The Versatility of Fluoroantimonic Acid in Chemical Industries
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful and versatile compound in the chemical industry. Its discovery in the mid-20th century marked a significant milestone in the field of acid chemistry, revolutionizing our understanding of superacidity and opening new avenues for chemical synthesis and catalysis.
The development of fluoroantimonic acid can be traced back to the pioneering work of Ronald Gillespie and his colleagues in the 1960s. Their research on strong acids led to the creation of this exceptionally potent superacid, which exhibits a Hammett acidity function (H0) of -31.3. This extreme acidity surpasses that of pure sulfuric acid by many orders of magnitude, making fluoroantimonic acid the strongest known superacid.
The unique properties of fluoroantimonic acid stem from its ability to protonate even very weak bases, including compounds that are typically considered non-basic. This characteristic has propelled its application in various industrial processes, particularly in the petrochemical sector, where it serves as a potent catalyst for hydrocarbon cracking and isomerization reactions.
As the chemical industry continues to evolve, the demand for more efficient and environmentally friendly processes has intensified. In this context, fluoroantimonic acid has garnered significant attention due to its potential to facilitate reactions under milder conditions, potentially reducing energy consumption and improving overall process efficiency.
The primary objective of exploring the versatility of fluoroantimonic acid in chemical industries is to unlock its full potential across a wide range of applications. This includes investigating its role in organic synthesis, where it can enable the formation of novel compounds and streamline existing synthetic routes. Additionally, researchers aim to harness its superacidic properties for the development of advanced materials, such as high-performance polymers and specialty chemicals.
Another crucial goal is to address the challenges associated with the handling and containment of fluoroantimonic acid. Due to its extreme corrosiveness and reactivity, developing safer handling protocols and innovative containment materials is paramount to expanding its industrial use. This objective aligns with the broader industry trend towards enhancing safety and sustainability in chemical processes.
Furthermore, the exploration of fluoroantimonic acid's versatility extends to its potential applications in emerging fields such as nanotechnology and energy storage. Researchers are investigating its ability to modify surface properties of materials and its role in the synthesis of advanced electrode materials for next-generation batteries.
As we delve deeper into the capabilities of fluoroantimonic acid, the overarching aim is to bridge the gap between fundamental research and practical industrial applications. This involves not only expanding our understanding of its chemical behavior but also developing scalable and economically viable processes that can leverage its unique properties to drive innovation across multiple sectors of the chemical industry.
The development of fluoroantimonic acid can be traced back to the pioneering work of Ronald Gillespie and his colleagues in the 1960s. Their research on strong acids led to the creation of this exceptionally potent superacid, which exhibits a Hammett acidity function (H0) of -31.3. This extreme acidity surpasses that of pure sulfuric acid by many orders of magnitude, making fluoroantimonic acid the strongest known superacid.
The unique properties of fluoroantimonic acid stem from its ability to protonate even very weak bases, including compounds that are typically considered non-basic. This characteristic has propelled its application in various industrial processes, particularly in the petrochemical sector, where it serves as a potent catalyst for hydrocarbon cracking and isomerization reactions.
As the chemical industry continues to evolve, the demand for more efficient and environmentally friendly processes has intensified. In this context, fluoroantimonic acid has garnered significant attention due to its potential to facilitate reactions under milder conditions, potentially reducing energy consumption and improving overall process efficiency.
The primary objective of exploring the versatility of fluoroantimonic acid in chemical industries is to unlock its full potential across a wide range of applications. This includes investigating its role in organic synthesis, where it can enable the formation of novel compounds and streamline existing synthetic routes. Additionally, researchers aim to harness its superacidic properties for the development of advanced materials, such as high-performance polymers and specialty chemicals.
Another crucial goal is to address the challenges associated with the handling and containment of fluoroantimonic acid. Due to its extreme corrosiveness and reactivity, developing safer handling protocols and innovative containment materials is paramount to expanding its industrial use. This objective aligns with the broader industry trend towards enhancing safety and sustainability in chemical processes.
Furthermore, the exploration of fluoroantimonic acid's versatility extends to its potential applications in emerging fields such as nanotechnology and energy storage. Researchers are investigating its ability to modify surface properties of materials and its role in the synthesis of advanced electrode materials for next-generation batteries.
As we delve deeper into the capabilities of fluoroantimonic acid, the overarching aim is to bridge the gap between fundamental research and practical industrial applications. This involves not only expanding our understanding of its chemical behavior but also developing scalable and economically viable processes that can leverage its unique properties to drive innovation across multiple sectors of the chemical industry.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various chemical industries due to its exceptional reactivity and unique properties. The market demand for this powerful compound has been steadily increasing, driven by its versatile applications across multiple sectors.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in catalytic cracking processes, particularly in the production of high-octane gasoline. Its ability to efficiently break down complex hydrocarbons has led to improved fuel quality and increased refinery yields. This application alone has created a substantial market, with major oil companies investing in research and development to optimize the use of fluoroantimonic acid in their refining processes.
The semiconductor industry has also embraced fluoroantimonic acid for its etching capabilities. As the demand for smaller and more powerful electronic devices continues to grow, manufacturers are increasingly relying on this superacid to achieve precise etching of silicon wafers and other semiconductor materials. This has resulted in a steady rise in demand from electronics manufacturers and foundries worldwide.
In the pharmaceutical sector, fluoroantimonic acid has found applications in the synthesis of complex organic compounds. Its strong acidic properties enable reactions that were previously challenging or impossible, opening new avenues for drug discovery and development. This has led to increased interest from pharmaceutical companies seeking to expand their chemical toolbox for creating novel therapeutic compounds.
The materials science field has also recognized the potential of fluoroantimonic acid in the development of advanced materials. Its use in the production of super-strong polymers and specialty ceramics has created niche markets with high growth potential. Research institutions and materials companies are actively exploring new applications, further driving demand for this versatile compound.
Environmental concerns have also contributed to the market demand for fluoroantimonic acid. Its use in the destruction of persistent organic pollutants and the treatment of hazardous waste has gained traction in recent years. As environmental regulations become more stringent globally, the demand for effective chemical treatment solutions is expected to rise, potentially expanding the market for fluoroantimonic acid in this sector.
Despite its numerous applications and growing demand, the production and handling of fluoroantimonic acid present significant challenges due to its extreme corrosiveness and reactivity. This has led to a relatively concentrated market, with only a few specialized chemical companies capable of producing and supplying the acid safely. As a result, the market is characterized by high entry barriers and premium pricing, which may limit widespread adoption in some industries.
In the petrochemical industry, fluoroantimonic acid plays a crucial role in catalytic cracking processes, particularly in the production of high-octane gasoline. Its ability to efficiently break down complex hydrocarbons has led to improved fuel quality and increased refinery yields. This application alone has created a substantial market, with major oil companies investing in research and development to optimize the use of fluoroantimonic acid in their refining processes.
The semiconductor industry has also embraced fluoroantimonic acid for its etching capabilities. As the demand for smaller and more powerful electronic devices continues to grow, manufacturers are increasingly relying on this superacid to achieve precise etching of silicon wafers and other semiconductor materials. This has resulted in a steady rise in demand from electronics manufacturers and foundries worldwide.
In the pharmaceutical sector, fluoroantimonic acid has found applications in the synthesis of complex organic compounds. Its strong acidic properties enable reactions that were previously challenging or impossible, opening new avenues for drug discovery and development. This has led to increased interest from pharmaceutical companies seeking to expand their chemical toolbox for creating novel therapeutic compounds.
The materials science field has also recognized the potential of fluoroantimonic acid in the development of advanced materials. Its use in the production of super-strong polymers and specialty ceramics has created niche markets with high growth potential. Research institutions and materials companies are actively exploring new applications, further driving demand for this versatile compound.
Environmental concerns have also contributed to the market demand for fluoroantimonic acid. Its use in the destruction of persistent organic pollutants and the treatment of hazardous waste has gained traction in recent years. As environmental regulations become more stringent globally, the demand for effective chemical treatment solutions is expected to rise, potentially expanding the market for fluoroantimonic acid in this sector.
Despite its numerous applications and growing demand, the production and handling of fluoroantimonic acid present significant challenges due to its extreme corrosiveness and reactivity. This has led to a relatively concentrated market, with only a few specialized chemical companies capable of producing and supplying the acid safely. As a result, the market is characterized by high entry barriers and premium pricing, which may limit widespread adoption in some industries.
Current State and Challenges in Synthesis
The synthesis of fluoroantimonic acid presents significant challenges due to its extreme reactivity and corrosive nature. Currently, the most common method involves the reaction of hydrogen fluoride (HF) with antimony pentafluoride (SbF5) in a carefully controlled environment. This process requires specialized equipment and rigorous safety protocols, limiting its production to specialized facilities.
One of the primary challenges in synthesizing fluoroantimonic acid is maintaining the purity of the reactants and products. Even trace amounts of water or other contaminants can significantly affect the acid's properties and reactivity. Researchers are continuously working on developing more efficient purification techniques to ensure the highest possible quality of the final product.
The storage and handling of fluoroantimonic acid pose additional challenges. Due to its extreme reactivity with most materials, including glass and many metals, specialized containers made of fluoropolymers like PTFE (Teflon) are required. These containers must be meticulously maintained and regularly inspected to prevent any potential leaks or degradation.
Scale-up of production remains a significant hurdle in the industrial application of fluoroantimonic acid. While laboratory-scale synthesis is well-established, transitioning to large-scale production introduces new complexities in terms of heat management, reactant mixing, and safety considerations. Engineers are exploring innovative reactor designs and process control systems to address these challenges.
Environmental and safety concerns also play a crucial role in the current state of fluoroantimonic acid synthesis. Stringent regulations govern its production, transport, and use due to its hazardous nature. Researchers are investigating greener synthesis routes and exploring the use of ionic liquids as potential alternatives to mitigate some of these concerns.
Recent advancements in computational chemistry have opened new avenues for optimizing the synthesis process. Molecular modeling and simulation techniques are being employed to better understand the reaction mechanisms and predict optimal conditions for synthesis. These computational approaches are complementing experimental efforts and accelerating the development of more efficient and safer production methods.
The development of in-situ characterization techniques is another area of active research. Real-time monitoring of the synthesis process could provide valuable insights into reaction kinetics and help identify potential issues before they escalate. Spectroscopic methods adapted for highly corrosive environments are being explored for this purpose.
As the demand for fluoroantimonic acid in various industrial applications continues to grow, addressing these challenges in synthesis remains a priority for researchers and chemical engineers. Collaborative efforts between academia and industry are driving innovation in this field, with the goal of making the production of this powerful superacid more efficient, safer, and environmentally sustainable.
One of the primary challenges in synthesizing fluoroantimonic acid is maintaining the purity of the reactants and products. Even trace amounts of water or other contaminants can significantly affect the acid's properties and reactivity. Researchers are continuously working on developing more efficient purification techniques to ensure the highest possible quality of the final product.
The storage and handling of fluoroantimonic acid pose additional challenges. Due to its extreme reactivity with most materials, including glass and many metals, specialized containers made of fluoropolymers like PTFE (Teflon) are required. These containers must be meticulously maintained and regularly inspected to prevent any potential leaks or degradation.
Scale-up of production remains a significant hurdle in the industrial application of fluoroantimonic acid. While laboratory-scale synthesis is well-established, transitioning to large-scale production introduces new complexities in terms of heat management, reactant mixing, and safety considerations. Engineers are exploring innovative reactor designs and process control systems to address these challenges.
Environmental and safety concerns also play a crucial role in the current state of fluoroantimonic acid synthesis. Stringent regulations govern its production, transport, and use due to its hazardous nature. Researchers are investigating greener synthesis routes and exploring the use of ionic liquids as potential alternatives to mitigate some of these concerns.
Recent advancements in computational chemistry have opened new avenues for optimizing the synthesis process. Molecular modeling and simulation techniques are being employed to better understand the reaction mechanisms and predict optimal conditions for synthesis. These computational approaches are complementing experimental efforts and accelerating the development of more efficient and safer production methods.
The development of in-situ characterization techniques is another area of active research. Real-time monitoring of the synthesis process could provide valuable insights into reaction kinetics and help identify potential issues before they escalate. Spectroscopic methods adapted for highly corrosive environments are being explored for this purpose.
As the demand for fluoroantimonic acid in various industrial applications continues to grow, addressing these challenges in synthesis remains a priority for researchers and chemical engineers. Collaborative efforts between academia and industry are driving innovation in this field, with the goal of making the production of this powerful superacid more efficient, safer, and environmentally sustainable.
Existing Handling and Storage Solutions
01 Chemical synthesis applications
Fluoroantimonic acid demonstrates versatility in chemical synthesis, particularly in reactions involving organic compounds. Its strong acidity and unique properties make it useful for catalyzing various reactions, including isomerizations, alkylations, and polymerizations. This superacid can facilitate reactions that are difficult or impossible with conventional acids, opening up new possibilities in organic synthesis.- Chemical synthesis applications: Fluoroantimonic acid demonstrates versatility in chemical synthesis due to its strong acidity. It can catalyze various reactions, including alkylations, acylations, and isomerizations. Its ability to protonate even weak bases makes it useful in generating reactive intermediates for organic synthesis.
- Materials processing: The acid's corrosive nature and high reactivity make it valuable in materials processing. It can be used for etching and cleaning surfaces, particularly in the semiconductor industry. Its ability to dissolve many materials allows for specialized applications in metal treatment and purification processes.
- Analytical chemistry techniques: Fluoroantimonic acid's unique properties make it useful in various analytical chemistry techniques. It can be employed in spectroscopy, chromatography, and other analytical methods where strong acid conditions are required. Its ability to protonate compounds can aid in structural elucidation and chemical analysis.
- Energy storage applications: The acid's high conductivity and electrochemical stability make it potentially useful in energy storage applications. It could be used in advanced battery technologies or as an electrolyte in certain types of fuel cells, where its strong acidic properties can enhance performance.
- Waste treatment and environmental remediation: Fluoroantimonic acid's strong oxidizing properties make it potentially useful in waste treatment and environmental remediation processes. It could be used to break down persistent organic pollutants or in the treatment of industrial effluents, though its use would require careful handling due to its corrosive nature.
02 Materials processing and treatment
The versatility of fluoroantimonic acid extends to materials processing and treatment. It can be used for etching and surface modification of various materials, including metals, semiconductors, and ceramics. Its strong acidic properties allow for efficient removal of oxide layers and other impurities, making it valuable in the preparation of high-purity materials for advanced applications.Expand Specific Solutions03 Analytical chemistry applications
Fluoroantimonic acid finds applications in analytical chemistry due to its unique properties. It can be used as a powerful solvent for dissolving and analyzing complex materials that are resistant to other acids. This superacid is also employed in spectroscopic techniques and chromatography, enabling the detection and characterization of challenging compounds in various fields of research.Expand Specific Solutions04 Energy storage and conversion
The versatility of fluoroantimonic acid extends to energy-related applications. It can be used in the development of advanced battery technologies, fuel cells, and other energy storage and conversion devices. The superacid's unique properties allow for the creation of novel electrolytes and electrode materials with enhanced performance characteristics.Expand Specific Solutions05 Industrial cleaning and decontamination
Fluoroantimonic acid demonstrates versatility in industrial cleaning and decontamination processes. Its strong acidic properties make it effective for removing stubborn contaminants, scale, and corrosion products from industrial equipment and surfaces. The superacid can be used in specialized cleaning formulations for challenging applications in various industries, including petrochemical, semiconductor, and manufacturing sectors.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Production
The versatility of fluoroantimonic acid in chemical industries is at a nascent stage of development, with a growing market potential due to its unique properties. The technology's maturity is still evolving, as evidenced by ongoing research and development efforts from key players. Companies like DAIKIN INDUSTRIES Ltd., DuPont de Nemours, Inc., and 3M Innovative Properties Co. are at the forefront of exploring applications in various sectors. Academic institutions such as Oxford University Innovation Ltd. and Yale University are contributing to fundamental research, while industrial giants like Honeywell International Technologies Ltd. and Central Glass Co., Ltd. are focusing on practical applications. The market size is expanding as new uses are discovered, particularly in advanced materials and specialty chemicals.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the production and handling of fluoroantimonic acid, utilizing specialized materials and equipment to contain this highly corrosive superacid. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under precise temperature and pressure conditions. DuPont's technology also includes advanced safety protocols and containment systems to manage the acid's extreme reactivity and potential hazards. The company has integrated this superacid into various industrial processes, particularly in the petrochemical sector, where it serves as a powerful catalyst for hydrocarbon isomerization and alkylation reactions.
Strengths: Extensive experience in handling hazardous materials, established safety protocols, and integration with existing industrial processes. Weaknesses: High production costs and limited applications due to the acid's extreme reactivity.
3M Innovative Properties Co.
Technical Solution: 3M has developed a novel approach to harnessing the power of fluoroantimonic acid in advanced materials synthesis. Their technology involves using the superacid as a catalyst in the production of high-performance fluoropolymers and specialty chemicals. 3M's process incorporates a unique containment system that allows for the controlled application of fluoroantimonic acid in microscale reactions, minimizing risks while maximizing efficiency. This method enables the creation of materials with exceptional chemical resistance and thermal stability, finding applications in aerospace, electronics, and industrial coatings. 3M has also pioneered techniques for recovering and recycling the acid, enhancing the sustainability of their processes.
Strengths: Innovative microscale application techniques, diverse product applications, and focus on sustainability. Weaknesses: Limited large-scale production capabilities and high initial investment costs.
Core Innovations in Superacid Chemistry
Fluorination processes
PatentActiveUS20230339829A1
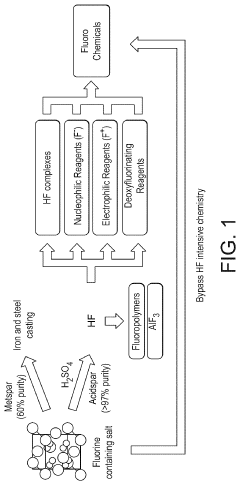
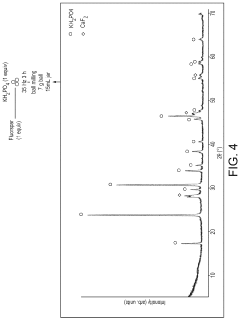
Innovation
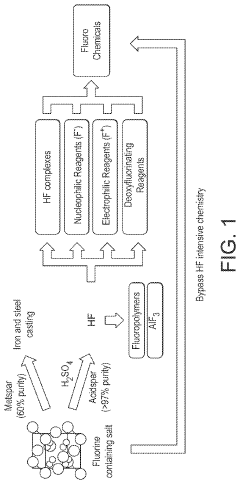
- A process involving the pulverization of calcium fluoride and fluorapatite with specific ionic compounds under mechanochemical conditions to form a fluorinating reagent, bypassing the need for hydrofluoric acid and enabling the direct conversion of these compounds into fluorochemicals.
Fluorination processes
PatentInactiveUS20240017996A1
Innovation
- A process involving the pulverization of calcium fluoride or fluorapatite with specific ionic compounds under mechanochemical conditions to form a fluorinating reagent, bypassing the need for hydrofluoric acid and enabling the direct conversion of these compounds into fluorochemicals.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges in chemical industries. Its extreme corrosiveness and reactivity necessitate stringent handling protocols to protect workers and prevent accidents. Personal protective equipment, including chemical-resistant suits, gloves, and respiratory protection, is mandatory when working with this substance.
Specialized containment systems are crucial to prevent leaks or spills, as fluoroantimonic acid can react violently with many materials, including glass and most metals. Storage and transport containers must be made of materials resistant to its corrosive effects, such as Teflon or certain high-grade alloys. Emergency response plans and specialized training for personnel are essential to mitigate risks associated with potential exposure or release.
Environmental considerations are equally critical. The acid's potential for environmental damage is severe, as it can cause rapid and extensive contamination of soil and water systems. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of any residues or byproducts. Air quality monitoring is necessary to detect and control any vapors or aerosols that may be released during handling or processing.
The use of fluoroantimonic acid also raises concerns about its long-term environmental impact. Its production and use may contribute to the release of fluorine compounds, which can persist in the environment and potentially affect ecosystems. Industries must invest in advanced emission control technologies and implement closed-loop systems to minimize environmental release.
Regulatory compliance is a key aspect of managing fluoroantimonic acid. Industries must adhere to strict guidelines set by environmental protection agencies and occupational safety organizations. This includes regular safety audits, environmental impact assessments, and detailed documentation of handling procedures and incident reports.
Research into safer alternatives and process improvements is ongoing to reduce the reliance on such hazardous substances. This includes exploring less corrosive catalysts or developing new synthetic routes that avoid the use of superacids altogether. Such efforts aim to balance the industrial benefits of fluoroantimonic acid with the imperative of environmental stewardship and worker safety.
Specialized containment systems are crucial to prevent leaks or spills, as fluoroantimonic acid can react violently with many materials, including glass and most metals. Storage and transport containers must be made of materials resistant to its corrosive effects, such as Teflon or certain high-grade alloys. Emergency response plans and specialized training for personnel are essential to mitigate risks associated with potential exposure or release.
Environmental considerations are equally critical. The acid's potential for environmental damage is severe, as it can cause rapid and extensive contamination of soil and water systems. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of any residues or byproducts. Air quality monitoring is necessary to detect and control any vapors or aerosols that may be released during handling or processing.
The use of fluoroantimonic acid also raises concerns about its long-term environmental impact. Its production and use may contribute to the release of fluorine compounds, which can persist in the environment and potentially affect ecosystems. Industries must invest in advanced emission control technologies and implement closed-loop systems to minimize environmental release.
Regulatory compliance is a key aspect of managing fluoroantimonic acid. Industries must adhere to strict guidelines set by environmental protection agencies and occupational safety organizations. This includes regular safety audits, environmental impact assessments, and detailed documentation of handling procedures and incident reports.
Research into safer alternatives and process improvements is ongoing to reduce the reliance on such hazardous substances. This includes exploring less corrosive catalysts or developing new synthetic routes that avoid the use of superacids altogether. Such efforts aim to balance the industrial benefits of fluoroantimonic acid with the imperative of environmental stewardship and worker safety.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly fluoroantimonic acid, is complex and multifaceted due to the substance's extreme corrosiveness and reactivity. Governments and international bodies have established stringent guidelines to ensure safe handling, storage, and disposal of this powerful chemical agent.
In the United States, the Environmental Protection Agency (EPA) regulates fluoroantimonic acid under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) sets strict workplace safety standards, mandating proper personal protective equipment and handling procedures. The Department of Transportation (DOT) classifies it as a hazardous material, subject to specific packaging and transportation requirements.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes rigorous controls on the production, import, and use of fluoroantimonic acid. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, fluoroantimonic acid is classified as a corrosive substance, requiring specific labeling and safety data sheets.
Many countries have implemented additional regulations governing the use of superacids in industrial processes. These often include mandatory risk assessments, emergency response plans, and regular inspections of facilities handling such substances. Some jurisdictions require special permits or licenses for companies working with fluoroantimonic acid.
The chemical industry has also developed voluntary standards and best practices for superacid usage. Organizations like the American Chemistry Council and the European Chemical Industry Council provide guidance on safe handling, storage, and disposal methods that often exceed regulatory requirements.
Research institutions and universities working with fluoroantimonic acid must adhere to strict protocols set by institutional review boards and safety committees. These typically include detailed standard operating procedures, specialized training for personnel, and robust waste management systems.
As awareness of environmental and health risks associated with superacids grows, regulatory frameworks continue to evolve. There is an increasing focus on promoting safer alternatives and minimizing the use of highly hazardous substances where possible. This trend is likely to result in even more stringent controls on fluoroantimonic acid usage in the future, potentially impacting its versatility in chemical industries.
In the United States, the Environmental Protection Agency (EPA) regulates fluoroantimonic acid under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) sets strict workplace safety standards, mandating proper personal protective equipment and handling procedures. The Department of Transportation (DOT) classifies it as a hazardous material, subject to specific packaging and transportation requirements.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes rigorous controls on the production, import, and use of fluoroantimonic acid. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, fluoroantimonic acid is classified as a corrosive substance, requiring specific labeling and safety data sheets.
Many countries have implemented additional regulations governing the use of superacids in industrial processes. These often include mandatory risk assessments, emergency response plans, and regular inspections of facilities handling such substances. Some jurisdictions require special permits or licenses for companies working with fluoroantimonic acid.
The chemical industry has also developed voluntary standards and best practices for superacid usage. Organizations like the American Chemistry Council and the European Chemical Industry Council provide guidance on safe handling, storage, and disposal methods that often exceed regulatory requirements.
Research institutions and universities working with fluoroantimonic acid must adhere to strict protocols set by institutional review boards and safety committees. These typically include detailed standard operating procedures, specialized training for personnel, and robust waste management systems.
As awareness of environmental and health risks associated with superacids grows, regulatory frameworks continue to evolve. There is an increasing focus on promoting safer alternatives and minimizing the use of highly hazardous substances where possible. This trend is likely to result in even more stringent controls on fluoroantimonic acid usage in the future, potentially impacting its versatility in chemical industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!