development of electrochemical technologies.
Safety Regulations for Sodium Acetate Handling
The handling of sodium acetate in electrochemical processes requires strict adherence to safety regulations to protect workers and the environment. These regulations typically cover various aspects of storage, handling, and disposal of the chemical.
Storage of sodium acetate should be in a cool, dry, and well-ventilated area, away from sources of heat, ignition, and direct sunlight. Containers must be tightly sealed and properly labeled. Incompatible materials, such as strong oxidizing agents, should be stored separately to prevent potential reactions.
Personal protective equipment (PPE) is essential when handling sodium acetate. This includes wearing safety goggles, chemical-resistant gloves, and appropriate protective clothing. In cases where dust or mist may be present, respiratory protection should be used. Proper training on the use of PPE and handling procedures is mandatory for all personnel working with the chemical.
Workplace safety measures should include the installation of eyewash stations and safety showers in areas where sodium acetate is handled. Adequate ventilation systems must be in place to control dust levels and maintain air quality. Regular monitoring of air quality and exposure levels should be conducted to ensure compliance with occupational exposure limits.
Spill response procedures must be established and communicated to all relevant personnel. This includes methods for containment, cleanup, and proper disposal of spilled material. Absorbent materials suitable for sodium acetate should be readily available in handling areas.
Waste disposal regulations for sodium acetate must be strictly followed. This may involve neutralization, dilution, or other treatment methods before disposal, depending on local environmental regulations. Proper documentation and tracking of waste disposal are essential for regulatory compliance.
Emergency response plans should be developed and regularly updated to address potential incidents involving sodium acetate. This includes procedures for fire suppression, as sodium acetate can decompose at high temperatures, releasing toxic fumes.
Transportation of sodium acetate must comply with relevant regulations for hazardous materials. This includes proper packaging, labeling, and documentation. Drivers transporting the chemical should be trained in emergency procedures and equipped with appropriate safety equipment.
Regular safety audits and inspections should be conducted to ensure ongoing compliance with all applicable regulations. This includes reviewing storage conditions, handling procedures, and emergency preparedness measures.
Employee training programs should be implemented to educate workers on the hazards associated with sodium acetate, proper handling techniques, and emergency procedures. Regular refresher courses should be provided to maintain awareness and competency.
Storage of sodium acetate should be in a cool, dry, and well-ventilated area, away from sources of heat, ignition, and direct sunlight. Containers must be tightly sealed and properly labeled. Incompatible materials, such as strong oxidizing agents, should be stored separately to prevent potential reactions.
Personal protective equipment (PPE) is essential when handling sodium acetate. This includes wearing safety goggles, chemical-resistant gloves, and appropriate protective clothing. In cases where dust or mist may be present, respiratory protection should be used. Proper training on the use of PPE and handling procedures is mandatory for all personnel working with the chemical.
Workplace safety measures should include the installation of eyewash stations and safety showers in areas where sodium acetate is handled. Adequate ventilation systems must be in place to control dust levels and maintain air quality. Regular monitoring of air quality and exposure levels should be conducted to ensure compliance with occupational exposure limits.
Spill response procedures must be established and communicated to all relevant personnel. This includes methods for containment, cleanup, and proper disposal of spilled material. Absorbent materials suitable for sodium acetate should be readily available in handling areas.
Waste disposal regulations for sodium acetate must be strictly followed. This may involve neutralization, dilution, or other treatment methods before disposal, depending on local environmental regulations. Proper documentation and tracking of waste disposal are essential for regulatory compliance.
Emergency response plans should be developed and regularly updated to address potential incidents involving sodium acetate. This includes procedures for fire suppression, as sodium acetate can decompose at high temperatures, releasing toxic fumes.
Transportation of sodium acetate must comply with relevant regulations for hazardous materials. This includes proper packaging, labeling, and documentation. Drivers transporting the chemical should be trained in emergency procedures and equipped with appropriate safety equipment.
Regular safety audits and inspections should be conducted to ensure ongoing compliance with all applicable regulations. This includes reviewing storage conditions, handling procedures, and emergency preparedness measures.
Employee training programs should be implemented to educate workers on the hazards associated with sodium acetate, proper handling techniques, and emergency procedures. Regular refresher courses should be provided to maintain awareness and competency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!