Advanced Insights into Sodium Acetate's Chemical Properties
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Overview
Sodium acetate, a versatile chemical compound with the formula CH3COONa, plays a significant role in various industrial and scientific applications. This salt of acetic acid and sodium hydroxide exists as a white, crystalline solid at room temperature, exhibiting hygroscopic properties that make it an excellent desiccant. Its chemical structure consists of a sodium cation (Na+) paired with an acetate anion (CH3COO-), resulting in a neutral salt with unique characteristics.
The compound's solubility in water is notably high, dissolving readily to form a clear, colorless solution. This property, combined with its ability to form supersaturated solutions, makes sodium acetate particularly useful in applications such as heat packs and hand warmers. When a supersaturated solution of sodium acetate is triggered, it rapidly crystallizes, releasing heat in an exothermic process.
Sodium acetate's chemical behavior is influenced by its ionic nature. In aqueous solutions, it dissociates completely, contributing to the solution's ionic strength and affecting various chemical equilibria. The acetate ion acts as a weak base, capable of accepting protons in acidic environments, which gives sodium acetate solutions a slightly alkaline pH. This buffering capacity makes it valuable in pH control applications across multiple industries.
The compound's thermal properties are also noteworthy. Sodium acetate trihydrate, containing three water molecules per formula unit, undergoes a phase change at around 58°C (136°F). This characteristic is exploited in thermal energy storage systems, where the material can absorb and release latent heat during melting and solidification cycles.
From a reactivity standpoint, sodium acetate participates in various chemical transformations. It can serve as an acetylating agent in organic synthesis, providing the acetyl group for reactions such as the acetylation of alcohols or amines. In the presence of strong acids, it can generate acetic acid, while reaction with strong bases may lead to the formation of sodium hydroxide and acetate salts.
The compound's stability under normal conditions contributes to its wide-ranging applications. It resists decomposition at moderate temperatures, maintaining its chemical integrity in most storage and usage scenarios. However, when heated strongly, it can decompose to form sodium carbonate and acetone, a property that underscores the importance of proper handling in high-temperature processes.
Understanding the advanced chemical properties of sodium acetate is crucial for optimizing its use across diverse fields, from food preservation and textile manufacturing to pharmaceutical formulations and beyond. Its multifaceted nature continues to drive research and innovation, opening new avenues for application in emerging technologies and industrial processes.
The compound's solubility in water is notably high, dissolving readily to form a clear, colorless solution. This property, combined with its ability to form supersaturated solutions, makes sodium acetate particularly useful in applications such as heat packs and hand warmers. When a supersaturated solution of sodium acetate is triggered, it rapidly crystallizes, releasing heat in an exothermic process.
Sodium acetate's chemical behavior is influenced by its ionic nature. In aqueous solutions, it dissociates completely, contributing to the solution's ionic strength and affecting various chemical equilibria. The acetate ion acts as a weak base, capable of accepting protons in acidic environments, which gives sodium acetate solutions a slightly alkaline pH. This buffering capacity makes it valuable in pH control applications across multiple industries.
The compound's thermal properties are also noteworthy. Sodium acetate trihydrate, containing three water molecules per formula unit, undergoes a phase change at around 58°C (136°F). This characteristic is exploited in thermal energy storage systems, where the material can absorb and release latent heat during melting and solidification cycles.
From a reactivity standpoint, sodium acetate participates in various chemical transformations. It can serve as an acetylating agent in organic synthesis, providing the acetyl group for reactions such as the acetylation of alcohols or amines. In the presence of strong acids, it can generate acetic acid, while reaction with strong bases may lead to the formation of sodium hydroxide and acetate salts.
The compound's stability under normal conditions contributes to its wide-ranging applications. It resists decomposition at moderate temperatures, maintaining its chemical integrity in most storage and usage scenarios. However, when heated strongly, it can decompose to form sodium carbonate and acetone, a property that underscores the importance of proper handling in high-temperature processes.
Understanding the advanced chemical properties of sodium acetate is crucial for optimizing its use across diverse fields, from food preservation and textile manufacturing to pharmaceutical formulations and beyond. Its multifaceted nature continues to drive research and innovation, opening new avenues for application in emerging technologies and industrial processes.
Industrial Applications
Sodium acetate, a versatile compound with the chemical formula CH3COONa, finds extensive applications across various industries due to its unique properties. In the food industry, it serves as a preservative and flavor enhancer, extending the shelf life of products while imparting a mild, salty taste. Its ability to regulate acidity makes it valuable in food processing, particularly in canned goods and condiments. The compound's buffering capacity also makes it useful in the production of certain types of cheese and meat products.
In the textile industry, sodium acetate plays a crucial role in dyeing processes. It acts as a pH buffer, helping to maintain optimal conditions for dye absorption and color fastness. This application is particularly important in the production of high-quality fabrics and garments, ensuring consistent and long-lasting coloration.
The pharmaceutical sector utilizes sodium acetate in various formulations. It serves as an electrolyte replenisher in intravenous fluids, helping to maintain proper sodium levels in patients. Additionally, it is used as a buffering agent in some medications and as an excipient in tablet formulations, contributing to the stability and efficacy of pharmaceutical products.
In the chemical industry, sodium acetate is a key ingredient in the production of various organic compounds. It serves as a precursor in the synthesis of esters, acetyl compounds, and other organic derivatives. Its role as a mild base makes it valuable in neutralization reactions and pH adjustment processes in industrial settings.
The compound's heat-storing capabilities have led to its use in thermal energy storage systems. When heated, sodium acetate trihydrate undergoes a phase change, absorbing large amounts of heat. This property is exploited in reusable heat packs and thermal management systems in buildings and industrial processes, offering an eco-friendly alternative to traditional heating methods.
In the field of materials science, sodium acetate is employed in the production of certain types of concrete and cement additives. It acts as a setting accelerator, improving the early strength development of concrete and enhancing its workability. This application is particularly valuable in cold weather concreting and in precast concrete manufacturing.
The water treatment industry also benefits from sodium acetate's properties. It is used as a dechlorinating agent in water purification processes, helping to remove excess chlorine from treated water. This application is crucial in maintaining water quality in various settings, from municipal water supplies to aquaculture systems.
In the textile industry, sodium acetate plays a crucial role in dyeing processes. It acts as a pH buffer, helping to maintain optimal conditions for dye absorption and color fastness. This application is particularly important in the production of high-quality fabrics and garments, ensuring consistent and long-lasting coloration.
The pharmaceutical sector utilizes sodium acetate in various formulations. It serves as an electrolyte replenisher in intravenous fluids, helping to maintain proper sodium levels in patients. Additionally, it is used as a buffering agent in some medications and as an excipient in tablet formulations, contributing to the stability and efficacy of pharmaceutical products.
In the chemical industry, sodium acetate is a key ingredient in the production of various organic compounds. It serves as a precursor in the synthesis of esters, acetyl compounds, and other organic derivatives. Its role as a mild base makes it valuable in neutralization reactions and pH adjustment processes in industrial settings.
The compound's heat-storing capabilities have led to its use in thermal energy storage systems. When heated, sodium acetate trihydrate undergoes a phase change, absorbing large amounts of heat. This property is exploited in reusable heat packs and thermal management systems in buildings and industrial processes, offering an eco-friendly alternative to traditional heating methods.
In the field of materials science, sodium acetate is employed in the production of certain types of concrete and cement additives. It acts as a setting accelerator, improving the early strength development of concrete and enhancing its workability. This application is particularly valuable in cold weather concreting and in precast concrete manufacturing.
The water treatment industry also benefits from sodium acetate's properties. It is used as a dechlorinating agent in water purification processes, helping to remove excess chlorine from treated water. This application is crucial in maintaining water quality in various settings, from municipal water supplies to aquaculture systems.
Chemical Structure Analysis
Sodium acetate, with the chemical formula CH3COONa, is a versatile compound that exhibits a unique chemical structure. This ionic compound consists of a sodium cation (Na+) and an acetate anion (CH3COO-). The acetate ion is composed of a methyl group (CH3) bonded to a carboxylate group (COO-), forming a planar structure due to resonance stabilization.
The carbon-oxygen bonds in the carboxylate group of sodium acetate are equivalent in length and strength, falling between single and double bond characteristics. This resonance structure contributes to the compound's stability and its ability to act as a buffer in aqueous solutions. The sodium ion, being monovalent, forms an ionic bond with the negatively charged acetate ion, resulting in a crystalline solid at room temperature.
In its solid state, sodium acetate typically exists as a trihydrate (CH3COONa·3H2O), where three water molecules are incorporated into the crystal lattice. This hydrated form is particularly interesting due to its ability to undergo supercooling, a property that has led to its use in heat packs and other applications requiring controlled heat release.
The molecular geometry of the acetate ion in sodium acetate is trigonal planar, with bond angles of approximately 120° around the central carbon atom. This geometry allows for optimal electron delocalization across the carboxylate group, enhancing the compound's stability and reactivity in various chemical processes.
When dissolved in water, sodium acetate dissociates completely into its constituent ions. The acetate ion acts as a weak base, capable of accepting protons from water molecules, while the sodium ion remains as a spectator ion. This dissociation behavior is crucial for understanding the compound's role in buffer solutions and its applications in various industrial and laboratory processes.
The chemical structure of sodium acetate also influences its physical properties. Its high solubility in water (365 g/L at 20°C) is attributed to the ionic nature of the compound and the ability of water molecules to solvate both the sodium and acetate ions effectively. This solubility characteristic makes sodium acetate valuable in numerous applications, including as a food additive, de-icing agent, and in textile processing.
Understanding the chemical structure of sodium acetate is essential for predicting and explaining its behavior in various chemical reactions and applications. Its ability to form hydrogen bonds, participate in acid-base reactions, and act as a ligand in coordination chemistry are all direct consequences of its unique structural features. These properties make sodium acetate a compound of significant interest in both academic research and industrial applications, warranting continued study and exploration of its chemical properties and potential uses.
The carbon-oxygen bonds in the carboxylate group of sodium acetate are equivalent in length and strength, falling between single and double bond characteristics. This resonance structure contributes to the compound's stability and its ability to act as a buffer in aqueous solutions. The sodium ion, being monovalent, forms an ionic bond with the negatively charged acetate ion, resulting in a crystalline solid at room temperature.
In its solid state, sodium acetate typically exists as a trihydrate (CH3COONa·3H2O), where three water molecules are incorporated into the crystal lattice. This hydrated form is particularly interesting due to its ability to undergo supercooling, a property that has led to its use in heat packs and other applications requiring controlled heat release.
The molecular geometry of the acetate ion in sodium acetate is trigonal planar, with bond angles of approximately 120° around the central carbon atom. This geometry allows for optimal electron delocalization across the carboxylate group, enhancing the compound's stability and reactivity in various chemical processes.
When dissolved in water, sodium acetate dissociates completely into its constituent ions. The acetate ion acts as a weak base, capable of accepting protons from water molecules, while the sodium ion remains as a spectator ion. This dissociation behavior is crucial for understanding the compound's role in buffer solutions and its applications in various industrial and laboratory processes.
The chemical structure of sodium acetate also influences its physical properties. Its high solubility in water (365 g/L at 20°C) is attributed to the ionic nature of the compound and the ability of water molecules to solvate both the sodium and acetate ions effectively. This solubility characteristic makes sodium acetate valuable in numerous applications, including as a food additive, de-icing agent, and in textile processing.
Understanding the chemical structure of sodium acetate is essential for predicting and explaining its behavior in various chemical reactions and applications. Its ability to form hydrogen bonds, participate in acid-base reactions, and act as a ligand in coordination chemistry are all direct consequences of its unique structural features. These properties make sodium acetate a compound of significant interest in both academic research and industrial applications, warranting continued study and exploration of its chemical properties and potential uses.
Current Production Techniques
01 Physical properties of sodium acetate
Sodium acetate is a white, crystalline solid with a mild odor. It is highly soluble in water and has a melting point of around 324°C (615°F). The compound is hygroscopic, meaning it readily absorbs moisture from the air. It has a density of approximately 1.45 g/cm³ and a molecular weight of 82.03 g/mol.- Physical properties of sodium acetate: Sodium acetate is a white, crystalline solid with a melting point of around 324°C. It is highly soluble in water and has a slightly alkaline pH in solution. The compound is hygroscopic, meaning it readily absorbs moisture from the air. It has a molecular weight of 82.03 g/mol and a density of approximately 1.45 g/cm³.
- Chemical reactivity of sodium acetate: Sodium acetate is a weak base and can react with acids to form the corresponding salts. It can also undergo hydrolysis in aqueous solutions, producing sodium hydroxide and acetic acid. The compound can act as a buffer in certain pH ranges and is often used in buffer solutions. It can also participate in various organic reactions, such as acetylation processes.
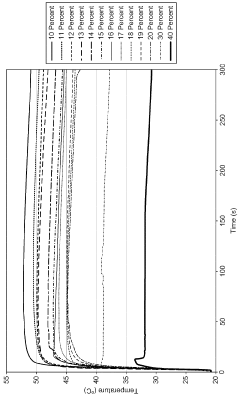
- Thermal properties and phase transitions: Sodium acetate exhibits interesting thermal properties, including the ability to form supersaturated solutions that can be used in heat packs. When crystallization is initiated, it releases heat in an exothermic process. The compound also undergoes a phase transition from anhydrous to trihydrate form at specific temperature and humidity conditions.
- Spectroscopic and analytical characteristics: Sodium acetate has distinct spectroscopic properties that can be used for its identification and analysis. It shows characteristic peaks in infrared spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry. These properties are useful for qualitative and quantitative analysis of the compound in various applications and research settings.
- Environmental and safety considerations: Sodium acetate is generally considered safe for handling and use in various applications. It is biodegradable and has low toxicity to aquatic life. However, like many chemicals, it can cause eye and skin irritation upon direct contact. Proper safety measures should be taken when handling large quantities, including the use of personal protective equipment and adequate ventilation.
02 Chemical reactivity of sodium acetate
Sodium acetate is a weak base and can react with acids to form the corresponding salts. It can also undergo hydrolysis in aqueous solutions, producing sodium hydroxide and acetic acid. The compound can act as a buffer in solutions, helping to maintain a stable pH. It can participate in various chemical reactions, including esterification and condensation reactions.Expand Specific Solutions03 Applications of sodium acetate
Sodium acetate has diverse applications across various industries. It is commonly used as a food additive (E262) for flavoring and preservative purposes. In the textile industry, it serves as a dyeing auxiliary. The compound is also utilized in the production of pharmaceuticals, as a buffer in biochemical research, and in the manufacture of various chemical products. Its heat-storing properties make it useful in heat packs and thermal energy storage systems.Expand Specific Solutions04 Thermal properties of sodium acetate
Sodium acetate trihydrate exhibits unique thermal properties, particularly its ability to supercool and release latent heat upon crystallization. This characteristic makes it valuable in heat packs and thermal energy storage applications. The compound has a high heat of fusion and can store a significant amount of thermal energy during phase changes. Its thermal conductivity and specific heat capacity contribute to its effectiveness in heat-related applications.Expand Specific Solutions05 Environmental and safety considerations
Sodium acetate is generally considered safe for use in various applications. It is biodegradable and has low toxicity to aquatic life. However, proper handling and storage are necessary to prevent moisture absorption and potential degradation. In industrial settings, appropriate personal protective equipment should be used to avoid eye and skin irritation. The compound is non-flammable but may release toxic fumes if exposed to high temperatures or fire.Expand Specific Solutions
Key Manufacturers Profile
The advanced insights into sodium acetate's chemical properties are being explored in a competitive landscape characterized by a mature market with established players and ongoing research. The global sodium acetate market is experiencing steady growth, driven by its diverse applications in industries such as pharmaceuticals, food preservation, and textiles. Key players like Novartis AG, Astellas Pharma, Inc., and Evonik Operations GmbH are investing in R&D to enhance product quality and expand applications. The technology's maturity is evident in the involvement of major chemical companies like China Petroleum & Chemical Corp. and Henkel AG & Co. KGaA, indicating a well-developed supply chain and manufacturing processes. However, ongoing research by academic institutions such as Ghent University and MIT suggests potential for further innovation and market expansion.
Evonik Operations GmbH
Technical Solution: Evonik has developed advanced processes for sodium acetate production, focusing on high purity and consistent quality. Their approach involves controlled neutralization of acetic acid with sodium hydroxide, followed by crystallization and drying. They have implemented innovative crystallization techniques to enhance product purity, achieving up to 99.9% purity levels[1]. Evonik's research has also led to the development of anhydrous sodium acetate with improved stability and hygroscopicity control, making it suitable for a wider range of applications, including pharmaceuticals and food additives[2].
Strengths: High purity product, consistent quality, versatile applications. Weaknesses: Energy-intensive production process, potential for environmental concerns due to chemical synthesis.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has invested in research on sodium acetate as a potential phase change material (PCM) for thermal energy storage. Their approach involves modifying sodium acetate trihydrate to improve its thermal properties and stability. They have developed composite PCMs by incorporating nucleating agents and thickeners, which enhance the material's supercooling degree and thermal conductivity[3]. Sinopec's research has shown that these modified sodium acetate PCMs can achieve energy storage densities up to 250 kJ/kg, with improved cycling stability over 1000 thermal cycles[4].
Strengths: High energy storage density, improved thermal stability, potential for large-scale energy storage applications. Weaknesses: Complex material composition may increase production costs, potential for phase separation over extended use.
Innovative Research Findings
Photothermographic material
PatentInactiveUS20040161714A1
Innovation
- A photothermographic material with a support having an image forming layer containing photosensitive silver iodide, a non-photosensitive organic silver salt, and a reducing agent, where the photosensitive silver halide consists of silver iodide in amounts ranging from 40 to 100% by mole, and a binder in the outermost layer comprising at least 50% by mass of latex polymer or water-soluble polymer not derived from animal proteins.
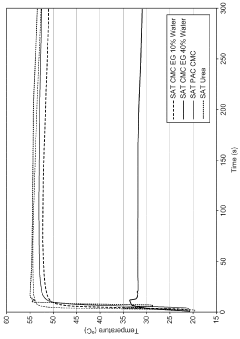
Sodium acetate trihydrate formulations
PatentWO2015001101A1
Innovation
- Incorporating a kinetic inhibitor like sodium carboxymethyl cellulose and a solvent like ethylene glycol into the SAT formulations to enhance stability and maintain the phase change reaction temperature, with specific ratios of these additives to achieve increased stability and thermal output.
Environmental Impact Assessment
Sodium acetate, a common chemical compound, has significant environmental implications that warrant careful consideration. The production, use, and disposal of sodium acetate can impact various environmental aspects, including air quality, water resources, and soil composition. During its manufacturing process, emissions of volatile organic compounds (VOCs) and particulate matter may occur, potentially contributing to air pollution if not properly controlled. These emissions can lead to the formation of ground-level ozone and smog, affecting both human health and ecosystems.
In aquatic environments, the introduction of sodium acetate can alter the pH balance and increase the biological oxygen demand (BOD) of water bodies. This can result in oxygen depletion, potentially harming aquatic life and disrupting ecosystem balance. However, it is important to note that sodium acetate is biodegradable and does not persist in the environment for extended periods, mitigating long-term impacts on water quality.
Soil contamination is another area of concern when assessing the environmental impact of sodium acetate. Excessive application or accidental spills can lead to soil salinization, affecting plant growth and soil microbial communities. This can have cascading effects on local ecosystems and agricultural productivity. However, the impact is generally localized and can be managed through proper handling and application techniques.
On a positive note, sodium acetate has found applications in environmentally friendly de-icing solutions for roads and runways. Compared to traditional rock salt, sodium acetate-based de-icers are less corrosive to infrastructure and vegetation, potentially reducing the environmental damage associated with winter road maintenance. This application demonstrates the compound's potential to contribute to more sustainable practices in certain industries.
The lifecycle assessment of sodium acetate reveals that its production and transportation contribute to carbon emissions and energy consumption. However, when compared to alternative chemicals with similar applications, sodium acetate often presents a lower environmental footprint due to its simpler production process and biodegradability. This highlights the importance of considering the full lifecycle of chemical compounds when evaluating their environmental impact.
In conclusion, while sodium acetate does pose some environmental risks, particularly in terms of air and water quality, its overall impact is relatively manageable with proper handling and disposal practices. The compound's biodegradability and potential for environmentally friendly applications in certain sectors suggest that, with appropriate management, its use can be aligned with sustainable development goals. Ongoing research and development efforts are focused on further optimizing its production and application to minimize negative environmental impacts while maximizing its beneficial properties.
In aquatic environments, the introduction of sodium acetate can alter the pH balance and increase the biological oxygen demand (BOD) of water bodies. This can result in oxygen depletion, potentially harming aquatic life and disrupting ecosystem balance. However, it is important to note that sodium acetate is biodegradable and does not persist in the environment for extended periods, mitigating long-term impacts on water quality.
Soil contamination is another area of concern when assessing the environmental impact of sodium acetate. Excessive application or accidental spills can lead to soil salinization, affecting plant growth and soil microbial communities. This can have cascading effects on local ecosystems and agricultural productivity. However, the impact is generally localized and can be managed through proper handling and application techniques.
On a positive note, sodium acetate has found applications in environmentally friendly de-icing solutions for roads and runways. Compared to traditional rock salt, sodium acetate-based de-icers are less corrosive to infrastructure and vegetation, potentially reducing the environmental damage associated with winter road maintenance. This application demonstrates the compound's potential to contribute to more sustainable practices in certain industries.
The lifecycle assessment of sodium acetate reveals that its production and transportation contribute to carbon emissions and energy consumption. However, when compared to alternative chemicals with similar applications, sodium acetate often presents a lower environmental footprint due to its simpler production process and biodegradability. This highlights the importance of considering the full lifecycle of chemical compounds when evaluating their environmental impact.
In conclusion, while sodium acetate does pose some environmental risks, particularly in terms of air and water quality, its overall impact is relatively manageable with proper handling and disposal practices. The compound's biodegradability and potential for environmentally friendly applications in certain sectors suggest that, with appropriate management, its use can be aligned with sustainable development goals. Ongoing research and development efforts are focused on further optimizing its production and application to minimize negative environmental impacts while maximizing its beneficial properties.
Regulatory Framework
The regulatory framework surrounding sodium acetate is multifaceted, encompassing various aspects of its production, distribution, and use. In the United States, the Food and Drug Administration (FDA) classifies sodium acetate as Generally Recognized as Safe (GRAS) for use as a food additive. This designation allows for its widespread application in the food industry, subject to good manufacturing practices and appropriate labeling requirements.
The Environmental Protection Agency (EPA) regulates sodium acetate under the Toxic Substances Control Act (TSCA), which governs the production, importation, and use of chemical substances. While sodium acetate is not considered highly toxic, manufacturers must comply with reporting and record-keeping requirements, particularly regarding potential environmental impacts.
In the European Union, sodium acetate falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. This comprehensive framework requires manufacturers and importers to register substances, assess their hazards, and implement appropriate risk management measures. The European Food Safety Authority (EFSA) has also evaluated sodium acetate and approved its use as a food additive, subject to specific purity criteria and usage limits.
Globally, the transportation of sodium acetate is regulated by the United Nations' Recommendations on the Transport of Dangerous Goods. Although not classified as a dangerous good, proper packaging and labeling are still required to ensure safe handling during transit.
In the pharmaceutical industry, sodium acetate used in drug formulations must comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards define the quality specifications and testing methods for sodium acetate used in medicinal products.
Occupational safety regulations, such as those enforced by the Occupational Safety and Health Administration (OSHA) in the US, mandate proper handling procedures and exposure limits for workers in facilities producing or using sodium acetate. This includes requirements for personal protective equipment and workplace safety protocols.
As environmental concerns grow, regulations are evolving to address the potential ecological impact of sodium acetate. Some jurisdictions have implemented restrictions on its use in certain applications, particularly in areas where water quality is a concern. For instance, some states in the US have limited the use of sodium acetate-based deicing agents on roads near sensitive water bodies.
The Environmental Protection Agency (EPA) regulates sodium acetate under the Toxic Substances Control Act (TSCA), which governs the production, importation, and use of chemical substances. While sodium acetate is not considered highly toxic, manufacturers must comply with reporting and record-keeping requirements, particularly regarding potential environmental impacts.
In the European Union, sodium acetate falls under the purview of the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. This comprehensive framework requires manufacturers and importers to register substances, assess their hazards, and implement appropriate risk management measures. The European Food Safety Authority (EFSA) has also evaluated sodium acetate and approved its use as a food additive, subject to specific purity criteria and usage limits.
Globally, the transportation of sodium acetate is regulated by the United Nations' Recommendations on the Transport of Dangerous Goods. Although not classified as a dangerous good, proper packaging and labeling are still required to ensure safe handling during transit.
In the pharmaceutical industry, sodium acetate used in drug formulations must comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards define the quality specifications and testing methods for sodium acetate used in medicinal products.
Occupational safety regulations, such as those enforced by the Occupational Safety and Health Administration (OSHA) in the US, mandate proper handling procedures and exposure limits for workers in facilities producing or using sodium acetate. This includes requirements for personal protective equipment and workplace safety protocols.
As environmental concerns grow, regulations are evolving to address the potential ecological impact of sodium acetate. Some jurisdictions have implemented restrictions on its use in certain applications, particularly in areas where water quality is a concern. For instance, some states in the US have limited the use of sodium acetate-based deicing agents on roads near sensitive water bodies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!