Sodium Acetate’s Role in Nanotechnology Material Innovation
JUN 30, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Nanotech Background and Objectives
Sodium acetate has emerged as a pivotal compound in the realm of nanotechnology, offering unique properties that have catalyzed significant advancements in material innovation. The journey of sodium acetate in nanotechnology began in the early 2000s when researchers discovered its potential as a precursor for synthesizing various nanostructures. This discovery marked the beginning of a new era in materials science, where sodium acetate's role transitioned from a simple organic salt to a key player in nanomaterial fabrication.
The evolution of sodium acetate's application in nanotechnology has been driven by the increasing demand for advanced materials with enhanced properties. As industries sought materials with improved strength, conductivity, and reactivity, sodium acetate-based nanostructures emerged as promising candidates. This trend has been particularly evident in sectors such as electronics, energy storage, and biomedical engineering, where the unique characteristics of sodium acetate-derived nanomaterials have opened new avenues for innovation.
Over the past decade, research efforts have intensified to explore the full potential of sodium acetate in nanotechnology. Scientists have made significant strides in understanding the mechanisms by which sodium acetate facilitates the formation of nanostructures, leading to more controlled and efficient synthesis methods. These advancements have not only expanded the range of achievable nanostructures but also improved their quality and reproducibility.
The current technological landscape sees sodium acetate playing a crucial role in the development of novel nanomaterials with tailored properties. From carbon nanotubes to metal oxide nanoparticles, sodium acetate has proven to be a versatile precursor, capable of producing a wide array of nanostructures with diverse applications. Its ability to act as both a reducing agent and a structure-directing agent has made it indispensable in many nanofabrication processes.
Looking ahead, the objectives for sodium acetate in nanotechnology are multifaceted. Researchers aim to further optimize synthesis protocols to achieve even greater control over nanostructure morphology and composition. There is also a growing focus on developing environmentally friendly and cost-effective production methods, leveraging sodium acetate's inherent advantages as a non-toxic and readily available compound.
Another key objective is to expand the application scope of sodium acetate-based nanomaterials. This includes exploring their potential in emerging fields such as quantum computing, advanced catalysis, and next-generation energy storage solutions. Additionally, there is a push towards integrating these nanomaterials into existing technologies to enhance performance and efficiency across various industrial sectors.
As we move forward, the role of sodium acetate in nanotechnology material innovation is expected to grow exponentially. With ongoing research and development, it is poised to contribute significantly to addressing global challenges in energy, healthcare, and environmental sustainability through the creation of advanced nanomaterials.
The evolution of sodium acetate's application in nanotechnology has been driven by the increasing demand for advanced materials with enhanced properties. As industries sought materials with improved strength, conductivity, and reactivity, sodium acetate-based nanostructures emerged as promising candidates. This trend has been particularly evident in sectors such as electronics, energy storage, and biomedical engineering, where the unique characteristics of sodium acetate-derived nanomaterials have opened new avenues for innovation.
Over the past decade, research efforts have intensified to explore the full potential of sodium acetate in nanotechnology. Scientists have made significant strides in understanding the mechanisms by which sodium acetate facilitates the formation of nanostructures, leading to more controlled and efficient synthesis methods. These advancements have not only expanded the range of achievable nanostructures but also improved their quality and reproducibility.
The current technological landscape sees sodium acetate playing a crucial role in the development of novel nanomaterials with tailored properties. From carbon nanotubes to metal oxide nanoparticles, sodium acetate has proven to be a versatile precursor, capable of producing a wide array of nanostructures with diverse applications. Its ability to act as both a reducing agent and a structure-directing agent has made it indispensable in many nanofabrication processes.
Looking ahead, the objectives for sodium acetate in nanotechnology are multifaceted. Researchers aim to further optimize synthesis protocols to achieve even greater control over nanostructure morphology and composition. There is also a growing focus on developing environmentally friendly and cost-effective production methods, leveraging sodium acetate's inherent advantages as a non-toxic and readily available compound.
Another key objective is to expand the application scope of sodium acetate-based nanomaterials. This includes exploring their potential in emerging fields such as quantum computing, advanced catalysis, and next-generation energy storage solutions. Additionally, there is a push towards integrating these nanomaterials into existing technologies to enhance performance and efficiency across various industrial sectors.
As we move forward, the role of sodium acetate in nanotechnology material innovation is expected to grow exponentially. With ongoing research and development, it is poised to contribute significantly to addressing global challenges in energy, healthcare, and environmental sustainability through the creation of advanced nanomaterials.
Market Demand for Sodium Acetate-based Nanomaterials
The market demand for sodium acetate-based nanomaterials has been steadily growing, driven by their unique properties and versatile applications across various industries. These nanomaterials offer enhanced performance characteristics, including improved thermal stability, electrical conductivity, and mechanical strength, making them attractive for a wide range of applications.
In the electronics industry, sodium acetate-based nanomaterials are increasingly sought after for their potential in developing advanced energy storage devices, such as supercapacitors and batteries. The growing demand for high-performance, long-lasting energy storage solutions in portable electronics and electric vehicles is a significant driver for this market segment.
The healthcare sector represents another substantial market for sodium acetate-based nanomaterials. These materials show promise in drug delivery systems, biosensors, and tissue engineering applications. The ability to precisely control the size and surface properties of these nanomaterials makes them particularly valuable for targeted drug delivery and diagnostic tools.
Environmental applications are also fueling the demand for sodium acetate-based nanomaterials. Their use in water purification systems and air filtration technologies is gaining traction due to their high surface area and adsorption capabilities. As global concerns about water scarcity and air pollution continue to rise, the market for these nanomaterials in environmental remediation is expected to expand significantly.
The construction industry is another sector showing increased interest in sodium acetate-based nanomaterials. These materials can enhance the strength and durability of concrete and other building materials, leading to more resilient and sustainable infrastructure. The growing focus on green building practices and energy-efficient construction is likely to drive further demand in this area.
In the textile industry, sodium acetate-based nanomaterials are being explored for their potential to create smart fabrics with enhanced properties such as water repellency, UV protection, and antimicrobial characteristics. This aligns with the growing consumer demand for high-performance, multifunctional textiles in both fashion and technical applications.
The automotive sector is also a key driver of market demand for these nanomaterials. Their use in lightweight composites, coatings, and lubricants can contribute to improved fuel efficiency and vehicle performance. As the automotive industry continues to focus on sustainability and electrification, the demand for innovative materials that can address these challenges is expected to grow.
While the market for sodium acetate-based nanomaterials is promising, it is important to note that challenges such as scalability, cost-effectiveness, and regulatory compliance need to be addressed to fully realize their market potential. As research and development efforts continue to overcome these hurdles, the market demand is expected to expand further, opening up new opportunities across various industries.
In the electronics industry, sodium acetate-based nanomaterials are increasingly sought after for their potential in developing advanced energy storage devices, such as supercapacitors and batteries. The growing demand for high-performance, long-lasting energy storage solutions in portable electronics and electric vehicles is a significant driver for this market segment.
The healthcare sector represents another substantial market for sodium acetate-based nanomaterials. These materials show promise in drug delivery systems, biosensors, and tissue engineering applications. The ability to precisely control the size and surface properties of these nanomaterials makes them particularly valuable for targeted drug delivery and diagnostic tools.
Environmental applications are also fueling the demand for sodium acetate-based nanomaterials. Their use in water purification systems and air filtration technologies is gaining traction due to their high surface area and adsorption capabilities. As global concerns about water scarcity and air pollution continue to rise, the market for these nanomaterials in environmental remediation is expected to expand significantly.
The construction industry is another sector showing increased interest in sodium acetate-based nanomaterials. These materials can enhance the strength and durability of concrete and other building materials, leading to more resilient and sustainable infrastructure. The growing focus on green building practices and energy-efficient construction is likely to drive further demand in this area.
In the textile industry, sodium acetate-based nanomaterials are being explored for their potential to create smart fabrics with enhanced properties such as water repellency, UV protection, and antimicrobial characteristics. This aligns with the growing consumer demand for high-performance, multifunctional textiles in both fashion and technical applications.
The automotive sector is also a key driver of market demand for these nanomaterials. Their use in lightweight composites, coatings, and lubricants can contribute to improved fuel efficiency and vehicle performance. As the automotive industry continues to focus on sustainability and electrification, the demand for innovative materials that can address these challenges is expected to grow.
While the market for sodium acetate-based nanomaterials is promising, it is important to note that challenges such as scalability, cost-effectiveness, and regulatory compliance need to be addressed to fully realize their market potential. As research and development efforts continue to overcome these hurdles, the market demand is expected to expand further, opening up new opportunities across various industries.
Current Challenges in Sodium Acetate Nanotech Applications
Despite the promising potential of sodium acetate in nanotechnology material innovation, several significant challenges currently hinder its widespread application and full utilization. These obstacles span across various aspects of research, development, and implementation.
One of the primary challenges lies in the controlled synthesis and manipulation of sodium acetate-based nanomaterials. While sodium acetate offers unique properties for nanostructure formation, achieving precise control over size, shape, and composition remains difficult. This lack of control can lead to inconsistent material properties and performance, limiting the reliability and reproducibility of sodium acetate nanotech applications.
Another major hurdle is the stability of sodium acetate nanomaterials under various environmental conditions. Many potential applications require these materials to maintain their structural integrity and functional properties across a range of temperatures, pH levels, and chemical environments. However, sodium acetate-based nanostructures often exhibit sensitivity to these factors, potentially compromising their long-term stability and effectiveness in real-world scenarios.
The scalability of sodium acetate nanotech production presents another significant challenge. While laboratory-scale synthesis may yield promising results, translating these processes to industrial-scale production often encounters difficulties. Issues such as maintaining uniform quality, optimizing reaction conditions, and developing cost-effective manufacturing methods need to be addressed to enable commercial viability.
Furthermore, the integration of sodium acetate nanomaterials into existing products and systems poses technical challenges. Compatibility issues with other materials, potential interactions that may alter desired properties, and the need for specialized handling and processing techniques all contribute to the complexity of incorporating these innovative materials into practical applications.
The environmental impact and safety concerns associated with sodium acetate nanomaterials also present ongoing challenges. As with many nanomaterials, the potential long-term effects on human health and ecosystems are not fully understood. Addressing these concerns requires extensive toxicological studies and the development of appropriate safety protocols, which can be time-consuming and resource-intensive.
Lastly, the regulatory landscape surrounding nanomaterials, including those based on sodium acetate, remains complex and evolving. Navigating the regulatory requirements for approval and commercialization of nanotech products can be challenging, particularly given the novel nature of these materials and the potential gaps in existing regulatory frameworks.
Overcoming these challenges will require concerted efforts in fundamental research, engineering innovation, and interdisciplinary collaboration. Addressing these issues is crucial for unlocking the full potential of sodium acetate in nanotechnology material innovation and enabling its widespread adoption across various industries and applications.
One of the primary challenges lies in the controlled synthesis and manipulation of sodium acetate-based nanomaterials. While sodium acetate offers unique properties for nanostructure formation, achieving precise control over size, shape, and composition remains difficult. This lack of control can lead to inconsistent material properties and performance, limiting the reliability and reproducibility of sodium acetate nanotech applications.
Another major hurdle is the stability of sodium acetate nanomaterials under various environmental conditions. Many potential applications require these materials to maintain their structural integrity and functional properties across a range of temperatures, pH levels, and chemical environments. However, sodium acetate-based nanostructures often exhibit sensitivity to these factors, potentially compromising their long-term stability and effectiveness in real-world scenarios.
The scalability of sodium acetate nanotech production presents another significant challenge. While laboratory-scale synthesis may yield promising results, translating these processes to industrial-scale production often encounters difficulties. Issues such as maintaining uniform quality, optimizing reaction conditions, and developing cost-effective manufacturing methods need to be addressed to enable commercial viability.
Furthermore, the integration of sodium acetate nanomaterials into existing products and systems poses technical challenges. Compatibility issues with other materials, potential interactions that may alter desired properties, and the need for specialized handling and processing techniques all contribute to the complexity of incorporating these innovative materials into practical applications.
The environmental impact and safety concerns associated with sodium acetate nanomaterials also present ongoing challenges. As with many nanomaterials, the potential long-term effects on human health and ecosystems are not fully understood. Addressing these concerns requires extensive toxicological studies and the development of appropriate safety protocols, which can be time-consuming and resource-intensive.
Lastly, the regulatory landscape surrounding nanomaterials, including those based on sodium acetate, remains complex and evolving. Navigating the regulatory requirements for approval and commercialization of nanotech products can be challenging, particularly given the novel nature of these materials and the potential gaps in existing regulatory frameworks.
Overcoming these challenges will require concerted efforts in fundamental research, engineering innovation, and interdisciplinary collaboration. Addressing these issues is crucial for unlocking the full potential of sodium acetate in nanotechnology material innovation and enabling its widespread adoption across various industries and applications.
Existing Sodium Acetate Nanotech Solutions
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as esterification, saponification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.- Sodium acetate in chemical processes: Sodium acetate is widely used in various chemical processes, including as a catalyst, pH regulator, and reagent in organic synthesis. It plays a crucial role in industrial applications, such as the production of pharmaceuticals, dyes, and other chemical compounds.
- Sodium acetate in heat storage applications: Sodium acetate trihydrate is utilized as a phase change material for thermal energy storage. It has a high latent heat of fusion and can store and release heat energy during phase transitions, making it suitable for use in heating and cooling systems, as well as in thermal management solutions.
- Sodium acetate in food preservation: Sodium acetate is employed as a food preservative and flavoring agent. It helps to control acidity, enhance flavor, and extend the shelf life of various food products. Its use in food applications is regulated and approved by food safety authorities.
- Sodium acetate in textile and leather industries: Sodium acetate finds applications in textile and leather processing. It is used as a dyeing auxiliary, pH regulator, and in the treatment of leather. The compound helps improve the quality and durability of textiles and leather products.
- Sodium acetate in environmental applications: Sodium acetate is utilized in environmental remediation and wastewater treatment processes. It serves as a carbon source for microbial growth in biological treatment systems and can be used to neutralize acidic waste streams. The compound also finds applications in air pollution control and soil remediation.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It undergoes phase changes at specific temperatures, allowing it to store and release heat effectively. This property is exploited in heating pads, building materials, and energy storage solutions.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate finds applications in the food and beverage industry as a preservative, flavoring agent, and acidity regulator. It helps extend shelf life, enhance taste, and maintain product stability in various food products.Expand Specific Solutions04 Application in textile and leather processing
In the textile and leather industries, sodium acetate is used for dyeing, tanning, and finishing processes. It acts as a mordant, pH regulator, and helps improve color fastness and leather quality.Expand Specific Solutions05 Use in environmental and waste treatment
Sodium acetate is employed in environmental applications such as wastewater treatment, soil remediation, and air pollution control. It can act as a carbon source for microbial processes, neutralize acidic waste, and assist in the removal of contaminants.Expand Specific Solutions
Key Players in Sodium Acetate Nanotechnology Research
The sodium acetate nanotechnology material innovation field is in an early development stage, with growing market potential as research advances. The market size is expanding, driven by increasing applications in energy storage, electronics, and biomedical sectors. Technological maturity varies across different applications, with some areas more advanced than others. Key players like Sunamp Ltd., KIST Corp., and Faradion Ltd. are making significant strides in energy storage applications, while universities such as the National University of Singapore and China Agricultural University are contributing to fundamental research. Companies like Wanhua Chemical Group and Nantong Alchemy Biotech are exploring industrial applications. Overall, the field shows promise but requires further development to reach full commercial potential.
Sunamp Ltd.
Technical Solution: Sunamp Ltd. has developed innovative phase change materials (PCMs) using sodium acetate trihydrate for thermal energy storage. Their technology involves encapsulating sodium acetate in a nanoscale matrix, enhancing its thermal properties and stability. This nanostructured PCM demonstrates improved heat transfer rates and cycling stability compared to bulk sodium acetate [1]. The company has successfully integrated this material into their thermal batteries, achieving energy densities up to 4 times higher than traditional water-based systems [2]. Sunamp's approach also includes surface modification of nanoparticles to prevent agglomeration and enhance the overall performance of the PCM [3].
Strengths: High energy density, improved thermal stability, and enhanced heat transfer. Weaknesses: Potential high production costs and scalability challenges for large-scale applications.
KIST Corp. (South Korea)
Technical Solution: KIST Corp. has developed a novel approach using sodium acetate in nanotechnology for advanced energy storage materials. Their research focuses on creating sodium-ion batteries with enhanced performance using sodium acetate-derived carbon anodes. The process involves pyrolysis of sodium acetate to produce hard carbon nanostructures with optimized porosity and surface area [4]. These nanostructured carbons exhibit high sodium storage capacity and excellent cycling stability. KIST's technology also incorporates sodium acetate as a precursor for producing sodium-rich cathode materials, resulting in batteries with improved energy density and longer lifespan [5].
Strengths: Sustainable and cost-effective sodium-ion battery technology, high performance anodes and cathodes. Weaknesses: Still in research phase, may face challenges in scaling up production.
Breakthrough Sodium Acetate Nanotech Innovations
Continuous process for the preparation of sodium titanate nanotubes
PatentActiveEP1988059A3
Innovation
- A continuous process using multiple reactors connected in series, operated at different temperatures, with mechanical stirring to increase the solids content and control the specific surface area of sodium titanate nanotubes, allowing for high-yield production of nanotubes with controlled dimensions and surface area.
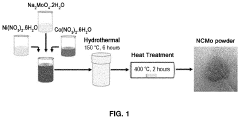
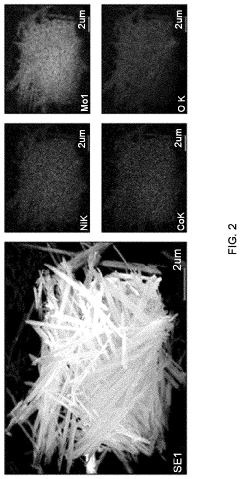
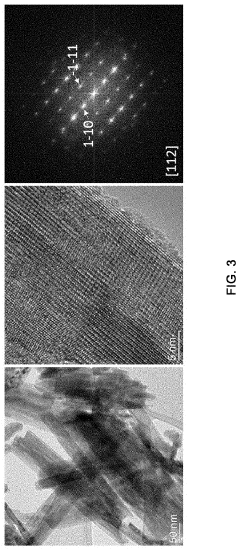
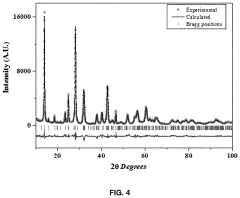
Anode electrode active material for sodium secondary battery comprising nickel cobalt molybdenum oxide, anode electrode for sodium secondary battery comprising same, sodium secondary battery including anode electrode for sodium secondary battery, and method for manufacturing same
PatentActiveUS20230146801A1
Innovation
- A novel anode electrode material composed of nickel cobalt molybdenum oxide (NixCo1-xMoO4) synthesized via a one-pot hydrothermal process, forming a single-phase nanorod structure that enables stable intercalation/deintercalation of sodium ions without significant volume change, reducing electrode damage and enhancing electrochemical characteristics.
Environmental Impact of Sodium Acetate Nanomaterials
The environmental impact of sodium acetate nanomaterials is a critical consideration in the development and application of these innovative materials. As nanotechnology continues to advance, the potential ecological consequences of sodium acetate-based nanomaterials must be thoroughly evaluated to ensure sustainable and responsible implementation.
One of the primary concerns regarding sodium acetate nanomaterials is their potential release into aquatic ecosystems. These nanoparticles may enter water bodies through various pathways, including industrial effluents, consumer product disposal, and atmospheric deposition. Once in aquatic environments, sodium acetate nanomaterials can interact with organisms and potentially disrupt ecological balance. Studies have shown that certain aquatic species may accumulate these nanoparticles, leading to bioaccumulation and potential biomagnification up the food chain.
The fate and behavior of sodium acetate nanomaterials in soil ecosystems are also of significant interest. These materials may alter soil properties, affecting nutrient cycling and microbial communities. Research has indicated that sodium acetate nanoparticles can influence soil pH and ionic strength, potentially impacting plant growth and soil fertility. Furthermore, the interaction between these nanomaterials and soil organic matter may lead to changes in soil structure and water retention capabilities.
Air quality is another environmental aspect to consider when assessing the impact of sodium acetate nanomaterials. The production and handling of these materials may result in the release of nanoparticles into the atmosphere. Airborne sodium acetate nanoparticles can potentially contribute to air pollution and may pose inhalation risks to both humans and wildlife. The long-term effects of chronic exposure to these airborne nanoparticles on respiratory health and ecosystem functioning require further investigation.
The biodegradability and persistence of sodium acetate nanomaterials in the environment are crucial factors in determining their overall ecological impact. While sodium acetate itself is generally considered biodegradable, the nanoscale properties of these materials may alter their degradation patterns. Some studies suggest that certain sodium acetate nanostructures may exhibit increased environmental persistence compared to their bulk counterparts, potentially leading to long-term accumulation in ecosystems.
To mitigate potential environmental risks, it is essential to develop comprehensive risk assessment frameworks specifically tailored for sodium acetate nanomaterials. These frameworks should consider the unique properties of these materials, their potential transformations in different environmental compartments, and their interactions with biota. Additionally, the development of eco-friendly synthesis methods and the implementation of effective waste management strategies for sodium acetate nanomaterials are crucial steps towards minimizing their environmental footprint.
One of the primary concerns regarding sodium acetate nanomaterials is their potential release into aquatic ecosystems. These nanoparticles may enter water bodies through various pathways, including industrial effluents, consumer product disposal, and atmospheric deposition. Once in aquatic environments, sodium acetate nanomaterials can interact with organisms and potentially disrupt ecological balance. Studies have shown that certain aquatic species may accumulate these nanoparticles, leading to bioaccumulation and potential biomagnification up the food chain.
The fate and behavior of sodium acetate nanomaterials in soil ecosystems are also of significant interest. These materials may alter soil properties, affecting nutrient cycling and microbial communities. Research has indicated that sodium acetate nanoparticles can influence soil pH and ionic strength, potentially impacting plant growth and soil fertility. Furthermore, the interaction between these nanomaterials and soil organic matter may lead to changes in soil structure and water retention capabilities.
Air quality is another environmental aspect to consider when assessing the impact of sodium acetate nanomaterials. The production and handling of these materials may result in the release of nanoparticles into the atmosphere. Airborne sodium acetate nanoparticles can potentially contribute to air pollution and may pose inhalation risks to both humans and wildlife. The long-term effects of chronic exposure to these airborne nanoparticles on respiratory health and ecosystem functioning require further investigation.
The biodegradability and persistence of sodium acetate nanomaterials in the environment are crucial factors in determining their overall ecological impact. While sodium acetate itself is generally considered biodegradable, the nanoscale properties of these materials may alter their degradation patterns. Some studies suggest that certain sodium acetate nanostructures may exhibit increased environmental persistence compared to their bulk counterparts, potentially leading to long-term accumulation in ecosystems.
To mitigate potential environmental risks, it is essential to develop comprehensive risk assessment frameworks specifically tailored for sodium acetate nanomaterials. These frameworks should consider the unique properties of these materials, their potential transformations in different environmental compartments, and their interactions with biota. Additionally, the development of eco-friendly synthesis methods and the implementation of effective waste management strategies for sodium acetate nanomaterials are crucial steps towards minimizing their environmental footprint.
Scalability of Sodium Acetate Nanotech Production
The scalability of sodium acetate nanotech production is a critical factor in determining the widespread adoption and commercial viability of this innovative material. As research progresses, several key aspects have emerged that influence the ability to scale up production from laboratory to industrial levels.
One of the primary considerations is the development of efficient synthesis methods that can be easily adapted to large-scale manufacturing processes. Current approaches, such as sol-gel techniques and hydrothermal synthesis, have shown promise in producing sodium acetate-based nanomaterials with controlled morphologies and properties. However, these methods often require precise control of reaction conditions, which can be challenging to maintain in large-scale reactors.
To address this challenge, researchers are exploring continuous flow synthesis techniques that allow for better control of reaction parameters and improved reproducibility. These methods have the potential to significantly increase production rates while maintaining product quality. Additionally, the use of microfluidic devices for nanoparticle synthesis has shown promising results in terms of scalability and uniformity of the produced materials.
Another crucial aspect of scaling up sodium acetate nanotech production is the development of cost-effective and sustainable raw material sourcing strategies. As demand for these materials increases, ensuring a stable and economical supply of high-purity sodium acetate and other precursors becomes essential. This may involve collaborations with chemical manufacturers to optimize production processes and reduce costs.
The scalability of purification and post-processing steps is equally important in the production chain. Techniques such as centrifugation, filtration, and dialysis, which are commonly used in laboratory-scale synthesis, may not be practical for industrial-scale production. As a result, there is ongoing research into developing more efficient separation and purification methods, such as membrane-based technologies and continuous flow purification systems.
Environmental considerations and waste management also play a significant role in scaling up production. Developing green synthesis routes that minimize the use of harmful solvents and reduce waste generation is crucial for sustainable large-scale manufacturing. This includes exploring the potential for recycling and reusing reaction byproducts, as well as implementing closed-loop production systems.
As the field progresses, addressing these scalability challenges will be crucial for realizing the full potential of sodium acetate-based nanomaterials in various applications, from energy storage to biomedical technologies. Continued research and development efforts, coupled with industry collaborations, will be essential in overcoming these hurdles and paving the way for widespread commercialization of these innovative materials.
One of the primary considerations is the development of efficient synthesis methods that can be easily adapted to large-scale manufacturing processes. Current approaches, such as sol-gel techniques and hydrothermal synthesis, have shown promise in producing sodium acetate-based nanomaterials with controlled morphologies and properties. However, these methods often require precise control of reaction conditions, which can be challenging to maintain in large-scale reactors.
To address this challenge, researchers are exploring continuous flow synthesis techniques that allow for better control of reaction parameters and improved reproducibility. These methods have the potential to significantly increase production rates while maintaining product quality. Additionally, the use of microfluidic devices for nanoparticle synthesis has shown promising results in terms of scalability and uniformity of the produced materials.
Another crucial aspect of scaling up sodium acetate nanotech production is the development of cost-effective and sustainable raw material sourcing strategies. As demand for these materials increases, ensuring a stable and economical supply of high-purity sodium acetate and other precursors becomes essential. This may involve collaborations with chemical manufacturers to optimize production processes and reduce costs.
The scalability of purification and post-processing steps is equally important in the production chain. Techniques such as centrifugation, filtration, and dialysis, which are commonly used in laboratory-scale synthesis, may not be practical for industrial-scale production. As a result, there is ongoing research into developing more efficient separation and purification methods, such as membrane-based technologies and continuous flow purification systems.
Environmental considerations and waste management also play a significant role in scaling up production. Developing green synthesis routes that minimize the use of harmful solvents and reduce waste generation is crucial for sustainable large-scale manufacturing. This includes exploring the potential for recycling and reusing reaction byproducts, as well as implementing closed-loop production systems.
As the field progresses, addressing these scalability challenges will be crucial for realizing the full potential of sodium acetate-based nanomaterials in various applications, from energy storage to biomedical technologies. Continued research and development efforts, coupled with industry collaborations, will be essential in overcoming these hurdles and paving the way for widespread commercialization of these innovative materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!