Sodium Acetate: Frontiers in Resource‑Conscious Processing
Sodium Acetate Overview and Research Objectives
Sodium acetate, a versatile compound with the chemical formula CH3COONa, has garnered significant attention in recent years due to its wide-ranging applications and potential for resource-conscious processing. This salt of acetic acid and sodium has been utilized across various industries, including food preservation, textile manufacturing, and as a buffering agent in numerous chemical processes. The historical development of sodium acetate can be traced back to its discovery in the early 19th century, with its industrial production methods evolving significantly over time.
The current technological landscape surrounding sodium acetate is characterized by a growing emphasis on sustainable production methods and novel applications. As global concerns about resource depletion and environmental impact intensify, researchers and industry professionals are increasingly focused on developing more efficient and eco-friendly processes for sodium acetate synthesis and utilization. This shift towards sustainability aligns with broader trends in green chemistry and circular economy principles, driving innovation in the field.
The primary objectives of this research on sodium acetate frontiers in resource-conscious processing are multifaceted. Firstly, there is a pressing need to optimize existing production methods to reduce energy consumption and minimize waste generation. This involves exploring alternative feedstocks, improving reaction efficiencies, and developing more effective purification techniques. Secondly, researchers aim to expand the application scope of sodium acetate, particularly in areas that can contribute to sustainability goals, such as renewable energy storage systems and biodegradable materials.
Another critical research objective is to investigate the potential of sodium acetate in addressing specific environmental challenges. For instance, its properties as a phase change material make it a promising candidate for thermal energy storage applications, which could play a crucial role in enhancing the efficiency of renewable energy systems. Additionally, there is growing interest in exploring sodium acetate's potential in carbon capture and utilization technologies, aligning with global efforts to mitigate climate change.
The technological evolution in this field is expected to focus on several key areas. These include the development of novel catalysts to improve reaction kinetics and selectivity, the integration of advanced process control systems to optimize production parameters, and the exploration of biotechnological approaches for sodium acetate synthesis using renewable resources. Furthermore, the research aims to elucidate the fundamental physicochemical properties of sodium acetate under various conditions, enabling more precise and efficient use in diverse applications.
Market Analysis for Sodium Acetate Applications
The sodium acetate market has shown significant growth in recent years, driven by its versatile applications across various industries. The global sodium acetate market size was valued at approximately $180 million in 2020 and is projected to reach $250 million by 2027, growing at a CAGR of around 5% during the forecast period. This growth is primarily attributed to the increasing demand for sodium acetate in the food and beverage industry, where it serves as a preservative and flavoring agent.
In the food sector, sodium acetate is widely used as an acidity regulator and preservative in a range of products, including bakery items, snacks, and processed meats. The growing consumer preference for convenience foods and the expansion of the packaged food industry are key factors driving the demand for sodium acetate in this segment. Additionally, the rising awareness of food safety and the need for extended shelf life of products further contribute to market growth.
The pharmaceutical industry represents another significant market for sodium acetate. It is used in various medical applications, including hemodialysis solutions and as a buffering agent in intravenous fluids. The increasing prevalence of chronic diseases and the growing aging population are expected to boost the demand for sodium acetate in pharmaceutical formulations.
In the textile industry, sodium acetate finds applications in dyeing processes and as a mordant for certain dyes. The steady growth of the textile sector, particularly in developing economies, is likely to create new opportunities for sodium acetate manufacturers. Moreover, the chemical industry utilizes sodium acetate as a raw material in the production of various chemicals and as a catalyst in certain reactions.
Geographically, Asia Pacific is expected to be the fastest-growing market for sodium acetate, driven by rapid industrialization, urbanization, and the expansion of end-use industries in countries like China and India. North America and Europe are mature markets but continue to show steady growth due to ongoing research and development activities and the adoption of sodium acetate in new applications.
However, the market faces challenges such as fluctuating raw material prices and the availability of substitutes for certain applications. Manufacturers are focusing on developing eco-friendly production processes and exploring new application areas to maintain their competitive edge in the market. The trend towards sustainable and bio-based chemicals is also influencing the sodium acetate market, with increasing research into green production methods and renewable raw material sources.
Current Challenges in Sodium Acetate Production
The production of sodium acetate faces several significant challenges in the current landscape of resource-conscious processing. One of the primary issues is the energy-intensive nature of traditional manufacturing methods. The conventional process involves the reaction of acetic acid with sodium hydroxide or sodium carbonate, which requires substantial heat input and often relies on fossil fuel-based energy sources. This not only contributes to high production costs but also raises environmental concerns due to the associated carbon emissions.
Raw material sourcing presents another hurdle in sodium acetate production. The increasing demand for acetic acid, a key precursor, has led to supply chain pressures and price volatility. Moreover, the quality and purity of raw materials can significantly impact the final product, necessitating stringent quality control measures that add complexity to the production process.
Water consumption is a critical issue in sodium acetate manufacturing. The crystallization and purification stages typically require large volumes of water, which is becoming an increasingly scarce resource in many regions. This not only raises sustainability concerns but also adds to operational costs and potential regulatory challenges in water-stressed areas.
Waste management and byproduct utilization represent ongoing challenges in the industry. The production process generates various waste streams, including mother liquors and off-spec products, which require proper handling and disposal. Developing efficient recycling methods and finding value-added applications for byproducts are essential for improving the overall resource efficiency of sodium acetate production.
Scale-up and process optimization continue to be areas of focus for manufacturers. Achieving consistent product quality while increasing production capacity often requires significant investment in equipment and process control systems. Balancing these capital expenditures with market demands and pricing pressures remains a delicate challenge for producers.
The industry also faces regulatory hurdles, particularly concerning environmental and safety standards. Compliance with evolving regulations on emissions, waste disposal, and product purity adds layers of complexity to the production process and can impact operational costs.
Lastly, the development of more sustainable and cost-effective production routes is an ongoing challenge. Research into bio-based precursors, green chemistry approaches, and novel catalytic processes shows promise but requires further development to become commercially viable alternatives to traditional methods. Overcoming these technological barriers while maintaining product quality and economic feasibility is crucial for the future of sodium acetate production in a resource-conscious world.
Resource-Efficient Sodium Acetate Production Methods
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications, including the production of pharmaceuticals, textiles, and other chemical compounds.- Use of sodium acetate in heat storage materials: Sodium acetate is utilized in heat storage materials due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage applications. These materials can be used in various heating and cooling systems to improve energy efficiency.
- Sodium acetate in food preservation and packaging: Sodium acetate is employed in food preservation and packaging solutions. It acts as a preservative and pH regulator, extending the shelf life of food products. Additionally, it can be incorporated into packaging materials to create active packaging systems that help maintain food quality and safety.
- Production methods for sodium acetate: Various methods are used to produce sodium acetate, including the reaction of acetic acid with sodium hydroxide or sodium carbonate. Some processes involve the use of catalysts or specific reaction conditions to improve yield and purity. These production methods aim to optimize efficiency and reduce costs in industrial-scale manufacturing.
- Sodium acetate in textile and fiber treatment: Sodium acetate finds applications in textile and fiber treatment processes. It can be used as a buffering agent in dyeing and finishing operations, helping to maintain optimal pH levels. Additionally, it may be employed in fiber modification techniques to enhance certain properties of textiles.
- Use of sodium acetate in environmental applications: Sodium acetate is utilized in various environmental applications, including wastewater treatment and air pollution control. It can serve as a carbon source for biological processes in wastewater treatment plants. In air pollution control, it may be used in scrubbing systems to neutralize acidic gases or as a de-icing agent with reduced environmental impact.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes a phase change at specific temperatures, allowing it to store and release latent heat. This property is exploited in heat packs, building materials for temperature regulation, and energy storage systems.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate serves as a food additive, acting as a preservative, acidity regulator, and flavoring agent. It is used in various food products to enhance shelf life, control pH, and impart a mild salty taste. Its application extends to beverages, condiments, and processed foods.Expand Specific Solutions04 Application in wastewater treatment
Sodium acetate is employed in wastewater treatment processes, particularly in biological treatment systems. It serves as a carbon source for microorganisms involved in denitrification and other biodegradation processes. This application helps in the removal of nitrogen compounds and organic pollutants from wastewater.Expand Specific Solutions05 Use in textile and leather industries
Sodium acetate finds applications in textile and leather processing. It is used as a dyeing auxiliary, helping to improve dye fixation and color fastness. In leather tanning, it serves as a buffering agent and assists in the penetration of tanning agents. These properties contribute to enhanced product quality in these industries.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The research on sodium acetate processing is in an early development stage, with a growing market driven by increasing demand for sustainable and resource-efficient solutions. The technology's maturity is still evolving, with key players like Shanghai Institute of Pharmaceutical Industry, China State Institute of Pharmaceutical Industry, and Zhejiang Yishu Environmental Protection Technology Co., Ltd. leading innovation efforts. These companies are focusing on developing advanced processing techniques to improve efficiency and reduce environmental impact. The competitive landscape is characterized by a mix of established chemical firms and emerging specialized players, indicating potential for significant advancements in the near future.
Institute of Process Engineering, Chinese Academy of Sciences
University of Strathclyde
Innovative Technologies in Sodium Acetate Synthesis
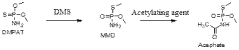
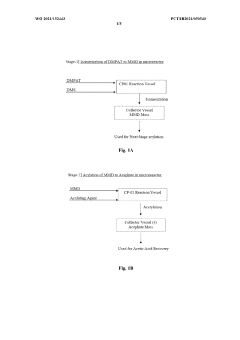
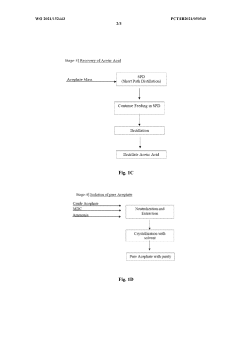
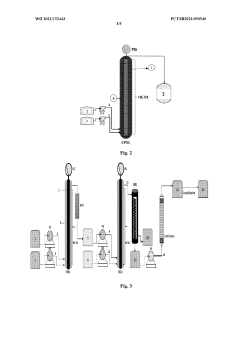
- A continuous flow process in a microreactor system that isomerizes O, O-dimethyl phosphoramidothioate using dimethyl sulphate to produce O,S-dimethyl phosphoramidothioate, followed by acetylation to produce acephate without solvents, allowing for improved thermal control and increased production capacity by connecting multiple reactors.
- An assay cassette with a fluid conduit and multiple chambers containing immobilized analyte-specific probes, allowing for sequential measurement and control of reagents, including washing, labeling, and triggering fluids, to simplify and automate the assay process, reducing the need for specialized expertise and equipment.
Environmental Impact Assessment of Production Processes
The environmental impact assessment of sodium acetate production processes is a critical aspect of resource-conscious processing. This evaluation encompasses various factors, including energy consumption, water usage, waste generation, and greenhouse gas emissions.
Energy consumption in sodium acetate production is a significant concern. The process typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate, which requires substantial thermal energy. Efforts to optimize energy efficiency have led to the development of more advanced reactor designs and heat recovery systems. These innovations aim to reduce the overall energy footprint of the production process.
Water usage is another crucial environmental factor. The production of sodium acetate often involves aqueous solutions, and water is used for cooling and cleaning purposes. Implementing closed-loop water systems and improving water treatment technologies can significantly reduce the water consumption and minimize the discharge of potentially harmful effluents.
Waste generation is a key consideration in the environmental impact assessment. The production process may generate by-products and waste materials that require proper handling and disposal. Implementing waste reduction strategies, such as recycling and reprocessing of by-products, can help minimize the environmental burden associated with sodium acetate production.
Greenhouse gas emissions, particularly carbon dioxide, are a concern in sodium acetate production. The energy-intensive nature of the process contributes to these emissions, primarily through the combustion of fossil fuels for heat and electricity generation. Transitioning to renewable energy sources and implementing carbon capture technologies can help mitigate these emissions.
The assessment also considers the sourcing of raw materials. Sustainable procurement practices for acetic acid and sodium-based compounds can reduce the overall environmental impact of the production chain. This includes considering the transportation distances of raw materials and the environmental practices of suppliers.
Life cycle assessment (LCA) methodologies are increasingly being applied to sodium acetate production. These comprehensive analyses evaluate the environmental impacts from raw material extraction through to product disposal, providing a holistic view of the process's environmental footprint.
Regulatory compliance is an essential aspect of the environmental impact assessment. Producers must adhere to local, national, and international environmental regulations, which may include limits on emissions, waste disposal practices, and resource consumption. Staying ahead of regulatory requirements often drives innovation in cleaner production technologies.
Regulatory Framework for Sodium Acetate Manufacturing
The regulatory framework for sodium acetate manufacturing is a critical aspect of the industry, ensuring product safety, environmental protection, and quality standards. In the United States, the Food and Drug Administration (FDA) oversees the regulation of sodium acetate as a food additive and pharmaceutical ingredient. The FDA has classified sodium acetate as Generally Recognized as Safe (GRAS) for use in food products, subject to specific limitations and conditions.
The Environmental Protection Agency (EPA) plays a crucial role in regulating the environmental impact of sodium acetate production. Manufacturers must comply with the Clean Air Act and Clean Water Act, which set limits on emissions and effluents from industrial processes. The EPA also enforces the Resource Conservation and Recovery Act (RCRA), governing the management of hazardous waste generated during production.
Occupational safety in sodium acetate manufacturing falls under the purview of the Occupational Safety and Health Administration (OSHA). OSHA standards mandate proper handling procedures, personal protective equipment, and workplace safety measures to protect workers from potential hazards associated with chemical manufacturing.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation applies to sodium acetate production and import within EU member states. Manufacturers and importers must register substances with the European Chemicals Agency (ECHA) and provide safety data to ensure compliance with human health and environmental protection standards.
In the context of resource-conscious processing, regulatory bodies are increasingly focusing on sustainable manufacturing practices. This includes promoting energy efficiency, waste reduction, and the use of renewable resources in chemical production. The EU's Industrial Emissions Directive (IED) and the United States' Pollution Prevention Act are examples of regulatory frameworks encouraging cleaner production methods.
Emerging regulations are also addressing the circular economy concept, emphasizing the importance of recycling and reusing materials in the chemical industry. This trend is likely to impact sodium acetate manufacturing, pushing for more efficient use of raw materials and the development of closed-loop production systems.
As global concerns about climate change intensify, carbon emissions regulations are becoming more stringent. Sodium acetate manufacturers may face increasing pressure to reduce their carbon footprint through process optimization, energy-efficient technologies, and the adoption of renewable energy sources.