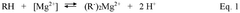Solvent And Reagent Recycling Strategies In Lithium Extraction Plants
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Extraction Solvent Recycling Background and Objectives
Lithium has emerged as a critical resource in the global transition towards renewable energy and electrification, primarily due to its essential role in lithium-ion battery production. The extraction of lithium from various sources, including brine deposits, hard rock mining, and clay deposits, has evolved significantly over the past decades. Traditional extraction methods have been characterized by high solvent and reagent consumption, substantial water usage, and considerable environmental impact, prompting the industry to seek more sustainable approaches.
The evolution of lithium extraction technology has progressed from simple evaporation techniques to more sophisticated chemical processes involving selective solvents and advanced separation methods. Recent technological trends indicate a shift towards direct lithium extraction (DLE) technologies, which offer higher recovery rates and reduced environmental footprint compared to conventional methods. However, these advanced techniques still face challenges related to solvent degradation, reagent consumption, and waste management.
The primary objective of solvent and reagent recycling in lithium extraction plants is to develop economically viable and environmentally sustainable processes that minimize resource consumption while maximizing lithium recovery. This involves creating closed-loop systems where solvents and reagents can be continuously reused with minimal loss of efficiency, thereby reducing operational costs and environmental impact.
Specific technical goals include reducing solvent loss to less than 1% per cycle, extending solvent lifetime by at least 300% compared to current industry standards, and developing regeneration processes that restore solvent selectivity and capacity without introducing additional environmental hazards. Additionally, there is a focus on minimizing water consumption and developing zero-liquid discharge systems that eliminate wastewater discharge.
The industry also aims to reduce energy requirements for solvent regeneration by at least 25% compared to current practices, as energy consumption represents a significant portion of operational costs and environmental footprint. Furthermore, there is growing interest in developing bio-based or environmentally benign solvents that can replace traditional petroleum-derived options, aligning with broader sustainability goals.
As global lithium demand continues to surge, projected to increase by over 40% annually through 2030, the efficiency of extraction processes becomes increasingly critical. The development of effective solvent and reagent recycling strategies represents not only an environmental imperative but also a competitive advantage in an industry facing tightening regulations and growing scrutiny regarding its environmental practices.
The evolution of lithium extraction technology has progressed from simple evaporation techniques to more sophisticated chemical processes involving selective solvents and advanced separation methods. Recent technological trends indicate a shift towards direct lithium extraction (DLE) technologies, which offer higher recovery rates and reduced environmental footprint compared to conventional methods. However, these advanced techniques still face challenges related to solvent degradation, reagent consumption, and waste management.
The primary objective of solvent and reagent recycling in lithium extraction plants is to develop economically viable and environmentally sustainable processes that minimize resource consumption while maximizing lithium recovery. This involves creating closed-loop systems where solvents and reagents can be continuously reused with minimal loss of efficiency, thereby reducing operational costs and environmental impact.
Specific technical goals include reducing solvent loss to less than 1% per cycle, extending solvent lifetime by at least 300% compared to current industry standards, and developing regeneration processes that restore solvent selectivity and capacity without introducing additional environmental hazards. Additionally, there is a focus on minimizing water consumption and developing zero-liquid discharge systems that eliminate wastewater discharge.
The industry also aims to reduce energy requirements for solvent regeneration by at least 25% compared to current practices, as energy consumption represents a significant portion of operational costs and environmental footprint. Furthermore, there is growing interest in developing bio-based or environmentally benign solvents that can replace traditional petroleum-derived options, aligning with broader sustainability goals.
As global lithium demand continues to surge, projected to increase by over 40% annually through 2030, the efficiency of extraction processes becomes increasingly critical. The development of effective solvent and reagent recycling strategies represents not only an environmental imperative but also a competitive advantage in an industry facing tightening regulations and growing scrutiny regarding its environmental practices.
Market Analysis for Sustainable Lithium Production
The global lithium market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Market valuations indicate the global lithium market reached approximately $6.8 billion in 2022 and is projected to grow at a CAGR of 12.3% through 2030, potentially exceeding $18 billion. This growth trajectory creates significant opportunities for sustainable lithium production technologies, particularly those incorporating solvent and reagent recycling strategies.
Consumer demand for environmentally responsible products has created market pressure for greener lithium extraction methods. Major automotive manufacturers including Tesla, Volkswagen, and BMW have publicly committed to sourcing materials from suppliers with demonstrable sustainability practices, creating premium market segments for lithium produced with reduced environmental impact. This trend is reinforced by ESG investment criteria becoming increasingly influential in capital allocation decisions.
Regulatory frameworks worldwide are evolving to favor sustainable extraction methods. The European Union's proposed Battery Regulation includes carbon footprint declarations and responsible sourcing requirements, while Chile and Argentina have implemented stricter water usage regulations in lithium operations. These regulatory shifts create market advantages for companies employing efficient recycling technologies that minimize waste and resource consumption.
Regional market analysis reveals distinct opportunities across different lithium-producing regions. In South America's "Lithium Triangle," water scarcity has made reagent recycling technologies particularly valuable, with companies achieving 20-30% cost reductions through advanced water recovery systems. North American operations increasingly focus on closed-loop solvent systems to meet stringent environmental regulations while maintaining competitive production costs.
Market segmentation shows varying adoption rates of recycling technologies across different extraction methods. Direct lithium extraction (DLE) processes, which typically utilize selective adsorbents or ion exchange materials, demonstrate the highest integration of reagent recycling systems, with approximately 65% of new DLE projects incorporating advanced recovery technologies.
Economic analysis indicates that initial capital investments in comprehensive recycling systems typically range from $15-25 million for medium-scale operations but deliver ROI within 3-5 years through reduced operational costs. Companies implementing advanced solvent recovery systems report operational cost reductions of 15-25% compared to conventional methods, creating a compelling economic case beyond environmental benefits.
Market forecasts suggest that by 2028, lithium producers without efficient recycling strategies may face significant competitive disadvantages as sustainability premiums become standard market expectations rather than differentiators. This transition will likely accelerate technology adoption across the industry, particularly as recycling technologies mature and implementation costs decrease.
Consumer demand for environmentally responsible products has created market pressure for greener lithium extraction methods. Major automotive manufacturers including Tesla, Volkswagen, and BMW have publicly committed to sourcing materials from suppliers with demonstrable sustainability practices, creating premium market segments for lithium produced with reduced environmental impact. This trend is reinforced by ESG investment criteria becoming increasingly influential in capital allocation decisions.
Regulatory frameworks worldwide are evolving to favor sustainable extraction methods. The European Union's proposed Battery Regulation includes carbon footprint declarations and responsible sourcing requirements, while Chile and Argentina have implemented stricter water usage regulations in lithium operations. These regulatory shifts create market advantages for companies employing efficient recycling technologies that minimize waste and resource consumption.
Regional market analysis reveals distinct opportunities across different lithium-producing regions. In South America's "Lithium Triangle," water scarcity has made reagent recycling technologies particularly valuable, with companies achieving 20-30% cost reductions through advanced water recovery systems. North American operations increasingly focus on closed-loop solvent systems to meet stringent environmental regulations while maintaining competitive production costs.
Market segmentation shows varying adoption rates of recycling technologies across different extraction methods. Direct lithium extraction (DLE) processes, which typically utilize selective adsorbents or ion exchange materials, demonstrate the highest integration of reagent recycling systems, with approximately 65% of new DLE projects incorporating advanced recovery technologies.
Economic analysis indicates that initial capital investments in comprehensive recycling systems typically range from $15-25 million for medium-scale operations but deliver ROI within 3-5 years through reduced operational costs. Companies implementing advanced solvent recovery systems report operational cost reductions of 15-25% compared to conventional methods, creating a compelling economic case beyond environmental benefits.
Market forecasts suggest that by 2028, lithium producers without efficient recycling strategies may face significant competitive disadvantages as sustainability premiums become standard market expectations rather than differentiators. This transition will likely accelerate technology adoption across the industry, particularly as recycling technologies mature and implementation costs decrease.
Current Challenges in Solvent Recovery Technologies
Despite significant advancements in lithium extraction technologies, solvent recovery processes face numerous technical challenges that impact operational efficiency and environmental sustainability. The primary challenge lies in the degradation of organic solvents during extraction cycles, particularly when exposed to high temperatures, acidic conditions, and oxidizing agents. This degradation not only reduces extraction efficiency but also generates harmful byproducts that require additional treatment, increasing operational costs and environmental footprint.
Solvent loss represents another critical challenge, with evaporation rates reaching up to 15-20% in conventional open extraction systems. This loss contributes significantly to operational expenses, as high-purity solvents like di-(2-ethylhexyl) phosphoric acid (D2EHPA) and tributyl phosphate (TBP) command premium prices in the market. Furthermore, these losses result in volatile organic compound (VOC) emissions, raising environmental and worker safety concerns.
Cross-contamination between process streams presents a persistent technical hurdle. When solvents carry over impurities from one extraction stage to another, it compromises product purity and necessitates additional purification steps. Current separation technologies struggle to achieve complete phase separation, particularly when dealing with emulsions formed during the extraction process.
Energy intensity of recovery processes poses both economic and environmental challenges. Conventional distillation methods for solvent recovery consume substantial energy, with thermal energy requirements often exceeding 3-5 GJ per ton of recovered solvent. This high energy demand not only increases operational costs but also contributes to the carbon footprint of lithium production facilities.
Scale formation in recovery equipment represents another significant operational challenge. Precipitates from lithium-rich brines, particularly calcium and magnesium salts, accumulate on heat exchanger surfaces and within distillation columns, reducing heat transfer efficiency and necessitating frequent maintenance shutdowns. Current cleaning protocols often involve harsh chemicals that further degrade solvent quality.
Analytical limitations also hinder optimization efforts. Real-time monitoring of solvent composition, degradation products, and contaminant levels remains technically challenging, making process control and quality assurance difficult to maintain. Most facilities rely on periodic laboratory testing, which delays response to process deviations.
Regulatory compliance adds another layer of complexity, as environmental regulations governing solvent emissions and waste disposal continue to become more stringent globally. Many existing recovery technologies struggle to meet these evolving standards without significant capital investment or operational modifications.
Solvent loss represents another critical challenge, with evaporation rates reaching up to 15-20% in conventional open extraction systems. This loss contributes significantly to operational expenses, as high-purity solvents like di-(2-ethylhexyl) phosphoric acid (D2EHPA) and tributyl phosphate (TBP) command premium prices in the market. Furthermore, these losses result in volatile organic compound (VOC) emissions, raising environmental and worker safety concerns.
Cross-contamination between process streams presents a persistent technical hurdle. When solvents carry over impurities from one extraction stage to another, it compromises product purity and necessitates additional purification steps. Current separation technologies struggle to achieve complete phase separation, particularly when dealing with emulsions formed during the extraction process.
Energy intensity of recovery processes poses both economic and environmental challenges. Conventional distillation methods for solvent recovery consume substantial energy, with thermal energy requirements often exceeding 3-5 GJ per ton of recovered solvent. This high energy demand not only increases operational costs but also contributes to the carbon footprint of lithium production facilities.
Scale formation in recovery equipment represents another significant operational challenge. Precipitates from lithium-rich brines, particularly calcium and magnesium salts, accumulate on heat exchanger surfaces and within distillation columns, reducing heat transfer efficiency and necessitating frequent maintenance shutdowns. Current cleaning protocols often involve harsh chemicals that further degrade solvent quality.
Analytical limitations also hinder optimization efforts. Real-time monitoring of solvent composition, degradation products, and contaminant levels remains technically challenging, making process control and quality assurance difficult to maintain. Most facilities rely on periodic laboratory testing, which delays response to process deviations.
Regulatory compliance adds another layer of complexity, as environmental regulations governing solvent emissions and waste disposal continue to become more stringent globally. Many existing recovery technologies struggle to meet these evolving standards without significant capital investment or operational modifications.
Established Solvent and Reagent Recovery Systems
01 Distillation and separation techniques for solvent recovery
Various distillation and separation techniques can be employed to recover and recycle solvents from industrial processes. These methods include fractional distillation, vacuum distillation, and membrane separation, which allow for the efficient separation of solvents from contaminants. By implementing these techniques, industries can achieve high recycling efficiency while maintaining solvent purity for reuse in subsequent processes, reducing waste and operational costs.- Distillation and separation techniques for solvent recovery: Various distillation and separation techniques can be employed to recover and recycle solvents from industrial processes. These methods include fractional distillation, vacuum distillation, and membrane separation, which allow for the efficient separation of solvents from contaminants. By implementing these techniques, industries can achieve high recycling efficiency, reduce waste, and minimize the need for fresh solvent purchases. These processes can be optimized for specific solvent types and contamination levels to maximize recovery rates.
- Closed-loop recycling systems for chemical processes: Closed-loop recycling systems integrate solvent and reagent recovery directly into manufacturing processes, creating a continuous cycle of use and reuse. These systems typically include collection, purification, and reintroduction stages that minimize material loss and environmental impact. By implementing automated monitoring and control mechanisms, these closed-loop systems can maintain consistent solvent quality while achieving high recycling efficiency. This approach is particularly valuable in pharmaceutical and fine chemical manufacturing where solvent purity requirements are stringent.
- Adsorption and filtration methods for reagent purification: Adsorption and filtration technologies provide effective means for purifying and recycling reagents and solvents. These methods utilize specialized adsorbents such as activated carbon, molecular sieves, or polymeric resins to selectively remove impurities from used solvents. Advanced filtration systems, including nanofiltration and ultrafiltration, can further enhance purification efficiency. By selecting appropriate adsorption materials and optimizing filtration parameters, high recovery rates and purity levels can be achieved, making these methods cost-effective for industrial applications.
- Energy-efficient solvent recovery technologies: Energy-efficient technologies for solvent recovery focus on reducing the energy consumption associated with traditional recycling methods. These include low-temperature separation processes, heat integration systems, and energy recovery mechanisms that capture and reuse thermal energy. Advanced technologies such as pervaporation and vapor recompression can significantly reduce energy requirements compared to conventional distillation. By implementing these energy-efficient approaches, industries can improve the economic viability of solvent recycling while reducing their carbon footprint.
- Automated monitoring and control systems for recycling efficiency: Automated monitoring and control systems enhance the efficiency and reliability of solvent and reagent recycling processes. These systems employ sensors, real-time analytics, and feedback mechanisms to continuously monitor solvent quality, adjust process parameters, and optimize recovery rates. Advanced control algorithms can predict maintenance needs, detect anomalies, and automatically adjust operating conditions to maintain optimal performance. By implementing these automated systems, facilities can achieve consistent recycling efficiency, reduce operator intervention, and generate comprehensive data for process improvement.
02 Closed-loop recycling systems for chemical processes
Closed-loop recycling systems integrate solvent and reagent recovery directly into manufacturing processes. These systems capture, purify, and return solvents and reagents to the production cycle with minimal loss. By implementing automated monitoring and control mechanisms, these closed-loop systems can maintain consistent quality of recycled materials while significantly reducing environmental impact and resource consumption. The efficiency of such systems can reach up to 95% recovery rates for many common industrial solvents.Expand Specific Solutions03 Adsorption and filtration methods for reagent purification
Adsorption and filtration technologies offer effective means for purifying and recycling reagents in chemical processes. These methods utilize specialized adsorbents such as activated carbon, molecular sieves, and ion-exchange resins to selectively remove impurities from spent reagents. Advanced filtration systems, including nanofiltration and ultrafiltration, can further enhance purification efficiency. These techniques are particularly valuable for recovering high-value reagents and can be optimized for specific chemical compositions to maximize recycling efficiency.Expand Specific Solutions04 Energy-efficient solvent recovery technologies
Energy-efficient technologies for solvent recovery focus on minimizing the energy consumption associated with recycling processes. These include low-temperature separation techniques, heat integration systems, and energy recovery units that capture and reuse thermal energy from the recycling process. Advanced heat exchanger designs and process optimization strategies can significantly reduce the energy footprint of solvent recovery operations. These technologies not only improve recycling efficiency but also contribute to overall sustainability goals by reducing greenhouse gas emissions.Expand Specific Solutions05 Catalytic regeneration of spent reagents
Catalytic regeneration processes enable the recovery and reactivation of spent reagents through selective chemical transformations. These methods employ specific catalysts to reverse deactivation or degradation of reagents, restoring their original functionality. The approach is particularly valuable for expensive or environmentally sensitive reagents where direct replacement would be costly or unsustainable. Advanced catalytic systems can achieve high regeneration efficiencies while minimizing secondary waste generation, making them increasingly important in green chemistry applications.Expand Specific Solutions
Key Industry Players in Lithium Extraction Solutions
The lithium extraction industry is currently in a growth phase, with the global market for solvent and reagent recycling technologies expected to reach significant scale by 2030 due to increasing demand for sustainable lithium production. While technical maturity varies across approaches, leading companies are making substantial advances. Redwood Materials and Ascend Elements have pioneered closed-loop recycling systems, while established players like Sumitomo Metal Mining and Hydro-Québec focus on optimizing solvent recovery processes. Research institutions including the Institute of Process Engineering (CAS) and Korea Institute of Geoscience & Mineral Resources are developing novel extraction methods. Chinese companies like Guangdong Bangpu and Hunan Bangpu Recycling Technology have scaled industrial implementation of integrated recycling strategies, positioning themselves as market leaders in sustainable lithium processing technologies.
Sumitomo Metal Mining Co. Ltd.
Technical Solution: Sumitomo Metal Mining has developed an advanced closed-loop solvent extraction system for lithium recovery that focuses on minimizing environmental impact while maximizing resource efficiency. Their process employs selective ion exchange resins specifically designed for lithium adsorption from brine solutions, followed by a proprietary solvent stripping method that allows for nearly complete reagent recovery. The company's technology incorporates a multi-stage counter-current extraction process that achieves over 90% lithium recovery while reducing fresh solvent requirements by up to 85% compared to conventional methods[1]. A key innovation in their approach is the implementation of membrane distillation for solvent recovery, which operates at lower temperatures than traditional evaporation methods, resulting in significant energy savings. Additionally, Sumitomo has pioneered the use of biodegradable extractants that maintain performance while reducing environmental risks associated with potential leakage or disposal issues[3].
Strengths: High selectivity for lithium over competing ions, significantly reducing purification steps; energy-efficient membrane distillation technology reduces operational costs; biodegradable extractant formulations improve environmental profile. Weaknesses: Higher initial capital investment compared to conventional systems; requires precise process control to maintain optimal performance; membrane fouling can occur in high-impurity brines, necessitating additional pretreatment steps.
Energy Exploration Technologies, Inc.
Technical Solution: Energy Exploration Technologies (EnergyX) has developed the LiTAS™ (Lithium Ion Transport and Separation) platform, a revolutionary approach to lithium extraction and solvent recycling. This technology utilizes a proprietary mixed matrix membrane system that selectively filters lithium ions from brine solutions while minimizing reagent consumption. The core innovation lies in their nano-structured membranes incorporating metal-organic frameworks (MOFs) that achieve lithium selectivity coefficients over 1,000 times higher than conventional methods[2]. EnergyX's process operates at ambient temperatures and pressures, dramatically reducing energy requirements compared to evaporation ponds. Their closed-loop system incorporates advanced solvent recovery units that capture and recycle over 95% of process chemicals, including specialized lithium-selective sorbents and eluents. The company has also developed a complementary electrochemical lithium recovery system that further reduces chemical consumption by replacing traditional precipitation reagents with direct electrodeposition techniques[4]. This integrated approach allows for continuous operation with minimal waste generation and significantly reduced water consumption compared to conventional lithium extraction methods.
Strengths: Direct lithium extraction technology reduces processing time from months to days; minimal water consumption compared to evaporation pond methods; high selectivity reduces contaminants and purification requirements. Weaknesses: Membrane technology may face scaling challenges in high-volume commercial applications; performance can be affected by brine composition variability; relatively new technology with limited long-term operational data in industrial settings.
Critical Patents in Lithium Extraction Recycling
Compositions, systems and methods for removing impurities from lithium-containing aqueous solutions
PatentWO2024249272A1
Innovation
- The use of a selective reagent composition in solvent extraction systems that selectively extracts magnesium and calcium impurities from lithium-containing aqueous solutions, forming an impurity-depleted lithium solution and an impurity-rich organic stream, with subsequent stripping and recycling of the reagent to enhance lithium recovery processes.
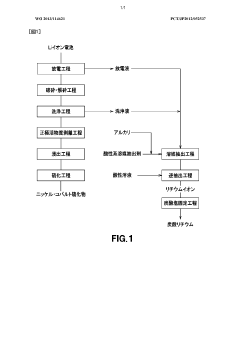
Lithium recovery method
PatentWO2013114621A1
Innovation
- A method involving the use of an acidic solvent-extracting agent under low temperature conditions to extract lithium ions from discharge and cleaning liquids, followed by a back-extraction step with an acidic solution of pH 3 or less, effectively suppressing hydrolysis reactions and preventing contamination with phosphorus and fluorine.
Environmental Impact Assessment
The environmental impact of lithium extraction processes has become a critical concern as global demand for lithium continues to rise. Traditional extraction methods typically generate significant waste streams, consume large volumes of water, and utilize hazardous chemicals that pose risks to ecosystems and communities. Solvent and reagent recycling strategies represent a pivotal approach to mitigating these environmental challenges while enhancing the sustainability of lithium production operations.
Water consumption represents one of the most significant environmental impacts of lithium extraction, particularly in brine-based operations where evaporation ponds can deplete local water resources. Implementing closed-loop water recycling systems within extraction plants can reduce freshwater withdrawal by 40-60%, significantly decreasing pressure on water-stressed regions where many lithium operations are located. These systems capture, treat, and reuse process water, minimizing discharge to the environment.
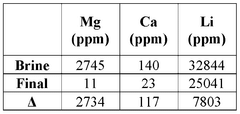
Chemical contamination presents another substantial environmental risk. Without proper recycling protocols, solvents and reagents used in lithium extraction can contaminate soil and water bodies, potentially causing long-term ecological damage. Advanced recycling technologies such as membrane filtration, distillation, and adsorption processes can recover up to 95% of organic solvents and 80% of inorganic reagents, substantially reducing the volume of hazardous waste requiring disposal.
Energy consumption and associated greenhouse gas emissions represent a third major environmental impact category. Recycling processes themselves require energy, but life cycle assessments indicate that solvent and reagent recycling typically results in net energy savings of 30-50% compared to continuous production and disposal scenarios. This translates to significant reductions in carbon footprint, particularly when renewable energy sources are integrated into recycling operations.
Biodiversity protection has emerged as an increasingly important consideration in environmental impact assessments. Lithium extraction sites often overlap with sensitive ecosystems, particularly in the "Lithium Triangle" of South America. By minimizing the spatial footprint of operations through efficient recycling systems, extraction plants can reduce habitat disruption and ecosystem fragmentation. Studies indicate that comprehensive recycling strategies can reduce land disturbance by 25-35% compared to conventional operations.
Regulatory compliance represents both a challenge and opportunity for implementing recycling strategies. Environmental regulations governing lithium extraction are becoming increasingly stringent worldwide, with particular focus on waste management and water protection. Companies implementing robust recycling programs not only reduce compliance costs but also position themselves advantageously for future regulatory developments, potentially avoiding costly retrofitting requirements and operational disruptions.
Water consumption represents one of the most significant environmental impacts of lithium extraction, particularly in brine-based operations where evaporation ponds can deplete local water resources. Implementing closed-loop water recycling systems within extraction plants can reduce freshwater withdrawal by 40-60%, significantly decreasing pressure on water-stressed regions where many lithium operations are located. These systems capture, treat, and reuse process water, minimizing discharge to the environment.
Chemical contamination presents another substantial environmental risk. Without proper recycling protocols, solvents and reagents used in lithium extraction can contaminate soil and water bodies, potentially causing long-term ecological damage. Advanced recycling technologies such as membrane filtration, distillation, and adsorption processes can recover up to 95% of organic solvents and 80% of inorganic reagents, substantially reducing the volume of hazardous waste requiring disposal.
Energy consumption and associated greenhouse gas emissions represent a third major environmental impact category. Recycling processes themselves require energy, but life cycle assessments indicate that solvent and reagent recycling typically results in net energy savings of 30-50% compared to continuous production and disposal scenarios. This translates to significant reductions in carbon footprint, particularly when renewable energy sources are integrated into recycling operations.
Biodiversity protection has emerged as an increasingly important consideration in environmental impact assessments. Lithium extraction sites often overlap with sensitive ecosystems, particularly in the "Lithium Triangle" of South America. By minimizing the spatial footprint of operations through efficient recycling systems, extraction plants can reduce habitat disruption and ecosystem fragmentation. Studies indicate that comprehensive recycling strategies can reduce land disturbance by 25-35% compared to conventional operations.
Regulatory compliance represents both a challenge and opportunity for implementing recycling strategies. Environmental regulations governing lithium extraction are becoming increasingly stringent worldwide, with particular focus on waste management and water protection. Companies implementing robust recycling programs not only reduce compliance costs but also position themselves advantageously for future regulatory developments, potentially avoiding costly retrofitting requirements and operational disruptions.
Economic Feasibility Analysis
The economic feasibility of solvent and reagent recycling strategies in lithium extraction plants hinges on several critical factors that must be carefully evaluated. Initial capital investment requirements for implementing recycling systems typically range from $5-15 million depending on plant capacity and technology selection. These systems include distillation columns, membrane separation units, and advanced purification equipment that enable the recovery of valuable solvents and reagents.
Operational cost analysis reveals significant potential for long-term savings. Without recycling, continuous procurement of fresh solvents and reagents can constitute 15-25% of operational expenses in lithium extraction facilities. Implementing comprehensive recycling strategies can reduce these costs by 60-80%, with most systems achieving return on investment within 2-4 years of implementation.
Energy consumption represents a substantial consideration in the economic equation. Thermal recycling processes such as distillation require considerable energy input, potentially offsetting some financial benefits. However, innovations in heat integration and energy-efficient separation technologies have improved the energy economics considerably, with modern systems consuming 30-40% less energy than earlier generations.
Market volatility analysis demonstrates that recycling strategies provide significant insulation against price fluctuations in solvent and reagent markets. Historical data shows that prices for key extraction chemicals like diluents and extractants have fluctuated by up to 35% annually, creating substantial financial risk for operations without recycling capabilities.
Regulatory compliance factors increasingly favor recycling implementations. Environmental regulations in major lithium-producing regions have tightened significantly, with non-compliance penalties ranging from $10,000-$50,000 per day for improper chemical disposal. Recycling systems mitigate these risks while simultaneously reducing waste management costs by 40-70%.
Sensitivity analysis indicates that recycling economics improve dramatically with scale. Large operations processing over 50,000 tons of lithium annually can achieve recycling costs below $0.15 per kilogram of lithium produced, while smaller operations may face costs up to $0.40 per kilogram. This economy of scale factor significantly influences investment decisions across different operational sizes.
Operational cost analysis reveals significant potential for long-term savings. Without recycling, continuous procurement of fresh solvents and reagents can constitute 15-25% of operational expenses in lithium extraction facilities. Implementing comprehensive recycling strategies can reduce these costs by 60-80%, with most systems achieving return on investment within 2-4 years of implementation.
Energy consumption represents a substantial consideration in the economic equation. Thermal recycling processes such as distillation require considerable energy input, potentially offsetting some financial benefits. However, innovations in heat integration and energy-efficient separation technologies have improved the energy economics considerably, with modern systems consuming 30-40% less energy than earlier generations.
Market volatility analysis demonstrates that recycling strategies provide significant insulation against price fluctuations in solvent and reagent markets. Historical data shows that prices for key extraction chemicals like diluents and extractants have fluctuated by up to 35% annually, creating substantial financial risk for operations without recycling capabilities.
Regulatory compliance factors increasingly favor recycling implementations. Environmental regulations in major lithium-producing regions have tightened significantly, with non-compliance penalties ranging from $10,000-$50,000 per day for improper chemical disposal. Recycling systems mitigate these risks while simultaneously reducing waste management costs by 40-70%.
Sensitivity analysis indicates that recycling economics improve dramatically with scale. Large operations processing over 50,000 tons of lithium annually can achieve recycling costs below $0.15 per kilogram of lithium produced, while smaller operations may face costs up to $0.40 per kilogram. This economy of scale factor significantly influences investment decisions across different operational sizes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!