Testing Neodymium Magnet Effects on Biomedical Device Reliability
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neodymium Magnet Interference Background and Objectives
Neodymium magnets, discovered in the 1980s by General Motors and Sumitomo Special Metals, represent a significant advancement in permanent magnet technology. These rare-earth magnets, composed primarily of neodymium, iron, and boron (Nd₂Fe₁₄B), possess magnetic field strengths up to ten times greater than traditional ferrite magnets. Their exceptional magnetic properties have led to widespread adoption across various industries, including consumer electronics, automotive applications, and notably, the biomedical sector.
The integration of neodymium magnets in biomedical devices has evolved considerably over the past three decades. Initially limited to external applications, these powerful magnets now feature prominently in implantable medical devices, diagnostic equipment, and therapeutic systems. This technological progression has enabled miniaturization of devices, enhanced functionality, and improved patient outcomes across numerous medical specialties.
However, the increasing prevalence of neodymium magnets in both medical and non-medical environments has raised significant concerns regarding electromagnetic interference (EMI) and its potential impact on biomedical device reliability. Historical incidents of device malfunction due to magnetic interference have highlighted the critical nature of this technical challenge, particularly as the deployment of implantable medical devices continues to expand globally.
The magnetic field strength of neodymium magnets, measured in tesla (T) or gauss (G), can significantly exceed safety thresholds established for many biomedical devices. Contemporary research indicates that even relatively small neodymium magnets can generate field strengths of 0.5-1.5 tesla at their surface—sufficient to interfere with or potentially damage sensitive electronic components in medical devices at distances of several centimeters.
Current technological trends point toward continued miniaturization of both magnets and biomedical devices, creating an increasingly complex electromagnetic environment that demands robust testing methodologies and interference mitigation strategies. The convergence of these technologies necessitates a comprehensive understanding of interaction mechanisms and failure modes to ensure patient safety and device reliability.
The primary objectives of this technical research are threefold: first, to systematically characterize the electromagnetic interference patterns generated by various grades and configurations of neodymium magnets; second, to establish standardized testing protocols that accurately simulate real-world exposure scenarios; and third, to develop evidence-based guidelines for both biomedical device manufacturers and healthcare providers regarding safe operational parameters in the presence of strong magnetic fields.
Additionally, this research aims to identify emerging technologies and materials that may offer enhanced electromagnetic compatibility while maintaining or improving device performance characteristics. By anticipating future developments in both magnet technology and biomedical applications, this investigation seeks to provide a foundation for proactive rather than reactive approaches to ensuring device reliability.
The integration of neodymium magnets in biomedical devices has evolved considerably over the past three decades. Initially limited to external applications, these powerful magnets now feature prominently in implantable medical devices, diagnostic equipment, and therapeutic systems. This technological progression has enabled miniaturization of devices, enhanced functionality, and improved patient outcomes across numerous medical specialties.
However, the increasing prevalence of neodymium magnets in both medical and non-medical environments has raised significant concerns regarding electromagnetic interference (EMI) and its potential impact on biomedical device reliability. Historical incidents of device malfunction due to magnetic interference have highlighted the critical nature of this technical challenge, particularly as the deployment of implantable medical devices continues to expand globally.
The magnetic field strength of neodymium magnets, measured in tesla (T) or gauss (G), can significantly exceed safety thresholds established for many biomedical devices. Contemporary research indicates that even relatively small neodymium magnets can generate field strengths of 0.5-1.5 tesla at their surface—sufficient to interfere with or potentially damage sensitive electronic components in medical devices at distances of several centimeters.
Current technological trends point toward continued miniaturization of both magnets and biomedical devices, creating an increasingly complex electromagnetic environment that demands robust testing methodologies and interference mitigation strategies. The convergence of these technologies necessitates a comprehensive understanding of interaction mechanisms and failure modes to ensure patient safety and device reliability.
The primary objectives of this technical research are threefold: first, to systematically characterize the electromagnetic interference patterns generated by various grades and configurations of neodymium magnets; second, to establish standardized testing protocols that accurately simulate real-world exposure scenarios; and third, to develop evidence-based guidelines for both biomedical device manufacturers and healthcare providers regarding safe operational parameters in the presence of strong magnetic fields.
Additionally, this research aims to identify emerging technologies and materials that may offer enhanced electromagnetic compatibility while maintaining or improving device performance characteristics. By anticipating future developments in both magnet technology and biomedical applications, this investigation seeks to provide a foundation for proactive rather than reactive approaches to ensuring device reliability.
Market Analysis of Magnet-Sensitive Biomedical Devices
The global market for magnet-sensitive biomedical devices has experienced significant growth over the past decade, driven by technological advancements in medical diagnostics and therapeutic applications. Currently valued at approximately $18.7 billion, this market segment is projected to reach $27.3 billion by 2028, representing a compound annual growth rate of 6.5%.
Implantable medical devices constitute the largest segment within this market, accounting for nearly 42% of the total market share. Cardiac pacemakers and implantable cardioverter-defibrillators (ICDs) dominate this category, with over 1.5 million new implantations occurring annually worldwide. The increasing prevalence of cardiovascular diseases, particularly among aging populations in developed economies, continues to fuel demand for these devices.
Magnetic resonance imaging (MRI) compatibility has emerged as a critical market driver, with approximately 75% of patients with implanted medical devices requiring an MRI scan during their device's lifetime. This has created substantial demand for devices that can maintain reliability despite exposure to strong magnetic fields, particularly those generated by neodymium magnets which are increasingly common in medical and consumer electronics.
Regional analysis reveals North America as the dominant market, holding 38% of the global share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth rate at 8.2% annually, attributed to expanding healthcare infrastructure and increasing adoption of advanced medical technologies in countries like China, India, and Japan.
Consumer wearable medical devices represent the fastest-growing segment, expanding at 9.7% annually. This category includes continuous glucose monitors, insulin pumps, and cardiac monitoring devices that must maintain functionality despite potential interference from environmental magnetic fields. Market research indicates that approximately 63% of consumers express concerns about electromagnetic interference affecting their medical devices.
The competitive landscape features both established medical device manufacturers and emerging technology companies. Major players include Medtronic (16.3% market share), Abbott Laboratories (12.8%), and Boston Scientific (10.5%), who have all invested significantly in developing magnet-resistant technologies. Emerging companies focusing specifically on magnetic interference mitigation solutions have attracted over $870 million in venture capital funding since 2019.
Regulatory considerations significantly impact market dynamics, with the FDA and European Medical Agency implementing increasingly stringent requirements for magnetic interference testing. Compliance with these standards adds approximately 18-24 months to development timelines and increases R&D costs by 15-20%, creating substantial barriers to market entry for smaller companies.
Implantable medical devices constitute the largest segment within this market, accounting for nearly 42% of the total market share. Cardiac pacemakers and implantable cardioverter-defibrillators (ICDs) dominate this category, with over 1.5 million new implantations occurring annually worldwide. The increasing prevalence of cardiovascular diseases, particularly among aging populations in developed economies, continues to fuel demand for these devices.
Magnetic resonance imaging (MRI) compatibility has emerged as a critical market driver, with approximately 75% of patients with implanted medical devices requiring an MRI scan during their device's lifetime. This has created substantial demand for devices that can maintain reliability despite exposure to strong magnetic fields, particularly those generated by neodymium magnets which are increasingly common in medical and consumer electronics.
Regional analysis reveals North America as the dominant market, holding 38% of the global share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth rate at 8.2% annually, attributed to expanding healthcare infrastructure and increasing adoption of advanced medical technologies in countries like China, India, and Japan.
Consumer wearable medical devices represent the fastest-growing segment, expanding at 9.7% annually. This category includes continuous glucose monitors, insulin pumps, and cardiac monitoring devices that must maintain functionality despite potential interference from environmental magnetic fields. Market research indicates that approximately 63% of consumers express concerns about electromagnetic interference affecting their medical devices.
The competitive landscape features both established medical device manufacturers and emerging technology companies. Major players include Medtronic (16.3% market share), Abbott Laboratories (12.8%), and Boston Scientific (10.5%), who have all invested significantly in developing magnet-resistant technologies. Emerging companies focusing specifically on magnetic interference mitigation solutions have attracted over $870 million in venture capital funding since 2019.
Regulatory considerations significantly impact market dynamics, with the FDA and European Medical Agency implementing increasingly stringent requirements for magnetic interference testing. Compliance with these standards adds approximately 18-24 months to development timelines and increases R&D costs by 15-20%, creating substantial barriers to market entry for smaller companies.
Current Challenges in Electromagnetic Compatibility Testing
Electromagnetic Compatibility (EMC) testing for biomedical devices faces significant challenges when evaluating the effects of neodymium magnets. The increasing prevalence of these powerful permanent magnets in medical environments creates complex testing scenarios that current standardized protocols struggle to address adequately. Traditional EMC testing frameworks were developed before the widespread adoption of high-strength rare earth magnets in both medical and consumer devices, resulting in testing gaps.
One primary challenge is the accurate simulation of real-world magnetic field exposures. Neodymium magnets produce strong, localized magnetic fields that can vary dramatically with minimal distance changes, making it difficult to create reproducible test conditions that reflect actual clinical environments. Testing facilities often lack specialized equipment capable of generating and precisely controlling magnetic field gradients comparable to those produced by neodymium magnets in proximity to biomedical devices.
The transient nature of magnetic interference presents another significant obstacle. Unlike continuous electromagnetic radiation, the effects of neodymium magnets on biomedical devices often manifest during movement or when magnetic fields suddenly change. Current testing protocols predominantly focus on static field exposure or standardized field variations, failing to capture the dynamic interactions that occur in practical scenarios, such as when a patient with an implanted medical device approaches equipment containing neodymium magnets.
Threshold determination represents a critical testing challenge. The minimum magnetic field strength that causes malfunction varies widely across different biomedical devices, and establishing universally applicable safety thresholds has proven difficult. This variability necessitates device-specific testing approaches, significantly increasing testing complexity and resource requirements.
The miniaturization trend in biomedical devices compounds these challenges. As devices become smaller and more sophisticated, their susceptibility to magnetic interference can change dramatically. Components packed more densely may experience enhanced coupling effects, while integrated shielding solutions become more difficult to implement without compromising device functionality or size advantages.
Regulatory frameworks have not kept pace with technological advancements in this area. Current standards like IEC 60601-1-2 provide general guidance for electromagnetic compatibility but lack specific protocols for testing against the unique interference patterns generated by neodymium magnets. This regulatory gap creates uncertainty for manufacturers regarding compliance requirements and appropriate testing methodologies.
Interdisciplinary expertise requirements further complicate testing efforts. Effective EMC testing for neodymium magnet effects requires collaboration between experts in magnetism, electronics, medical device engineering, and clinical practice—a combination rarely found within a single testing facility or development team.
One primary challenge is the accurate simulation of real-world magnetic field exposures. Neodymium magnets produce strong, localized magnetic fields that can vary dramatically with minimal distance changes, making it difficult to create reproducible test conditions that reflect actual clinical environments. Testing facilities often lack specialized equipment capable of generating and precisely controlling magnetic field gradients comparable to those produced by neodymium magnets in proximity to biomedical devices.
The transient nature of magnetic interference presents another significant obstacle. Unlike continuous electromagnetic radiation, the effects of neodymium magnets on biomedical devices often manifest during movement or when magnetic fields suddenly change. Current testing protocols predominantly focus on static field exposure or standardized field variations, failing to capture the dynamic interactions that occur in practical scenarios, such as when a patient with an implanted medical device approaches equipment containing neodymium magnets.
Threshold determination represents a critical testing challenge. The minimum magnetic field strength that causes malfunction varies widely across different biomedical devices, and establishing universally applicable safety thresholds has proven difficult. This variability necessitates device-specific testing approaches, significantly increasing testing complexity and resource requirements.
The miniaturization trend in biomedical devices compounds these challenges. As devices become smaller and more sophisticated, their susceptibility to magnetic interference can change dramatically. Components packed more densely may experience enhanced coupling effects, while integrated shielding solutions become more difficult to implement without compromising device functionality or size advantages.
Regulatory frameworks have not kept pace with technological advancements in this area. Current standards like IEC 60601-1-2 provide general guidance for electromagnetic compatibility but lack specific protocols for testing against the unique interference patterns generated by neodymium magnets. This regulatory gap creates uncertainty for manufacturers regarding compliance requirements and appropriate testing methodologies.
Interdisciplinary expertise requirements further complicate testing efforts. Effective EMC testing for neodymium magnet effects requires collaboration between experts in magnetism, electronics, medical device engineering, and clinical practice—a combination rarely found within a single testing facility or development team.
Established Testing Protocols and Standards
01 Corrosion resistance and coating technologies
Neodymium magnets are susceptible to corrosion which can significantly impact their reliability. Various coating technologies have been developed to enhance their corrosion resistance, including nickel plating, zinc plating, epoxy coatings, and specialized polymer layers. These protective coatings not only prevent oxidation but also extend the operational lifespan of the magnets in harsh environments while maintaining their magnetic properties.- Corrosion resistance and coating technologies: Neodymium magnets are susceptible to corrosion which can significantly impact their reliability. Various coating technologies have been developed to enhance corrosion resistance, including nickel plating, zinc plating, epoxy coatings, and specialized polymer layers. These protective coatings not only prevent oxidation but also maintain the magnetic properties over extended periods, even in harsh environmental conditions.
- Temperature stability and thermal performance: The reliability of neodymium magnets is heavily influenced by temperature variations. High temperatures can cause demagnetization and permanent loss of magnetic properties. Advanced formulations incorporate dysprosium and terbium to improve temperature stability and increase maximum operating temperatures. Thermal management systems and specialized heat-resistant compositions have been developed to maintain reliable performance in high-temperature applications.
- Mechanical stability and structural integrity: Neodymium magnets are brittle by nature, which affects their reliability in applications with mechanical stress or impact. Reinforcement techniques include embedding magnets in protective housings, using specialized mounting methods, and developing composite structures that distribute mechanical forces. These approaches prevent cracking, chipping, and structural failure while maintaining magnetic performance under mechanical stress.
- Long-term stability and aging characteristics: The long-term reliability of neodymium magnets depends on their resistance to aging effects. Factors affecting long-term stability include microstructural changes, internal oxidation, and gradual demagnetization. Advanced manufacturing processes focus on grain boundary optimization, precise composition control, and post-production treatments to enhance long-term magnetic stability and minimize flux loss over time.
- Testing methods and quality assurance: Ensuring the reliability of neodymium magnets requires comprehensive testing protocols and quality assurance measures. Advanced testing methods include accelerated aging tests, thermal cycling, vibration testing, and magnetic property verification under various environmental conditions. Non-destructive evaluation techniques help identify internal defects and predict potential reliability issues before deployment in critical applications.
02 Thermal stability and demagnetization resistance
The reliability of neodymium magnets is significantly affected by temperature fluctuations. Innovations focus on improving their thermal stability to prevent demagnetization at high temperatures. This includes developing specialized alloy compositions with dysprosium or terbium additions, optimized microstructures, and heat treatment processes that enhance the magnets' maximum operating temperature and reduce irreversible magnetic losses under thermal stress.Expand Specific Solutions03 Mechanical strength and structural integrity
Neodymium magnets are inherently brittle, which poses reliability challenges in applications with mechanical stress. Advancements in manufacturing processes aim to improve their mechanical strength through optimized sintering conditions, grain boundary engineering, and reinforcement techniques. Some solutions incorporate protective casings or structural supports to prevent chipping, cracking, and catastrophic failure during operation or handling.Expand Specific Solutions04 Long-term stability and aging resistance
The long-term reliability of neodymium magnets depends on their resistance to aging effects. Research focuses on minimizing flux loss over time through improved material composition and processing techniques. Stabilization treatments, controlled exposure to demagnetizing fields during manufacturing, and microstructural optimization help ensure consistent magnetic performance throughout the product lifecycle, particularly in precision applications requiring sustained magnetic field strength.Expand Specific Solutions05 Environmental adaptability and specialized applications
Enhancing the reliability of neodymium magnets in specialized environments requires tailored solutions. Innovations include magnets designed for extreme conditions such as high humidity, vibration, radiation, or vacuum environments. These adaptations involve specialized sealing techniques, composite structures, and application-specific designs that maintain magnetic performance while addressing unique environmental challenges in automotive, aerospace, medical, and renewable energy applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The neodymium magnet effects on biomedical device reliability landscape is currently in a growth phase, with the global market expanding due to increasing medical device applications. The technology maturity varies across applications, with companies demonstrating different specialization levels. MED-EL and Koninklijke Philips lead in medical device integration, while Cardiovascular Systems and TODOC focus on specific therapeutic applications. Research institutions like CNRS, Brown University, and Tokyo Institute of Technology provide fundamental scientific advancements. Material specialists including Advanced Technology & Materials, NEOMAX, and Jiangsu Guoyuan Rare Earth contribute specialized magnetic materials expertise. This diverse ecosystem reflects the technology's cross-disciplinary nature, spanning medical device manufacturing, materials science, and biomedical engineering.
MED-EL Elektromedizinische Geräte GmbH
Technical Solution: MED-EL has developed comprehensive testing protocols for their cochlear implants and other hearing solutions to evaluate neodymium magnet effects on device reliability. Their approach includes multi-layered magnetic shielding technology that incorporates specialized alloys to protect internal electronic components from external magnetic interference. The company implements a proprietary demagnetization resistance system that maintains magnet strength and orientation even when exposed to strong external magnetic fields up to 3 Tesla, allowing patients to safely undergo MRI procedures without device removal. MED-EL's testing methodology includes accelerated aging tests under various magnetic field strengths to simulate long-term exposure effects, and they've pioneered self-adjusting magnet systems that automatically reorient when temporarily displaced by external magnetic fields, returning to optimal position for sound transmission. Their implantable devices undergo rigorous testing in simulated biomedical environments to ensure consistent performance despite magnetic interference.
Strengths: Industry-leading MRI compatibility allowing scanning at 3 Tesla without magnet removal; advanced self-adjusting magnet technology that maintains optimal positioning; extensive clinical validation data supporting reliability claims. Weaknesses: Higher manufacturing costs associated with specialized magnetic shielding; potential for slight surgical complexity due to additional protective components; requires periodic recalibration in environments with persistent strong magnetic fields.
Koninklijke Philips NV
Technical Solution: Philips has engineered a sophisticated approach to testing neodymium magnet effects on their extensive portfolio of biomedical devices. Their methodology centers on a proprietary Magnetic Compatibility Assessment Framework that evaluates device performance across multiple magnetic exposure scenarios. The company utilizes advanced computational modeling to predict magnetic field interactions before physical testing, significantly reducing development cycles. Philips implements gradient-based testing protocols that incrementally increase magnetic field strength to identify precise thresholds where device performance might be compromised. Their biomedical devices incorporate adaptive magnetic shielding using composite materials that provide selective permeability, blocking harmful magnetic interference while allowing necessary electromagnetic functions to operate unimpeded. For implantable devices, Philips has developed a nano-structured coating technology that creates a magnetic buffer zone, preventing direct field interactions with sensitive electronic components while maintaining device miniaturization requirements. The company's testing facilities can simulate real-world clinical environments where multiple magnetic sources may be present simultaneously, providing more realistic reliability assessments.
Strengths: Comprehensive testing framework that addresses multiple device categories simultaneously; advanced computational modeling capabilities that predict failures before physical testing; extensive global clinical validation network. Weaknesses: Complex testing protocols require specialized equipment and expertise; higher development costs associated with comprehensive magnetic compatibility testing; potential overengineering of magnetic shielding in some lower-risk applications.
Critical Patents in Magnetic Shielding Technologies
Magnetic resonance imaging safe magnet structure and cochlear implant including same
PatentWO2025173820A1
Innovation
- A cochlear implant design with a first magnet unit having multiple pairs of magnetic poles arranged to cancel out magnetic fields, ensuring stability and enhancing adhesion while maintaining efficient wireless power and data transmission.
Implantable medical device with MRI and gradient field induced capture detection methods
PatentInactiveUS20060293591A1
Innovation
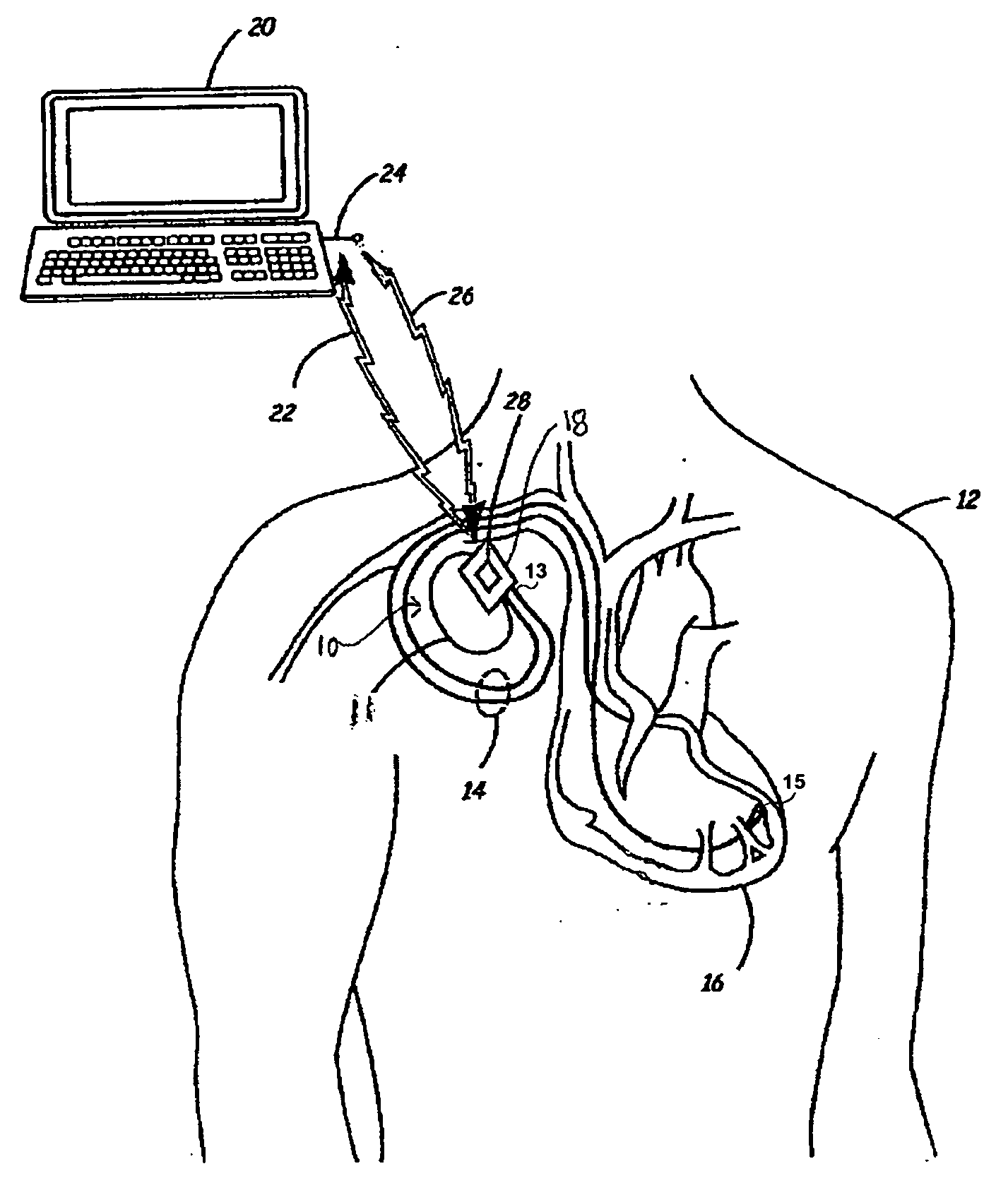
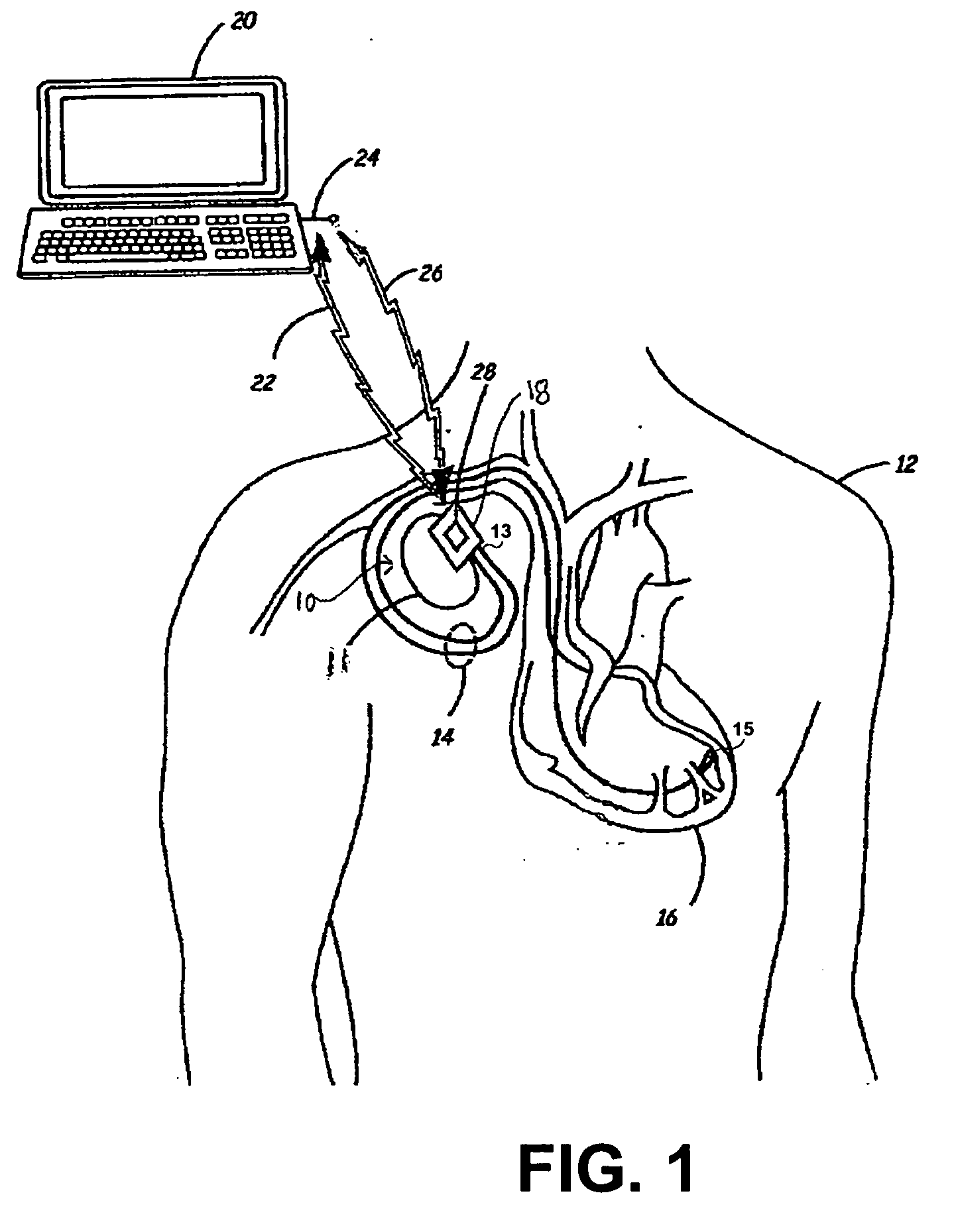
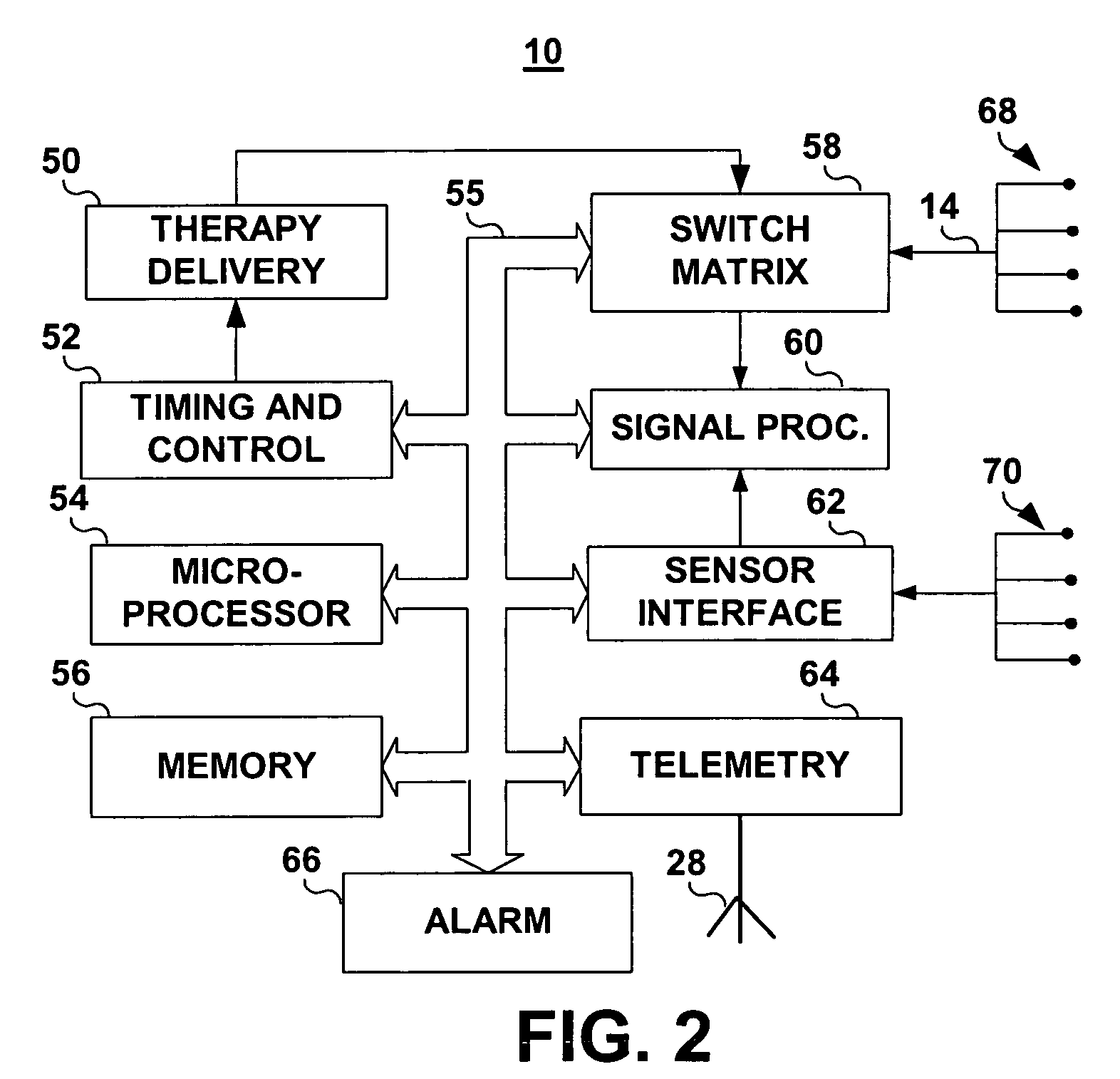
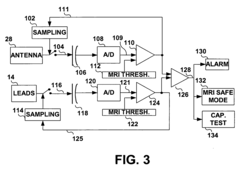
- An IMD equipped with MRI field detection circuitry that includes telemetry, sensing, processing, and control circuitry to detect the presence of an MRI field by comparing voltage signals from the telemetry antenna and lead conductors to predefined thresholds, triggering a safeguard response such as generating an alarm or implementing temporary operating parameters to prevent inadvertent tissue stimulation.
Safety and Risk Assessment Frameworks
The assessment of neodymium magnet effects on biomedical devices requires robust safety and risk assessment frameworks to ensure patient protection and device reliability. Current frameworks typically follow a multi-tiered approach that combines regulatory guidelines, industry standards, and scientific testing protocols.
The FDA's risk-based classification system provides the foundational structure for evaluating magnetic interference risks in medical devices. This framework categorizes devices based on potential harm levels, with Class III devices (life-supporting or life-sustaining) requiring the most rigorous testing for magnetic field interactions. Complementing this, the ISO 14708 series specifically addresses electromagnetic compatibility requirements for active implantable medical devices, providing standardized testing methodologies.
Risk assessment matrices have evolved to quantify both the probability and severity of magnetic interference events. These matrices typically evaluate factors including magnetic field strength thresholds, proximity duration, device criticality, and failure mode consequences. Modern frameworks increasingly incorporate Failure Mode and Effects Analysis (FMEA) to systematically identify potential failure points in medical devices when exposed to neodymium magnets.
The IEC 60601-1-2 standard has become particularly relevant, establishing electromagnetic compatibility requirements and test methods for medical electrical equipment. This standard defines specific test conditions for evaluating device performance in the presence of magnetic fields, including those generated by neodymium magnets.
Recent advancements in risk assessment frameworks include the implementation of probabilistic risk assessment (PRA) methodologies. These approaches utilize statistical models to quantify uncertainties in magnetic field exposure scenarios, providing more nuanced risk evaluations than traditional deterministic methods. Such frameworks often incorporate Monte Carlo simulations to model various exposure scenarios and their potential impacts on device functionality.
Post-market surveillance has also become an integral component of comprehensive risk assessment frameworks. Systematic collection and analysis of real-world data regarding magnetic interference incidents help refine risk models and identify previously unknown vulnerability patterns. This feedback loop enables continuous improvement of both testing protocols and device designs.
Emerging frameworks are increasingly adopting a "safety by design" philosophy, where magnetic interference considerations are integrated throughout the product development lifecycle rather than assessed only during final testing phases. This approach emphasizes proactive risk mitigation through design choices that inherently reduce susceptibility to magnetic field interference.
The FDA's risk-based classification system provides the foundational structure for evaluating magnetic interference risks in medical devices. This framework categorizes devices based on potential harm levels, with Class III devices (life-supporting or life-sustaining) requiring the most rigorous testing for magnetic field interactions. Complementing this, the ISO 14708 series specifically addresses electromagnetic compatibility requirements for active implantable medical devices, providing standardized testing methodologies.
Risk assessment matrices have evolved to quantify both the probability and severity of magnetic interference events. These matrices typically evaluate factors including magnetic field strength thresholds, proximity duration, device criticality, and failure mode consequences. Modern frameworks increasingly incorporate Failure Mode and Effects Analysis (FMEA) to systematically identify potential failure points in medical devices when exposed to neodymium magnets.
The IEC 60601-1-2 standard has become particularly relevant, establishing electromagnetic compatibility requirements and test methods for medical electrical equipment. This standard defines specific test conditions for evaluating device performance in the presence of magnetic fields, including those generated by neodymium magnets.
Recent advancements in risk assessment frameworks include the implementation of probabilistic risk assessment (PRA) methodologies. These approaches utilize statistical models to quantify uncertainties in magnetic field exposure scenarios, providing more nuanced risk evaluations than traditional deterministic methods. Such frameworks often incorporate Monte Carlo simulations to model various exposure scenarios and their potential impacts on device functionality.
Post-market surveillance has also become an integral component of comprehensive risk assessment frameworks. Systematic collection and analysis of real-world data regarding magnetic interference incidents help refine risk models and identify previously unknown vulnerability patterns. This feedback loop enables continuous improvement of both testing protocols and device designs.
Emerging frameworks are increasingly adopting a "safety by design" philosophy, where magnetic interference considerations are integrated throughout the product development lifecycle rather than assessed only during final testing phases. This approach emphasizes proactive risk mitigation through design choices that inherently reduce susceptibility to magnetic field interference.
Regulatory Compliance Requirements
Regulatory compliance for biomedical devices incorporating or tested with neodymium magnets falls under stringent frameworks established by multiple international bodies. The FDA (Food and Drug Administration) in the United States requires comprehensive testing under 21 CFR Part 820 Quality System Regulation, with specific attention to electromagnetic compatibility testing outlined in IEC 60601-1-2. Manufacturers must demonstrate that neodymium magnets do not interfere with device functionality or pose safety risks through rigorous validation protocols.
The European Union's Medical Device Regulation (MDR 2017/745) imposes additional requirements, particularly regarding risk management documentation that must explicitly address magnetic field interactions. Manufacturers must conduct thorough risk assessments following ISO 14971 methodology, with special consideration for potential magnetic interference with other medical equipment in clinical environments.
For implantable devices, ISO 10993 series standards govern biocompatibility testing, requiring evaluation of potential biological responses to magnetic materials. The ASTM F2052 standard specifically addresses magnetically induced displacement force on medical devices in the magnetic resonance environment, critical for devices that may be exposed to MRI procedures.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established parallel requirements with country-specific testing protocols that must be satisfied for market access. These typically include stability testing under various environmental conditions to ensure magnetic properties remain consistent throughout the device lifecycle.
Documentation requirements across all regulatory frameworks include detailed test reports demonstrating magnetic field strength measurements, interference patterns, and thermal effects. These must be maintained in the technical file and updated whenever design modifications occur. Post-market surveillance plans must specifically monitor for adverse events related to magnetic interactions.
Labeling requirements present another critical compliance area, with ISO 15223-1 symbols for magnetic materials mandatory on packaging. Warning statements regarding minimum safe distances from other electronic devices and contraindications for patients with certain implants must be clearly articulated in user documentation.
Regulatory bodies increasingly require electromagnetic compatibility testing under worst-case scenarios, including testing at elevated temperatures and after simulated aging processes to account for potential degradation of magnetic properties over time. This trend toward more comprehensive testing reflects growing awareness of the complex interactions between neodymium magnets and biomedical device components.
The European Union's Medical Device Regulation (MDR 2017/745) imposes additional requirements, particularly regarding risk management documentation that must explicitly address magnetic field interactions. Manufacturers must conduct thorough risk assessments following ISO 14971 methodology, with special consideration for potential magnetic interference with other medical equipment in clinical environments.
For implantable devices, ISO 10993 series standards govern biocompatibility testing, requiring evaluation of potential biological responses to magnetic materials. The ASTM F2052 standard specifically addresses magnetically induced displacement force on medical devices in the magnetic resonance environment, critical for devices that may be exposed to MRI procedures.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established parallel requirements with country-specific testing protocols that must be satisfied for market access. These typically include stability testing under various environmental conditions to ensure magnetic properties remain consistent throughout the device lifecycle.
Documentation requirements across all regulatory frameworks include detailed test reports demonstrating magnetic field strength measurements, interference patterns, and thermal effects. These must be maintained in the technical file and updated whenever design modifications occur. Post-market surveillance plans must specifically monitor for adverse events related to magnetic interactions.
Labeling requirements present another critical compliance area, with ISO 15223-1 symbols for magnetic materials mandatory on packaging. Warning statements regarding minimum safe distances from other electronic devices and contraindications for patients with certain implants must be clearly articulated in user documentation.
Regulatory bodies increasingly require electromagnetic compatibility testing under worst-case scenarios, including testing at elevated temperatures and after simulated aging processes to account for potential degradation of magnetic properties over time. This trend toward more comprehensive testing reflects growing awareness of the complex interactions between neodymium magnets and biomedical device components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!