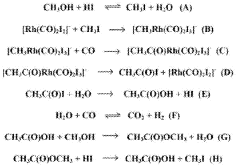Sodium Acetate in Pharmaceutical Manufacturing Processes
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate in Pharma: Background and Objectives
Sodium acetate has emerged as a crucial compound in pharmaceutical manufacturing processes, playing a significant role in various aspects of drug production. This versatile substance has a rich history dating back to its discovery in the early 19th century. Initially used in textile and leather industries, sodium acetate's potential in pharmaceuticals was gradually recognized, leading to its widespread adoption in the field.
The evolution of sodium acetate's use in pharmaceutical manufacturing has been driven by advancements in drug formulation techniques and the increasing demand for more efficient and cost-effective production methods. Over the years, researchers and industry professionals have explored its properties and applications, uncovering a wide range of benefits that have made it an indispensable component in many pharmaceutical processes.
One of the primary objectives of researching sodium acetate in pharmaceutical manufacturing is to optimize its utilization across various stages of drug production. This includes its role as a buffering agent, pH regulator, and stabilizer in drug formulations. By understanding the intricate interactions between sodium acetate and other pharmaceutical ingredients, researchers aim to enhance drug stability, bioavailability, and overall efficacy.
Another key goal is to investigate the potential of sodium acetate in improving the solubility and dissolution rates of poorly water-soluble drugs. This aspect has gained significant attention in recent years, as many new drug candidates face challenges related to low solubility, which can hinder their therapeutic effectiveness. Sodium acetate's unique properties make it a promising candidate for addressing these issues and potentially expanding the range of drugs that can be successfully formulated.
Furthermore, the research aims to explore sodium acetate's applications in novel drug delivery systems. As the pharmaceutical industry continues to innovate in areas such as controlled release formulations and targeted drug delivery, understanding how sodium acetate can be leveraged in these advanced systems becomes increasingly important. This includes investigating its role in matrix formation, coating processes, and other delivery mechanisms that can enhance drug performance and patient outcomes.
The ongoing research also focuses on the sustainability and environmental aspects of using sodium acetate in pharmaceutical manufacturing. As the industry moves towards more eco-friendly practices, there is a growing interest in exploring sodium acetate's potential as a green alternative to certain traditional excipients. This aligns with the broader trend of developing more sustainable pharmaceutical production processes and reducing the environmental footprint of drug manufacturing.
The evolution of sodium acetate's use in pharmaceutical manufacturing has been driven by advancements in drug formulation techniques and the increasing demand for more efficient and cost-effective production methods. Over the years, researchers and industry professionals have explored its properties and applications, uncovering a wide range of benefits that have made it an indispensable component in many pharmaceutical processes.
One of the primary objectives of researching sodium acetate in pharmaceutical manufacturing is to optimize its utilization across various stages of drug production. This includes its role as a buffering agent, pH regulator, and stabilizer in drug formulations. By understanding the intricate interactions between sodium acetate and other pharmaceutical ingredients, researchers aim to enhance drug stability, bioavailability, and overall efficacy.
Another key goal is to investigate the potential of sodium acetate in improving the solubility and dissolution rates of poorly water-soluble drugs. This aspect has gained significant attention in recent years, as many new drug candidates face challenges related to low solubility, which can hinder their therapeutic effectiveness. Sodium acetate's unique properties make it a promising candidate for addressing these issues and potentially expanding the range of drugs that can be successfully formulated.
Furthermore, the research aims to explore sodium acetate's applications in novel drug delivery systems. As the pharmaceutical industry continues to innovate in areas such as controlled release formulations and targeted drug delivery, understanding how sodium acetate can be leveraged in these advanced systems becomes increasingly important. This includes investigating its role in matrix formation, coating processes, and other delivery mechanisms that can enhance drug performance and patient outcomes.
The ongoing research also focuses on the sustainability and environmental aspects of using sodium acetate in pharmaceutical manufacturing. As the industry moves towards more eco-friendly practices, there is a growing interest in exploring sodium acetate's potential as a green alternative to certain traditional excipients. This aligns with the broader trend of developing more sustainable pharmaceutical production processes and reducing the environmental footprint of drug manufacturing.
Market Analysis for Sodium Acetate in Pharmaceuticals
The global market for sodium acetate in pharmaceutical manufacturing processes has been experiencing steady growth, driven by the increasing demand for pharmaceutical products worldwide. Sodium acetate plays a crucial role in various pharmaceutical applications, including as a buffering agent, pH adjuster, and preservative in drug formulations. The market size for sodium acetate in the pharmaceutical industry is projected to reach significant figures in the coming years, with a compound annual growth rate (CAGR) that reflects the expanding pharmaceutical sector.
One of the key factors contributing to the market growth is the rising prevalence of chronic diseases and the subsequent increase in drug production. As pharmaceutical companies strive to meet the growing demand for medications, the consumption of sodium acetate as an excipient and processing aid has correspondingly increased. Additionally, the trend towards personalized medicine and the development of novel drug delivery systems have created new opportunities for sodium acetate applications in pharmaceutical manufacturing.
The market demand for sodium acetate is also influenced by regulatory requirements and quality standards in pharmaceutical production. As regulatory bodies worldwide emphasize the importance of drug safety and efficacy, pharmaceutical manufacturers are increasingly relying on high-quality excipients like sodium acetate to ensure product stability and consistency. This has led to a growing preference for pharmaceutical-grade sodium acetate, which meets stringent purity and quality standards.
Geographically, the market for sodium acetate in pharmaceuticals is segmented across North America, Europe, Asia-Pacific, and the rest of the world. North America and Europe currently hold significant market shares due to their well-established pharmaceutical industries and advanced healthcare infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by the expanding pharmaceutical manufacturing capabilities in countries like China and India.
The competitive landscape of the sodium acetate market in pharmaceuticals is characterized by the presence of both large multinational chemical companies and specialized manufacturers. Key players are focusing on product innovation, quality improvements, and strategic partnerships to maintain their market positions. The increasing emphasis on sustainable manufacturing practices is also influencing market dynamics, with some companies exploring eco-friendly production methods for sodium acetate.
Looking ahead, the market for sodium acetate in pharmaceutical manufacturing processes is poised for continued growth. Factors such as the expansion of the biopharmaceutical sector, advancements in drug delivery technologies, and the increasing adoption of sodium acetate in novel pharmaceutical applications are expected to drive market demand. However, challenges such as price fluctuations of raw materials and the need for compliance with evolving regulatory standards may impact market growth and require adaptive strategies from industry players.
One of the key factors contributing to the market growth is the rising prevalence of chronic diseases and the subsequent increase in drug production. As pharmaceutical companies strive to meet the growing demand for medications, the consumption of sodium acetate as an excipient and processing aid has correspondingly increased. Additionally, the trend towards personalized medicine and the development of novel drug delivery systems have created new opportunities for sodium acetate applications in pharmaceutical manufacturing.
The market demand for sodium acetate is also influenced by regulatory requirements and quality standards in pharmaceutical production. As regulatory bodies worldwide emphasize the importance of drug safety and efficacy, pharmaceutical manufacturers are increasingly relying on high-quality excipients like sodium acetate to ensure product stability and consistency. This has led to a growing preference for pharmaceutical-grade sodium acetate, which meets stringent purity and quality standards.
Geographically, the market for sodium acetate in pharmaceuticals is segmented across North America, Europe, Asia-Pacific, and the rest of the world. North America and Europe currently hold significant market shares due to their well-established pharmaceutical industries and advanced healthcare infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by the expanding pharmaceutical manufacturing capabilities in countries like China and India.
The competitive landscape of the sodium acetate market in pharmaceuticals is characterized by the presence of both large multinational chemical companies and specialized manufacturers. Key players are focusing on product innovation, quality improvements, and strategic partnerships to maintain their market positions. The increasing emphasis on sustainable manufacturing practices is also influencing market dynamics, with some companies exploring eco-friendly production methods for sodium acetate.
Looking ahead, the market for sodium acetate in pharmaceutical manufacturing processes is poised for continued growth. Factors such as the expansion of the biopharmaceutical sector, advancements in drug delivery technologies, and the increasing adoption of sodium acetate in novel pharmaceutical applications are expected to drive market demand. However, challenges such as price fluctuations of raw materials and the need for compliance with evolving regulatory standards may impact market growth and require adaptive strategies from industry players.
Current Challenges in Sodium Acetate Utilization
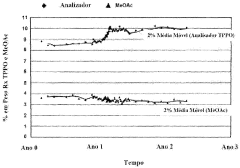
Despite the widespread use of sodium acetate in pharmaceutical manufacturing processes, several challenges persist in its utilization. One of the primary concerns is the variability in quality and purity of commercially available sodium acetate. This inconsistency can lead to unpredictable outcomes in drug formulations, potentially affecting the efficacy and safety of the final product. Manufacturers often struggle to maintain consistent batch-to-batch quality, which is crucial for regulatory compliance and product reliability.
Another significant challenge lies in the hygroscopic nature of sodium acetate. Its tendency to absorb moisture from the environment can lead to caking and clumping, making it difficult to handle and measure accurately. This property also necessitates stringent storage conditions, which can increase operational costs and complexity in pharmaceutical manufacturing facilities.
The pH-dependent solubility of sodium acetate presents additional complications in formulation development. While it is highly soluble in water, its solubility can be significantly affected by changes in pH, temperature, and the presence of other ions in the solution. This sensitivity can lead to precipitation or crystallization issues during various stages of the manufacturing process, potentially compromising product quality and yield.
Furthermore, the potential for sodium acetate to interact with other excipients or active pharmaceutical ingredients (APIs) poses a challenge in formulation design. These interactions may alter the stability, bioavailability, or therapeutic efficacy of the drug product. Formulators must carefully consider these potential interactions when developing new pharmaceutical compositions, often requiring extensive compatibility studies.
The environmental impact of sodium acetate production and disposal is an emerging concern in the pharmaceutical industry. As sustainability becomes increasingly important, manufacturers are facing pressure to develop more eco-friendly processes for producing and using sodium acetate. This includes finding ways to reduce energy consumption during production, minimize waste generation, and explore biodegradable alternatives where possible.
Lastly, regulatory compliance remains a persistent challenge in the use of sodium acetate. As global regulatory bodies continue to tighten their requirements for pharmaceutical excipients, manufacturers must invest in robust quality control systems and documentation processes to ensure compliance. This includes implementing thorough traceability measures, conducting regular stability studies, and maintaining detailed records of sourcing and processing.
Another significant challenge lies in the hygroscopic nature of sodium acetate. Its tendency to absorb moisture from the environment can lead to caking and clumping, making it difficult to handle and measure accurately. This property also necessitates stringent storage conditions, which can increase operational costs and complexity in pharmaceutical manufacturing facilities.
The pH-dependent solubility of sodium acetate presents additional complications in formulation development. While it is highly soluble in water, its solubility can be significantly affected by changes in pH, temperature, and the presence of other ions in the solution. This sensitivity can lead to precipitation or crystallization issues during various stages of the manufacturing process, potentially compromising product quality and yield.
Furthermore, the potential for sodium acetate to interact with other excipients or active pharmaceutical ingredients (APIs) poses a challenge in formulation design. These interactions may alter the stability, bioavailability, or therapeutic efficacy of the drug product. Formulators must carefully consider these potential interactions when developing new pharmaceutical compositions, often requiring extensive compatibility studies.
The environmental impact of sodium acetate production and disposal is an emerging concern in the pharmaceutical industry. As sustainability becomes increasingly important, manufacturers are facing pressure to develop more eco-friendly processes for producing and using sodium acetate. This includes finding ways to reduce energy consumption during production, minimize waste generation, and explore biodegradable alternatives where possible.
Lastly, regulatory compliance remains a persistent challenge in the use of sodium acetate. As global regulatory bodies continue to tighten their requirements for pharmaceutical excipients, manufacturers must invest in robust quality control systems and documentation processes to ensure compliance. This includes implementing thorough traceability measures, conducting regular stability studies, and maintaining detailed records of sourcing and processing.
Current Applications of Sodium Acetate in Drug Formulation
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications, including the production of pharmaceuticals, textiles, and other chemical compounds.- Use of sodium acetate in chemical processes: Sodium acetate is utilized in various chemical processes, including as a catalyst, pH regulator, or reagent. It plays a role in reactions such as esterification, acetylation, and neutralization. The compound's properties make it suitable for applications in industrial chemistry and laboratory settings.
- Application in heat storage and thermal management: Sodium acetate is employed in heat storage systems and thermal management solutions. Its phase change properties allow it to absorb and release heat effectively, making it useful in heat packs, building materials for temperature regulation, and energy storage applications.
- Use in food and beverage industry: Sodium acetate finds applications in the food and beverage industry as a preservative, flavoring agent, and acidity regulator. It helps extend shelf life, enhance taste, and maintain product quality in various food products and beverages.
- Application in textile and paper industries: Sodium acetate is used in textile and paper industries for various purposes. In textiles, it can be used as a mordant in dyeing processes or as a neutralizing agent. In paper production, it may serve as a pH buffer or in surface treatments.
- Use in pharmaceutical and personal care products: Sodium acetate is utilized in pharmaceutical formulations and personal care products. It can act as a buffering agent, pH adjuster, or electrolyte in various medications, topical preparations, and cosmetic products. Its properties contribute to product stability and effectiveness.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes a phase change at specific temperatures, allowing it to store and release latent heat. This property makes it useful in heat packs, building materials for temperature regulation, and energy storage systems.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate serves as a food additive and preservative in the food and beverage industry. It acts as a acidity regulator, flavoring agent, and antimicrobial preservative. Its use helps extend shelf life, enhance flavor profiles, and maintain product quality in various food products.Expand Specific Solutions04 Application in wastewater treatment
Sodium acetate is employed in wastewater treatment processes, particularly in biological treatment systems. It serves as a carbon source for microorganisms involved in denitrification and other biological processes. This application helps improve the efficiency of wastewater treatment and reduces environmental impact.Expand Specific Solutions05 Use in material science and nanotechnology
Sodium acetate finds applications in material science and nanotechnology. It is used in the synthesis of various nanoparticles, as a precursor in sol-gel processes, and in the preparation of advanced materials. These applications contribute to the development of novel materials with unique properties for diverse industries.Expand Specific Solutions
Key Players in Pharmaceutical Sodium Acetate Production
The research on sodium acetate in pharmaceutical manufacturing processes is in a mature stage, with a well-established market and diverse applications. The global market size for sodium acetate in pharmaceuticals is estimated to be significant, driven by its use as a buffering agent, preservative, and diuretic. Key players in this field include major chemical companies like BASF Corp., Eastman Chemical Co., and LyondellBasell Acetyls LLC, as well as specialized pharmaceutical manufacturers such as Momenta Pharmaceuticals, Inc. and Hikma Pharmaceuticals USA, Inc. These companies have developed advanced production techniques and quality control measures to meet the stringent requirements of pharmaceutical-grade sodium acetate. The competitive landscape is characterized by a focus on product purity, cost-effectiveness, and regulatory compliance.
China Petroleum & Chemical Corp.
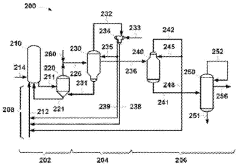
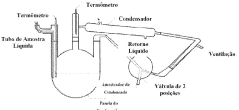
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced processes for sodium acetate production in pharmaceutical manufacturing. Their approach involves the direct reaction of acetic acid with sodium hydroxide or sodium carbonate, followed by crystallization and purification steps. Sinopec has implemented continuous flow reactors to improve efficiency and product quality[1]. They have also developed a novel spray drying technique that produces high-purity, fine sodium acetate particles suitable for pharmaceutical applications[2]. Additionally, Sinopec has invested in green chemistry initiatives, utilizing biomass-derived acetic acid as a feedstock for sodium acetate production, reducing environmental impact[3].
Strengths: Large-scale production capabilities, advanced process technologies, and integration with petrochemical feedstocks. Weaknesses: Potential environmental concerns associated with fossil fuel-based operations.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary process for high-purity sodium acetate production tailored for pharmaceutical applications. Their method utilizes a controlled neutralization reaction between glacial acetic acid and sodium hydroxide, followed by multiple crystallization and purification steps[4]. Celanese has implemented advanced process analytical technology (PAT) to ensure consistent product quality and meet stringent pharmaceutical standards[5]. They have also developed a novel anhydrous sodium acetate production method, which is particularly valuable for moisture-sensitive drug formulations[6]. Furthermore, Celanese has invested in sustainable practices, including water recycling and energy-efficient crystallization technologies in their sodium acetate production facilities.
Strengths: High-purity product offerings, expertise in acetic acid chemistry, and strong presence in pharmaceutical markets. Weaknesses: Potential cost pressures from raw material fluctuations.
Innovative Uses of Sodium Acetate in Drug Delivery
ACETIC ACID process
PatentActiveBR112019020678A2
Innovation
- Utilizing uncoated Raman probes and flow cells to measure initial concentrations of reference components, calculating an Adjustment Ratio to correct for coating effects, and adjusting process conditions based on adjusted component concentrations.
ACETIC ACID PRODUCTION PROCESS
PatentActiveBR112014031919A2
Innovation
- Continuous introduction of a complexing agent to interact with hydrogen iodide, forming a complex in the acetic acid production system.
- Integration of the complexing agent introduction step into the existing acetic acid production process, potentially simplifying the overall system.
- Potential reduction of corrosion and equipment wear by managing hydrogen iodide through complex formation.
Regulatory Framework for Sodium Acetate in Pharmaceuticals
The regulatory framework for sodium acetate in pharmaceuticals is a complex and evolving landscape that plays a crucial role in ensuring the safety, efficacy, and quality of pharmaceutical products. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national health authorities have established guidelines and regulations governing the use of sodium acetate in pharmaceutical manufacturing processes.
In the United States, sodium acetate is generally recognized as safe (GRAS) by the FDA when used as a food ingredient. However, its use in pharmaceutical applications is subject to more stringent regulations. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production, testing, and quality control of pharmaceutical ingredients, including sodium acetate. These regulations ensure that the manufacturing processes maintain consistent quality and purity standards.
The European Union has implemented similar regulations through the EMA. The European Pharmacopoeia, which sets quality standards for medicinal substances, includes monographs for sodium acetate, detailing specifications for its use in pharmaceutical formulations. Manufacturers must comply with these standards to ensure the quality and safety of their products.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed guidelines that aim to harmonize regulatory requirements across different regions. These guidelines cover various aspects of pharmaceutical development, including quality control and manufacturing processes, which are applicable to the use of sodium acetate in pharmaceutical production.
Regulatory bodies also require manufacturers to implement robust quality management systems and conduct thorough risk assessments when using sodium acetate in pharmaceutical processes. This includes validating analytical methods for testing sodium acetate purity and stability, as well as establishing appropriate specifications for its use in different formulations.
Furthermore, regulatory frameworks often mandate the documentation of all aspects of sodium acetate usage in pharmaceutical manufacturing. This includes maintaining detailed records of sourcing, testing, and incorporation into final products. Manufacturers must be prepared to provide this documentation during regulatory inspections or audits.
As the pharmaceutical industry continues to evolve, regulatory frameworks are also adapting to address new challenges and technologies. For instance, there is an increasing focus on the environmental impact of pharmaceutical manufacturing processes, which may lead to new regulations regarding the use and disposal of sodium acetate and other chemicals used in production.
In the United States, sodium acetate is generally recognized as safe (GRAS) by the FDA when used as a food ingredient. However, its use in pharmaceutical applications is subject to more stringent regulations. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide guidelines for the production, testing, and quality control of pharmaceutical ingredients, including sodium acetate. These regulations ensure that the manufacturing processes maintain consistent quality and purity standards.
The European Union has implemented similar regulations through the EMA. The European Pharmacopoeia, which sets quality standards for medicinal substances, includes monographs for sodium acetate, detailing specifications for its use in pharmaceutical formulations. Manufacturers must comply with these standards to ensure the quality and safety of their products.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has developed guidelines that aim to harmonize regulatory requirements across different regions. These guidelines cover various aspects of pharmaceutical development, including quality control and manufacturing processes, which are applicable to the use of sodium acetate in pharmaceutical production.
Regulatory bodies also require manufacturers to implement robust quality management systems and conduct thorough risk assessments when using sodium acetate in pharmaceutical processes. This includes validating analytical methods for testing sodium acetate purity and stability, as well as establishing appropriate specifications for its use in different formulations.
Furthermore, regulatory frameworks often mandate the documentation of all aspects of sodium acetate usage in pharmaceutical manufacturing. This includes maintaining detailed records of sourcing, testing, and incorporation into final products. Manufacturers must be prepared to provide this documentation during regulatory inspections or audits.
As the pharmaceutical industry continues to evolve, regulatory frameworks are also adapting to address new challenges and technologies. For instance, there is an increasing focus on the environmental impact of pharmaceutical manufacturing processes, which may lead to new regulations regarding the use and disposal of sodium acetate and other chemicals used in production.
Environmental Impact of Sodium Acetate in Drug Manufacturing
The environmental impact of sodium acetate in drug manufacturing is a critical consideration for pharmaceutical companies striving to balance production efficiency with ecological responsibility. Sodium acetate, a common ingredient in many pharmaceutical processes, has both direct and indirect effects on the environment throughout its lifecycle.
In the production phase, the synthesis of sodium acetate typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process requires energy and resources, contributing to carbon emissions and resource depletion. However, compared to many other chemical processes in pharmaceutical manufacturing, the production of sodium acetate is relatively less energy-intensive and generates fewer harmful byproducts.
During the manufacturing process, sodium acetate is often used as a buffering agent or pH regulator. Its use in this capacity helps to minimize the need for more environmentally harmful alternatives, potentially reducing the overall ecological footprint of drug production. Additionally, sodium acetate's high solubility in water facilitates easier handling and reduces the risk of environmental contamination through spills or improper disposal.
The disposal of sodium acetate and its byproducts presents another environmental consideration. When released into aquatic environments, sodium acetate can lead to increased biological oxygen demand (BOD) as microorganisms break down the compound. This can potentially impact aquatic ecosystems, particularly in cases of large-scale releases. However, sodium acetate is biodegradable and does not persist in the environment long-term, mitigating some of these concerns.
In terms of waste management, pharmaceutical companies employing sodium acetate must adhere to strict regulations regarding its disposal. Many facilities have implemented advanced wastewater treatment systems to remove or neutralize sodium acetate before release, further minimizing its environmental impact. Some companies have also explored recycling methods for sodium acetate, aiming to reduce waste and improve resource efficiency.
The transportation and storage of sodium acetate also contribute to its environmental footprint. As a relatively stable compound, it does not require special handling or storage conditions, which can reduce energy consumption and the risk of accidental releases during transport. However, the global distribution of sodium acetate for pharmaceutical use does contribute to transportation-related emissions.
Overall, while sodium acetate does have environmental implications in drug manufacturing, its impact is generally considered moderate compared to many other chemicals used in the pharmaceutical industry. Ongoing research and development efforts are focused on further optimizing its use and exploring more sustainable alternatives, aligning with the industry's growing commitment to environmental stewardship.
In the production phase, the synthesis of sodium acetate typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. This process requires energy and resources, contributing to carbon emissions and resource depletion. However, compared to many other chemical processes in pharmaceutical manufacturing, the production of sodium acetate is relatively less energy-intensive and generates fewer harmful byproducts.
During the manufacturing process, sodium acetate is often used as a buffering agent or pH regulator. Its use in this capacity helps to minimize the need for more environmentally harmful alternatives, potentially reducing the overall ecological footprint of drug production. Additionally, sodium acetate's high solubility in water facilitates easier handling and reduces the risk of environmental contamination through spills or improper disposal.
The disposal of sodium acetate and its byproducts presents another environmental consideration. When released into aquatic environments, sodium acetate can lead to increased biological oxygen demand (BOD) as microorganisms break down the compound. This can potentially impact aquatic ecosystems, particularly in cases of large-scale releases. However, sodium acetate is biodegradable and does not persist in the environment long-term, mitigating some of these concerns.
In terms of waste management, pharmaceutical companies employing sodium acetate must adhere to strict regulations regarding its disposal. Many facilities have implemented advanced wastewater treatment systems to remove or neutralize sodium acetate before release, further minimizing its environmental impact. Some companies have also explored recycling methods for sodium acetate, aiming to reduce waste and improve resource efficiency.
The transportation and storage of sodium acetate also contribute to its environmental footprint. As a relatively stable compound, it does not require special handling or storage conditions, which can reduce energy consumption and the risk of accidental releases during transport. However, the global distribution of sodium acetate for pharmaceutical use does contribute to transportation-related emissions.
Overall, while sodium acetate does have environmental implications in drug manufacturing, its impact is generally considered moderate compared to many other chemicals used in the pharmaceutical industry. Ongoing research and development efforts are focused on further optimizing its use and exploring more sustainable alternatives, aligning with the industry's growing commitment to environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!