The Future of Chemical Reactions with Fluoroantimonic Acid
JUN 23, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of intense scientific interest since its discovery in the mid-20th century. This compound, formed by mixing hydrogen fluoride and antimony pentafluoride, is recognized as one of the strongest acids known to science, with an acidity level far surpassing that of conventional strong acids like sulfuric acid.
The development of fluoroantimonic acid marks a significant milestone in the field of chemistry, particularly in the realm of superacids. Its extreme acidity, measured at -31.3 on the Hammett acidity function scale, has opened up new possibilities for chemical reactions that were previously thought impossible. This superacid's ability to protonate even extremely weak bases has revolutionized our understanding of acid-base chemistry and catalysis.
The primary objective of research into fluoroantimonic acid is to harness its unique properties for advancing chemical synthesis and industrial processes. Scientists aim to explore its potential in catalyzing reactions that are challenging or unfeasible with conventional acids, particularly in the petrochemical industry and in the synthesis of novel materials.
One of the key areas of focus is the acid's potential in hydrocarbon chemistry. Its extreme acidity allows for the protonation of alkanes, potentially leading to more efficient and selective methods for converting petroleum feedstocks into valuable products. This could have far-reaching implications for the energy sector and the production of plastics and other petrochemicals.
Another significant objective is to investigate the acid's role in generating highly reactive carbocations. These species are crucial intermediates in many organic reactions, and the ability to generate them more efficiently could lead to new synthetic pathways in pharmaceutical and materials science.
However, the extreme reactivity of fluoroantimonic acid also presents significant challenges. Its corrosive nature and sensitivity to moisture necessitate specialized handling techniques and equipment. Therefore, a parallel objective is to develop safer methods for utilizing this superacid, including the creation of more stable forms or supported versions that retain its reactivity while mitigating its hazardous properties.
As research progresses, there is growing interest in understanding the fundamental nature of superacidity at the molecular level. This includes studying the behavior of protons in extremely acidic environments and exploring the limits of acidity in chemical systems. Such fundamental research could lead to the development of even more powerful superacids or novel acid systems with tailored properties for specific applications.
The development of fluoroantimonic acid marks a significant milestone in the field of chemistry, particularly in the realm of superacids. Its extreme acidity, measured at -31.3 on the Hammett acidity function scale, has opened up new possibilities for chemical reactions that were previously thought impossible. This superacid's ability to protonate even extremely weak bases has revolutionized our understanding of acid-base chemistry and catalysis.
The primary objective of research into fluoroantimonic acid is to harness its unique properties for advancing chemical synthesis and industrial processes. Scientists aim to explore its potential in catalyzing reactions that are challenging or unfeasible with conventional acids, particularly in the petrochemical industry and in the synthesis of novel materials.
One of the key areas of focus is the acid's potential in hydrocarbon chemistry. Its extreme acidity allows for the protonation of alkanes, potentially leading to more efficient and selective methods for converting petroleum feedstocks into valuable products. This could have far-reaching implications for the energy sector and the production of plastics and other petrochemicals.
Another significant objective is to investigate the acid's role in generating highly reactive carbocations. These species are crucial intermediates in many organic reactions, and the ability to generate them more efficiently could lead to new synthetic pathways in pharmaceutical and materials science.
However, the extreme reactivity of fluoroantimonic acid also presents significant challenges. Its corrosive nature and sensitivity to moisture necessitate specialized handling techniques and equipment. Therefore, a parallel objective is to develop safer methods for utilizing this superacid, including the creation of more stable forms or supported versions that retain its reactivity while mitigating its hazardous properties.
As research progresses, there is growing interest in understanding the fundamental nature of superacidity at the molecular level. This includes studying the behavior of protons in extremely acidic environments and exploring the limits of acidity in chemical systems. Such fundamental research could lead to the development of even more powerful superacids or novel acid systems with tailored properties for specific applications.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, is experiencing significant growth driven by advancements in chemical synthesis and materials science. Fluoroantimonic acid, known as the strongest superacid, finds extensive use in various industrial processes due to its exceptional proton-donating ability and catalytic properties.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in isomerization and alkylation reactions, enhancing the production of high-octane gasoline components. This application alone accounts for a substantial portion of the superacid market, with increasing demand for cleaner and more efficient fuels driving further growth.
The electronics sector represents another key market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become indispensable as the industry pushes towards smaller and more powerful electronic devices. The ongoing trend of miniaturization in electronics is expected to sustain demand for superacids in this sector.
Emerging applications in nanotechnology and materials science are opening new avenues for superacid use. Researchers are exploring the potential of fluoroantimonic acid in the synthesis of novel materials, including advanced polymers and nanostructures. These applications, while currently niche, show promise for future market expansion.
The pharmaceutical industry is also increasingly utilizing superacids in the synthesis of complex drug molecules. Fluoroantimonic acid's ability to catalyze challenging reactions that are difficult or impossible with conventional acids is driving its adoption in drug discovery and development processes.
However, the market faces challenges related to the handling and disposal of superacids. The extreme corrosiveness and reactivity of fluoroantimonic acid necessitate specialized equipment and safety protocols, which can increase operational costs. Environmental concerns and stringent regulations regarding the use and disposal of such powerful acids also pose potential barriers to market growth.
Despite these challenges, the global market for superacid applications continues to expand. Technological advancements in containment and handling systems are gradually mitigating safety concerns, while ongoing research is uncovering new applications that leverage the unique properties of fluoroantimonic acid. As industries continue to seek more efficient and powerful chemical processes, the demand for superacids is expected to grow, with fluoroantimonic acid remaining at the forefront of this specialized market.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in isomerization and alkylation reactions, enhancing the production of high-octane gasoline components. This application alone accounts for a substantial portion of the superacid market, with increasing demand for cleaner and more efficient fuels driving further growth.
The electronics sector represents another key market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become indispensable as the industry pushes towards smaller and more powerful electronic devices. The ongoing trend of miniaturization in electronics is expected to sustain demand for superacids in this sector.
Emerging applications in nanotechnology and materials science are opening new avenues for superacid use. Researchers are exploring the potential of fluoroantimonic acid in the synthesis of novel materials, including advanced polymers and nanostructures. These applications, while currently niche, show promise for future market expansion.
The pharmaceutical industry is also increasingly utilizing superacids in the synthesis of complex drug molecules. Fluoroantimonic acid's ability to catalyze challenging reactions that are difficult or impossible with conventional acids is driving its adoption in drug discovery and development processes.
However, the market faces challenges related to the handling and disposal of superacids. The extreme corrosiveness and reactivity of fluoroantimonic acid necessitate specialized equipment and safety protocols, which can increase operational costs. Environmental concerns and stringent regulations regarding the use and disposal of such powerful acids also pose potential barriers to market growth.
Despite these challenges, the global market for superacid applications continues to expand. Technological advancements in containment and handling systems are gradually mitigating safety concerns, while ongoing research is uncovering new applications that leverage the unique properties of fluoroantimonic acid. As industries continue to seek more efficient and powerful chemical processes, the demand for superacids is expected to grow, with fluoroantimonic acid remaining at the forefront of this specialized market.
Current Challenges in Fluoroantimonic Acid Usage
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its usage and application. The primary obstacle lies in its extreme corrosiveness and reactivity, which severely limits the materials that can safely contain it. Traditional laboratory glassware and most metals are rapidly degraded by this powerful acid, necessitating the use of specialized containers made from fluoropolymers like PTFE (Teflon).
The handling and storage of fluoroantimonic acid pose considerable safety risks. Its highly hygroscopic nature means it reacts violently with water, producing dangerous hydrogen fluoride gas. This property not only complicates storage but also requires stringent safety protocols and specialized equipment for its manipulation. The acid's extreme reactivity also makes it challenging to transport, further limiting its accessibility for research and industrial applications.
Another significant challenge is the difficulty in controlling and moderating the acid's reactivity. Its superacidic nature can lead to undesired side reactions or over-fluorination of target compounds, making it challenging to achieve selective chemical transformations. This lack of selectivity limits its potential applications in organic synthesis and materials science, where precise control over reaction outcomes is crucial.
The environmental impact of fluoroantimonic acid usage is also a major concern. Its production and disposal involve highly toxic and environmentally harmful substances, including hydrogen fluoride and antimony pentafluoride. Developing safe and eco-friendly methods for its synthesis, use, and disposal remains a significant challenge for the chemical industry.
Furthermore, the high cost associated with the production and handling of fluoroantimonic acid restricts its widespread use in industrial processes. The specialized equipment, safety measures, and expertise required for its handling contribute to its economic limitations, making it impractical for large-scale applications in many sectors.
Lastly, there is a lack of comprehensive understanding of the full range of chemical reactions and transformations possible with fluoroantimonic acid. While its extreme acidity is well-documented, the potential for novel chemical syntheses or materials processing techniques remains largely unexplored. This knowledge gap hinders the development of innovative applications that could potentially justify the challenges associated with its use.
The handling and storage of fluoroantimonic acid pose considerable safety risks. Its highly hygroscopic nature means it reacts violently with water, producing dangerous hydrogen fluoride gas. This property not only complicates storage but also requires stringent safety protocols and specialized equipment for its manipulation. The acid's extreme reactivity also makes it challenging to transport, further limiting its accessibility for research and industrial applications.
Another significant challenge is the difficulty in controlling and moderating the acid's reactivity. Its superacidic nature can lead to undesired side reactions or over-fluorination of target compounds, making it challenging to achieve selective chemical transformations. This lack of selectivity limits its potential applications in organic synthesis and materials science, where precise control over reaction outcomes is crucial.
The environmental impact of fluoroantimonic acid usage is also a major concern. Its production and disposal involve highly toxic and environmentally harmful substances, including hydrogen fluoride and antimony pentafluoride. Developing safe and eco-friendly methods for its synthesis, use, and disposal remains a significant challenge for the chemical industry.
Furthermore, the high cost associated with the production and handling of fluoroantimonic acid restricts its widespread use in industrial processes. The specialized equipment, safety measures, and expertise required for its handling contribute to its economic limitations, making it impractical for large-scale applications in many sectors.
Lastly, there is a lack of comprehensive understanding of the full range of chemical reactions and transformations possible with fluoroantimonic acid. While its extreme acidity is well-documented, the potential for novel chemical syntheses or materials processing techniques remains largely unexplored. This knowledge gap hinders the development of innovative applications that could potentially justify the challenges associated with its use.
Existing Fluoroantimonic Acid Reaction Methodologies
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are challenging or impossible with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, and modify the properties of materials such as polymers and ceramics. The acid's unique properties enable the development of advanced materials with tailored surface characteristics.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and specialized titration procedures. Advanced instrumentation and methodologies are employed to investigate the acid's properties, reaction mechanisms, and interactions with other substances.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates processes such as alkylation, isomerization, and polymerization of hydrocarbons. The acid's extreme acidity enables it to catalyze reactions that are challenging or impossible with conventional acid catalysts.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It is used for etching, cleaning, and activating surfaces of metals, semiconductors, and other materials. The acid's strong oxidizing properties make it effective in removing contaminants and creating specific surface characteristics.Expand Specific Solutions04 Safety measures and handling procedures
Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal. Proper training and safety systems are essential to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and computational modeling. Such techniques are crucial for understanding the acid's properties, reaction mechanisms, and interactions with different substrates, which in turn aids in optimizing its applications and developing new uses.Expand Specific Solutions
Key Players in Superacid Research and Industry
The future of chemical reactions with fluoroantimonic acid is in a nascent stage of development, with significant potential for growth. The market size is currently limited due to the highly specialized nature of this superacid, but it shows promise in various industrial applications. The technology maturity is advancing, with key players like DuPont de Nemours, Inc., 3M Innovative Properties Co., and Central Glass Co., Ltd. leading research efforts. Universities such as Yale University and Jilin University are contributing to fundamental research, while companies like Honeywell International Technologies Ltd. and DAIKIN INDUSTRIES Ltd. are exploring practical applications. The competitive landscape is characterized by a mix of established chemical companies and academic institutions, indicating a collaborative approach to advancing this technology.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for handling and utilizing fluoroantimonic acid in chemical reactions. Their approach involves using specialized containment systems made of highly resistant materials like fluoropolymers. They have also created a novel catalytic system that incorporates fluoroantimonic acid for enhancing the efficiency of certain organic synthesis reactions, particularly in the production of high-performance polymers and specialty chemicals.
Strengths: Advanced containment technology, expertise in handling superacids, potential for more efficient chemical processes. Weaknesses: High costs associated with specialized equipment, safety concerns due to the extreme reactivity of fluoroantimonic acid.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has pioneered a method for using fluoroantimonic acid in the production of advanced fluoropolymers. Their technique involves a controlled reaction environment where fluoroantimonic acid acts as a super-catalyst, enabling the creation of novel fluorine-containing compounds with unique properties. They have also developed a recycling system for the acid, minimizing waste and improving the sustainability of the process.
Strengths: Innovative application in fluoropolymer synthesis, efficient acid recycling system. Weaknesses: Limited to specific applications in fluorine chemistry, potential environmental concerns related to fluorine compounds.
Innovative Approaches in Fluoroantimonic Acid Chemistry
Enantioselective phosphoramidite compounds and catalysts
PatentWO2005111050A2
Innovation
- The development of phosphoramidite compounds that form catalyst complexes with transition metals like iridium, rhodium, ruthenium, nickel, palladium, platinum, copper, or silver for enantioselective and regioselective reactions, including hydrogenation, transfer hydrogenation, and allylic substitution, using specific ligand structures to enhance enantioselectivity and modulate the steric and electronic environment.
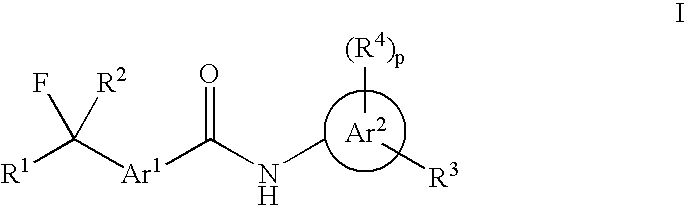
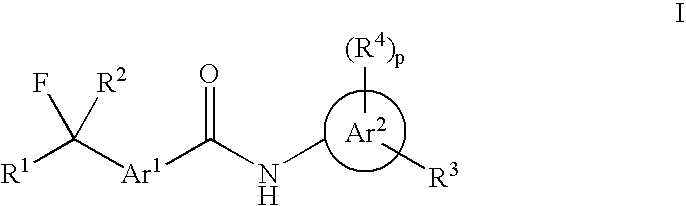
Fluorinated Arylamide Derivatives
PatentInactiveUS20090012075A1
Innovation
- Development of novel fluoroalkylarylamide derivatives that act as potent histone deacetylase inhibitors, capable of inducing terminal differentiation and apoptosis in neoplastic cells, and suitable for treating various diseases including cancer, autoimmune, and neurodegenerative conditions.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in any future applications. The extreme corrosiveness and reactivity of this compound necessitate stringent handling protocols and specialized containment systems to prevent accidental exposure or release.
Worker safety is paramount when dealing with fluoroantimonic acid. Personal protective equipment (PPE) must include fully encapsulating chemical-resistant suits, gloves, and respiratory protection. Facilities must be equipped with emergency showers, eyewash stations, and proper ventilation systems. Regular safety training and drills are essential for all personnel involved in its handling or use.
The environmental impact of fluoroantimonic acid is a major concern. Its highly reactive nature means that any release could have devastating effects on ecosystems. Strict containment and disposal procedures must be in place to prevent contamination of soil, water, or air. Neutralization techniques using appropriate bases should be developed and readily available in case of spills.
Long-term storage and transportation of fluoroantimonic acid pose additional challenges. Specialized containers made of materials resistant to extreme corrosion, such as PTFE or certain alloys, are required. These containers must be regularly inspected and replaced to ensure integrity. Transportation regulations for such hazardous materials must be strictly adhered to, with proper labeling and documentation.
Research into safer alternatives or methods to mitigate the risks associated with fluoroantimonic acid should be a priority. This could include developing less hazardous catalysts that can achieve similar chemical transformations, or exploring new reaction pathways that avoid the use of such extreme reagents altogether.
Regulatory compliance is another crucial aspect. As research and potential applications of fluoroantimonic acid progress, it is likely that new regulations will be implemented. Organizations working with this compound must stay informed about evolving safety standards and environmental regulations, and be prepared to adapt their practices accordingly.
In conclusion, while fluoroantimonic acid holds promise for future chemical reactions, its use comes with significant safety and environmental considerations. Addressing these challenges will require ongoing research, rigorous safety protocols, and a commitment to environmental stewardship from all stakeholders involved in its development and application.
Worker safety is paramount when dealing with fluoroantimonic acid. Personal protective equipment (PPE) must include fully encapsulating chemical-resistant suits, gloves, and respiratory protection. Facilities must be equipped with emergency showers, eyewash stations, and proper ventilation systems. Regular safety training and drills are essential for all personnel involved in its handling or use.
The environmental impact of fluoroantimonic acid is a major concern. Its highly reactive nature means that any release could have devastating effects on ecosystems. Strict containment and disposal procedures must be in place to prevent contamination of soil, water, or air. Neutralization techniques using appropriate bases should be developed and readily available in case of spills.
Long-term storage and transportation of fluoroantimonic acid pose additional challenges. Specialized containers made of materials resistant to extreme corrosion, such as PTFE or certain alloys, are required. These containers must be regularly inspected and replaced to ensure integrity. Transportation regulations for such hazardous materials must be strictly adhered to, with proper labeling and documentation.
Research into safer alternatives or methods to mitigate the risks associated with fluoroantimonic acid should be a priority. This could include developing less hazardous catalysts that can achieve similar chemical transformations, or exploring new reaction pathways that avoid the use of such extreme reagents altogether.
Regulatory compliance is another crucial aspect. As research and potential applications of fluoroantimonic acid progress, it is likely that new regulations will be implemented. Organizations working with this compound must stay informed about evolving safety standards and environmental regulations, and be prepared to adapt their practices accordingly.
In conclusion, while fluoroantimonic acid holds promise for future chemical reactions, its use comes with significant safety and environmental considerations. Addressing these challenges will require ongoing research, rigorous safety protocols, and a commitment to environmental stewardship from all stakeholders involved in its development and application.
Potential Industrial Applications
Fluoroantimonic acid, known as the world's strongest superacid, holds immense potential for various industrial applications due to its exceptional protonating ability and catalytic properties. In the petrochemical industry, this superacid could revolutionize the cracking and isomerization processes of hydrocarbons, potentially leading to more efficient fuel production and higher-quality petroleum products. Its extreme acidity may enable the conversion of lower-value hydrocarbons into more valuable compounds, enhancing the overall efficiency of oil refining processes.
In the field of materials science, fluoroantimonic acid could play a crucial role in the synthesis of novel materials. Its ability to protonate even weak bases suggests potential applications in the production of advanced polymers, super-strong adhesives, and highly resistant coatings. The acid's unique properties might also facilitate the development of new types of ion-exchange membranes, which could have significant implications for energy storage and water purification technologies.
The electronics industry could benefit from fluoroantimonic acid's etching capabilities. Its extreme corrosiveness could be harnessed for precision etching of semiconductor materials, potentially leading to the development of more advanced and miniaturized electronic components. This could contribute to the ongoing trend of device miniaturization and performance enhancement in the electronics sector.
In the pharmaceutical industry, fluoroantimonic acid might serve as a powerful tool for organic synthesis. Its strong protonating ability could enable the creation of complex organic molecules that are challenging to synthesize through conventional methods. This could potentially accelerate drug discovery processes and lead to the development of new classes of pharmaceutical compounds.
The nuclear industry might also find applications for fluoroantimonic acid in the processing and recycling of nuclear fuels. Its extreme acidity could potentially assist in the dissolution of highly resistant materials, aiding in the extraction and purification of valuable nuclear materials. However, the implementation of such applications would require extensive safety measures due to the acid's highly corrosive nature.
While the potential industrial applications of fluoroantimonic acid are promising, it is crucial to note that its extreme reactivity and corrosiveness pose significant challenges for practical use. Extensive research into containment materials, handling procedures, and safety protocols would be necessary before widespread industrial adoption could be considered. Additionally, the environmental impact of using such a powerful acid must be carefully evaluated to ensure sustainable and responsible implementation across various industries.
In the field of materials science, fluoroantimonic acid could play a crucial role in the synthesis of novel materials. Its ability to protonate even weak bases suggests potential applications in the production of advanced polymers, super-strong adhesives, and highly resistant coatings. The acid's unique properties might also facilitate the development of new types of ion-exchange membranes, which could have significant implications for energy storage and water purification technologies.
The electronics industry could benefit from fluoroantimonic acid's etching capabilities. Its extreme corrosiveness could be harnessed for precision etching of semiconductor materials, potentially leading to the development of more advanced and miniaturized electronic components. This could contribute to the ongoing trend of device miniaturization and performance enhancement in the electronics sector.
In the pharmaceutical industry, fluoroantimonic acid might serve as a powerful tool for organic synthesis. Its strong protonating ability could enable the creation of complex organic molecules that are challenging to synthesize through conventional methods. This could potentially accelerate drug discovery processes and lead to the development of new classes of pharmaceutical compounds.
The nuclear industry might also find applications for fluoroantimonic acid in the processing and recycling of nuclear fuels. Its extreme acidity could potentially assist in the dissolution of highly resistant materials, aiding in the extraction and purification of valuable nuclear materials. However, the implementation of such applications would require extensive safety measures due to the acid's highly corrosive nature.
While the potential industrial applications of fluoroantimonic acid are promising, it is crucial to note that its extreme reactivity and corrosiveness pose significant challenges for practical use. Extensive research into containment materials, handling procedures, and safety protocols would be necessary before widespread industrial adoption could be considered. Additionally, the environmental impact of using such a powerful acid must be carefully evaluated to ensure sustainable and responsible implementation across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!